
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 25 May 2023
Sec. Neonatology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1149401
This article is part of the Research Topic The Impact of Prenatal Cannabinoid Exposure on Offspring Development View all 6 articles
Introduction: Cannabis use among pregnant women has increased over time. Therefore, there is a great public health need to understand the consequences of in utero cannabis exposure. While several meta-analyses and reviews have summarized the evidence of in utero cannabis exposure on adverse obstetric outcomes (e.g., low birth weight and preterm birth) and long-term offspring development, there has not been a focus on in utero cannabis exposure and risk for structural birth defects.
Methods: We conducted a systematic review using PRISMA guidelines to evaluate the association between in utero cannabis exposure and structural birth defects.
Results: We identified 20 articles to include in our review and focused on interpreting findings from the 12 that adjusted for potential confounders. We report findings by seven organ systems. Within the 12 articles, four reported on cardiac malformations, three reported on central nervous system malformations, one reported on eye malformations, three reported on gastrointestinal malformations, one reported on genitourinary malformations, one reported on musculoskeletal malformations, and two reported on orofacial malformations.
Discussion: Findings on associations between in utero cannabis exposure and birth defects reported in more than two articles were mixed (i.e., findings for cardiac, gastrointestinal, central nervous system malformations). Findings for associations between in utero cannabis exposure and birth defects reported in two articles (i.e., orofacial malformations) or in a single article (eye, genitourinary, and musculoskeletal) suggested that cannabis exposure was not associated with these types of malformations, but strong conclusions cannot be drawn from such sparce research. We review the limitations and gaps in the existing literature and call for more research to rigorously evaluate associations between in utero cannabis exposure and structural birth defects.
Systematic Review Registration: identifier CRD42022308130.
Research has documented an increase in rates of cannabis use among pregnant people over time. Among a nationally representative sample of pregnant individuals in the United States, the prevalence of self-reported prenatal cannabis use in the past month increased from 3.4% in 2002–2003, to 7.0% in 2016–2017 (1). Prenatal cannabis use may increase even more rapidly as more US states legalize cannabis for recreational use (2–7). Moreover, cannabis use in pregnancy could impact fetal development because cannabis is lipid soluble and is able to cross the placenta and blood-brain barrier (8), and some previous studies have suggested a potential link between in-utero cannabis exposure and adverse offspring outcomes [e.g., (9)]. Therefore, there is a great public health need to understand the consequences of in utero cannabis exposure on offspring development. Several meta-analyses and reviews have summarized the evidence of in utero cannabis exposure on adverse obstetric outcomes (e.g., low birth weight and preterm birth) and long-term offspring development (8, 10–15). However, reviews to date have not focused on research regarding in utero cannabis exposure and risk for structural birth defects. The causes and risk factors for many structural birth defects remains unknown, and understanding preventable causes and risk factors for structural birth defects is particularly important given the strong association between birth defects and morbidity/mortality (16). Given this need, we conducted a systematic review using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to evaluate whether in utero cannabis exposure is associated with structural birth defects compared to pregnancies with no cannabis exposure (Prospective Register of Systematic Reviews [PROSPERO] registration number: CRD42022308130; (17)].
Web of Science and PubMed databases were searched for English language articles published before February 1, 2022 utilizing the following key words: “(Pregnancy OR Prenatal OR In utero OR Perinatal) AND (Cannabis OR Marijuana) AND (Birth defects OR Congenital malformations OR Congenital anomalies OR Central nervous system defect OR Neural tube defects OR Holoprosencephaly OR Microcephaly OR Ear defect OR Eye defect OR Gastrointestinal defect OR Biliary atresia OR Esophageal atresia OR Tracheoesophageal fistula OR Intestinal atresia OR Intestinal stenosis OR Pyloric stenosis OR Hypospadias OR Renal agenesis OR Renal hypoplasia OR Renal dysplasia OR Cardiac defect OR Musculoskeletal defect OR Congenital diaphragmatic hernia OR Gastroschisis OR Limb deficiency OR Omphalocele OR Orofacial defect OR Respiratory defect OR Choanal atresia OR Cleft lip OR Cleft palate).” The inclusion criteria were English-language articles and epidemiological studies. Animal studies and review articles were excluded as the focus of our review was strictly on human outcomes.
The search revealed 299 potentially relevant articles of which 48 were duplicates. We created an EndNote library of 251 non-duplicate articles. Two authors then independently reviewed the titles and abstracts of the articles in the EndNote library to exclude articles that did not meet the inclusion criteria. After their independent reviews, the two authors discussed disagreements and together decided to include 37 articles for a full text review. During the full text review, 17 additional articles were excluded for the following reasons: study design was a case study (18), a comparable study was conducted by the same authors using the same dataset (19–23), and the study did not specifically evaluate associations between cannabis exposure in pregnancy and birth defects [e.g., cannabis was included in a general substance use exposure variable or the outcome studied was not a birth defect; (24–34)]. Therefore, the final review included 20 articles (9, 22, 35–53). See Figure 1 for a PRISMA flow diagram illustrating our identification process of articles for our final review.
Of the 20 included articles, 8 were from prospective studies using recruited samples (35–42), and 12 were from retrospective cohort or case-control studies using health care records (9, 43–53). Samples sizes varied from 50 to 3,067,069. Earliest birth years for cohorts varied from 1968 to 1980. Only 3 of the articles reported on studies using urine toxicology tests (46–48); the rest reported on studies that relied on self-report to measure prenatal cannabis use. Of the 17 articles that reported on studies using self-reports to measure cannabis use, 16 had measures of self-reported cannabis use, and 1 had a measure of self-reported cannabis-related diagnoses (43). The outcome definitions varied across studies with some investigating associations with specific malformations and other studies investigating associations with any malformation. While 8 studies did not adjust for any potential confounders (38, 40, 41, 44, 46, 47, 49, 53), the rest adjusted for confounding, though the specific factors adjusted for varied across studies. Table 1 provides information about the characteristics of each individual study.
Table 2 includes information on adjusted associations between in utero cannabis exposure and specific birth defects. When examining associations, we only considered the 12 studies that adjusted for confounding, given the importance in doing so in assessing epidemiologic relationships (54). We included information about associations with specific malformations whenever available. However, given the rarity of specific malformations, most studies evaluated associations with organ specific malformations grouped together.
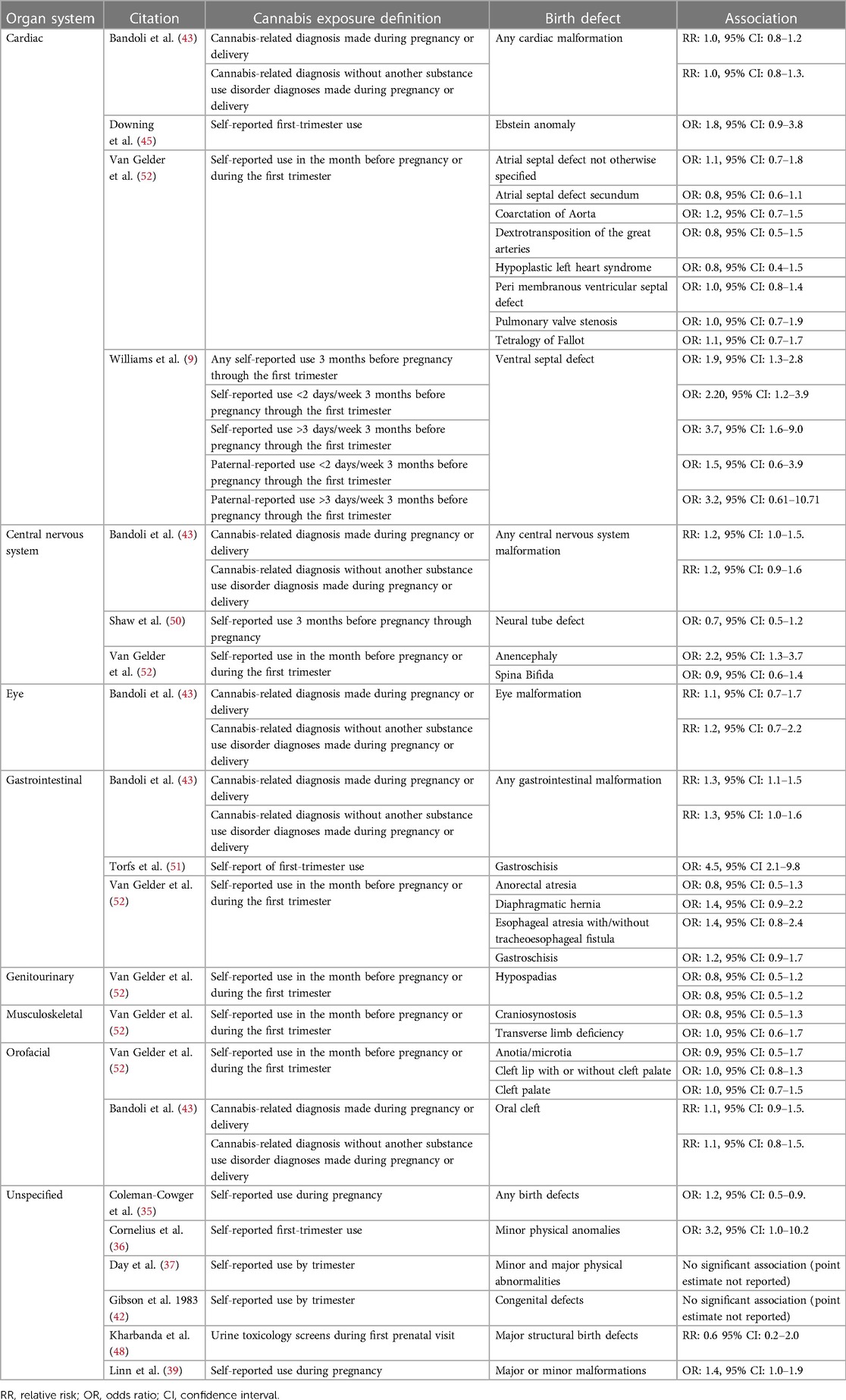
Table 2. Adjusted associations for specific birth defect, organized by organ system from 12 articles that adjust for confounding.
Results were inconsistent across the four articles reporting findings from studies assessing associations between in utero cannabis exposure and cardiac malformations (9, 43, 45, 52). One article (9) indicated a dose-response relationship between self-reported cannabis use three months before pregnancy through the first trimester and ventral septal defect [any use OR: 1.9, 95% CI: 1.3, 2.8; use <2 days/week OR: 2.20, 95% CI: 1.2, 3.9; use >3 days/week OR: 3.7, 95% CI: 1.6, 9.0; (9)]. Another study found increased odds of Ebstein anomaly associated with maternal self-reported first-trimester cannabis use, though the confidence interval around the estimate was wide and included the null [OR: 1.8, 95% CI: 0.9, 3.8; (45)]. Additionally, two other articles (43, 52) reported no elevated risk of any cardiac malformation among infants born to individuals with a cannabis-related diagnosis made during pregnancy or delivery [RR: 1.0, 95% CI: 0.8, 1.2; (43)] and no associations between maternal self-reported cannabis use in the month before pregnancy or during the first trimester of pregnancy and eight specific cardiac malformations [Table 2; (52)].
Three articles reported findings from studies assessing in utero cannabis exposure and central nervous system (CNS) malformations, and results were conflicting (43, 50, 52). Two studies focused on neural tube defects [NTD; (50, 52)]—Van Gelder et al. reported on two subtypes of NTD [anencephaly and spina bifida; (52)], while Shaw et al. focused on any NTD (50). Van Gelder et al. reported increased odds of anencephaly [odds ratio [OR]: 2.2, 95% CI: 1.3–3.7; (52)] but not spina bifida [OR: 0.9, 95% CI: 0.6–1.4; (52)] among infants born to individuals who self-reported cannabis use in the month before pregnancy or during the first trimester of pregnancy (52); and, Shaw et al. failed to find an association between self-reported cannabis use three months before pregnancy through pregnancy and any NTD [OR: 0.7, 95% CI: 0.5–1.2; (50)]. The third study (43) found an increased risk of any CNS malformations among infants born to individuals with a self-reported cannabis-related diagnosis [relative risk [RR]: 1.2, 95% CI: 1.0, 1.5; (43)].
One article reported on the association between in utero cannabis exposure and eye malformation (43). The study failed to find an association between a cannabis-related diagnosis made during pregnancy or delivery and eye malformation [RR: 1.1, 95% CI: 0.7, 1.7; (43)].
Three articles reported findings from studies assessing associations between in utero cannabis exposure and gastrointestinal malformations (43, 51, 52). The findings from these three studies were mixed. Two articles suggested in utero cannabis exposure was associated with increased risk of gastrointestinal malformations. Specifically, one article (43) reported an association between cannabis-related diagnoses made during pregnancy or delivery and any gastrointestinal malformation [RR: 1.3, 95% CI: 1.1, 1.5; (43)]; and, one article (51) reported an association between self-reported cannabis use in the first trimester and gastroschisis [OR: 4.5, 95% CI 2.1–9.8; (51)]. However, another article (52) did not find any significant associations between self-reported cannabis use in the month before pregnancy or during the first trimester and several specific gastrointestinal birth defects [Table 2; (51)], including gastroschisis [OR: 1.2, 95% CI: 0.9, 1.7 (52)].
One article reported on associations between in utero cannabis exposure and genitourinary malformations (52). This study failed to find an association between self-reported cannabis use in the month before pregnancy or during the first trimester and hypospadias [OR: 0.8, 95% CI: 0.5–1.2; (52)].
One article reported on association between in utero cannabis exposure and musculoskeletal malformations (52). The study failed to find associations between self-reported cannabis use in the month before pregnancy or during the first trimester and (a) craniosynostosis (OR: 0.8, 95% CI: 0.5–1.3) or (b) transverse limb deficiency [OR: 1.0, 95% CI: 0.6–1.7; (52)].
Associations between in utero cannabis exposure and specific orofacial malformations were reported on in two articles (43, 52). Both articles reported associations close to the null for each malformation [Table 2; (43, 52)]. Specifically, Van Gelder et al. reported associations close to the null for anotia/microtia (OR: 0.9, 95% CI: 0.5–1.7), cleft lip with or without cleft palate (OR: 1.0, 95% CI: 0.8–1.3), and cleft palate [OR: 1.0, 95% CI: 0.7–1.5; (52)]; and Bandoli et al. reported an association close to the null for oral cleft [RR: 1.1, 95% CI: 0.9, 1.5; (43)].
This systematic review found mixed and inconclusive associations between in utero cannabis exposure and risk for structural birth defects. Results were mixed among (a) the four articles reporting on adjusted associations with cardiac malformations (9, 43, 45, 52), (b) the three articles reporting on adjusted associations with central nervous system malformations (43, 50, 52), and (c) the three articles reporting on adjusted associations with gastrointestinal malformations (43, 51, 52). Some studies suggested in utero cannabis exposure was not associated with these types of birth defects; and, other articles suggesting that in utero cannabis exposure was associated with increased risk of these types of birth defects. Only two articles reported on adjusted associations with orofacial malformations (43, 52); and, only single articles reported on adjusted associations with eye malformation (43), genitourinary malformations (52), and musculoskeletal malformations (52). Though the articles reporting on associations with orofacial, eye, genitourinary, and musculoskeletal malformations all suggested that in utero cannabis exposure was not associated with these types of malformations (43, 52), strong conclusions cannot be drawn from these few studies that all had limitations.
There were several limitations of the included studies that may have contributed to the mixed findings on in utero cannabis exposure and birth defects. These limitations are similar to those of studies on in utero cannabis exposure and other outcomes, such as long-term neurodevelopmental and psychiatric problems (15). First, many of the studies had samples that were relatively small (e.g., 6 of the 20 studies had samples under 500) and reported findings with wide confidence intervals. Therefore, these studies had poor precision and likely were underpowered to detect associations that truly exist. Second, several studies (i.e., 16 of 20) utilized birth cohorts with births occurring more than 20 years ago, which could be problematic given increasing cannabis potency in recent years (55–57) and the proliferation of newer modes of administration (e.g., vaping, edibles) with potentially different risk profiles (58). Third, most studies utilized self-report data, which may underestimate cannabis exposure (59, 60). Therefore, these studies may have mistakenly classified exposed offspring as unexposed, reducing the likelihood of detecting a true association. Fourth, many studies did not address timing of exposure, which is particularly problematic when studying birth defects given that exposures early in pregnancy may be particularly risky for the development of major structural birth defects (61). Fifth, most studies did not assess associations with dose of cannabis exposure. This is a major limitation given that some research has supported a dose-response relationship between in utero cannabis exposure and birth defects (9), and research has shown dose-dependent associations between in utero cannabis exposure and other outcomes (8). Sixth, most studies did not adequately account for potential confounders, such as co-exposure to other substances. Despite the high co-occurrence of cannabis use and use of other substances, particularly tobacco and alcohol, in pregnancy 12 of the 20 studies did not take this into consideration. Therefore, observed associations between in utero cannabis exposure and birth defects could be attributable to exposure to a substance other than cannabis or could be explained by an interactive effect of cannabis use plus use of another substance (62, 63). We note that one study did find similar associations with and without limiting the sample to pregnancies with substance use disorder diagnoses other than cannabis-related diagnoses (43). Nonetheless, more research is needed to parse apart the effects of in utero cannabis exposure from exposure to other substances.
It is important to recognize that the mixed and inconclusive results on associations between in utero cannabis exposure and structural birth defects should not be interpreted as evidence suggesting cannabis use in pregnancy is safe. Rather these results indicate that the relationship between in utero cannabis exposure and structural birth defects is unknown and point to a critical need for future research. This need is particularly pressing given the documented increasing rates in prenatal cannabis use (1).
There are several important avenues for future research. First, samples should be sufficiently large to have adequate statistical power to identify associations if they truly exist. Second, studies with large sample sizes should evaluate associations with specific malformations within organ-specific malformation groups. Third, studies would benefit from including samples comprised of recent birth cohorts given changes in cannabis potency and modes of administration that have occurred in recent years. Fourth, utilizing biological measures (e.g., urine toxicology tests) in addition to self-reported cannabis use would reduce measurement error related to in utero cannabis exposure. Fifth, assessing the influence of timing of exposure and particularly focusing on first-trimester exposure is important. Sixth, it is also important for future studies to quantify the amount of prenatal cannabis exposure by considering the dose, frequency, potency, mode of administration and duration of use during pregnancy. Seventh, studies should utilize methods that rigorously evaluate the potential influence of confounding factors. Using conceptual models based on previous literature, researchers can identify potential factors that may confound associations between in utero cannabis exposure and birth defects. Researchers could also consider using advanced epidemiological methods that have been utilized to study other in utero exposures to help adjust for confounding factors, such as propensity scores, cannabis use before but not during pregnancy as a comparator, and comparisons of differentially exposed siblings [see Sujan et al. for a review of methods that have been used to study antidepressant medications during pregnancy (64)].
Importantly, no single study can implement all of these recommendations, particularly given common obstacles faced by researchers, such as funding limitations restricting the scope of studies, challenges enrolling participants, difficulty obtaining biological samples, and loss to follow-up. However, future research should try to incorporate as many of these recommendations as possible to reduce biases and maximize the overall quality of the studies. Rigorous, high-quality information on the potential consequences of in utero cannabis exposure is vital for individuals to make informed choices about cannabis use in pregnancy, as well as for families and providers caring for infants exposed to cannabis in utero.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
All authors conceptualized the study. AS and AP conducted the literature review and extracted information from the reviewed studies. All authors interpreted the findings. AS drafted the manuscript, and all authors provided critical revisions of the manuscript. KY and LA supervised AS. AS and KY supervised AP. All authors contributed to the article and approved the submitted version.
This study was supported by grants R01DA047405 funded by National Institute on Drug Abuse (NIDA) and R01DA48033 funded by NIDA and Office of the Director, NIH (OD). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical Cannabis use among pregnant women in the United States. JAMA. (2019) 322(2):167–9. doi: 10.1001/jama.2019.7982
2. Geiger A. Support for marijuana legalization continues to rise. (2016). Available at: http://www.pewresearch.org/fact-tank/2016/10/12/support-for-marijuana-legalization-continues-to-rise/
3. State Medical Marijuana Laws. (2017). Available at: http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
4. Gnofam M, Allshouse AA, Stickrath EH, Metz TD. Impact of marijuana legalization on prevalence of maternal marijuana use and perinatal outcomes. Am J Perinatol. (2020) 37(1):59–65. doi: 10.1055/s-0039-1696719
5. Skelton KR, Hecht AA, Benjamin-Neelon SE. Recreational Cannabis legalization in the US and maternal use during the preconception, prenatal, and postpartum periods. Int J Environ Res Public Health. (2020) 17(3):909. doi: 10.3390/ijerph17030909
6. Skelton KR, Hecht AA, Benjamin-Neelon SE. Association of recreational Cannabis legalization with maternal Cannabis use in the preconception, prenatal, and postpartum periods. JAMA Netw Open. (2021) 4(2):e210138. doi: 10.1001/jamanetworkopen.2021.0138
7. Young-Wolff KC, Adams SR, Padon A, Silver LD, Alexeeff SE, Van Den Eeden SK, et al. Association of cannabis retailer proximity and density with cannabis use among pregnant women in northern California after legalization of cannabis for recreational use. JAMA Netw Open. (2021) 4(3):e210694. doi: 10.1001/jamanetworkopen.2021.0694
8. Sharapova SR, Phillips E, Sirocco K, Kaminski JW, Leeb RT, Rolle I. Effects of prenatal marijuana exposure on neuropsychological outcomes in children aged 1–11 years: a systematic review. Paediatr Perinat Epidemiol. (2018) 32(6):512–32. doi: 10.1111/ppe.12505
9. Williams LJ, Correa A, Rasmussen S. Maternal lifestyle factors and risk for ventricular septal defects. Birth Defects Res A Clin Mol Teratol. (2004) 70(2):59–64. doi: 10.1002/bdra.10145
10. Nashed MG, Hardy DB, Laviolette SR. Prenatal cannabinoid exposure: emerging evidence of physiological and neuropsychiatric abnormalities. Front Psychiatry. (2021) 11:10. doi: 10.3389/fpsyt.2020.624275
11. Roncero C, Valriberas-Herrero I, Mezzatesta-Gava M, Villegas JL, Aguilar L, Grau-Lopez L. Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod Health. (2020) 17(1):9. doi: 10.1186/s12978-020-0880-9
12. Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes: a systematic review and meta-analysis. Obstet Gynecol. (2016) 128(4):713–23. doi: 10.1097/AOG.0000000000001649
13. Gunn JKL, Rosales CB, Center KE, Nunez A, Gibson SJ, Christ C, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. (2016) 6(4):e009986. doi: 10.1136/bmjopen-2015-009986
14. Singh S, Filion K, Abenhaim H, Eisenberg M. Prevalence and outcomes of prenatal recreational cannabis use in high-income countries: a scoping review. BJOG. (2020) 127(1):8–16. doi: 10.1111/1471-0528.15946
15. Sujan AC, Young-Wolff KC, Avalos LA. In-utero cannabis exposure and long-term psychiatric and neurodevelopmental outcomes: the limitations of existing literature and recommendations for future research. Birth Defects Res. (2022) 114(13):689–713. doi: 10.1002/bdr2.2060
16. Chung CS, Myrianthopoulos NC. Congenital anomalies: mortality and morbidity, burden and classification. Am J Med Genet. (1987) 27(3):505–23. doi: 10.1002/ajmg.1320270304
17. Sujan AC, Pal A, Avalos LA, Young-Wolff KC. Systematic review and meta-analyses of cannabis exposure during pregnancy and risk for birth defects in infants. PROSPERO CRD42022308130. (2022). Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022308130
18. Qazi QH, Mariano E, Milman DH, Beller E, Crombleholme W. Abnormalities in offspring associated with prenatal marihuana exposure. Dev Pharmacol Ther. (1985) 8(2):141–8. doi: 10.1159/000457031
19. Fried PA. Postnatal consequences of maternal marijuana use in humans. Ann N Y Acad Sci. (1989) 562:123–32. doi: 10.1111/j.1749-6632.1989.tb21011.x
20. Conner CS. Marijuana and alcohol use in pregnancy. Drug Intell Clin Pharm. (1984) 18(3):233–4.6365495
21. Zhang A, Marshall R, Kelsberg G. Clinical inquiry: what effects–if any–does marijuana use during pregnancy have on the fetus or child? J Fam Pract. (2017) 66(7):462–6.28700762
22. Day N, Sambamoorthi U, Taylor P, Richardson G, Robles N, Jhon Y, et al. Prenatal marijuana use and neonatal outcome. Neurotoxicol Teratol. (1991) 13(3):329–34. doi: 10.1016/0892-0362(91)90079-C
23. van Gelder MM, Reefhuis J, Caton AR, Werler MM, Druschel CM, Roeleveld N. Maternal periconceptional illicit drug use and the risk of congenital malformations. Epidemiology. (2009) 20(1):60–6. doi: 10.1097/EDE.0b013e31818e5930
24. Kennare R, Heard A, Chan A. Substance use during pregnancy: risk factors and obstetric and perinatal outcomes in South Australia. Aust N Z J Obstet Gynaecol. (2005) 45(3):220–5. doi: 10.1111/j.1479-828X.2005.00379.x
25. Ventura CV, Ventura LO, Miller MT, Cronemberger MF, Dias CS, Dias MJM, et al. Teratogen exposure and congenital ocular abnormalities in Brazilian patients with mobius sequence. Arq Bras Oftalmol. (2014) 77(5):300–4. doi: 10.5935/0004-2749.20140076
26. Wilson PD, Loffredo CA, Correa-Villaseñor A, Ferencz C. Attributable fraction for cardiac malformations. Am J Epidemiol. (1998) 148(5):414–23. doi: 10.1093/oxfordjournals.aje.a009666
27. Weinsheimer RL, Yanchar NL. Impact of maternal substance abuse and smoking on children with gastroschisis. J Pediatr Surg. (2008) 43(5):879–83. doi: 10.1016/j.jpedsurg.2007.12.032
28. Auger N, Rheaume MA, Low N, Lee GE, Ayoub A, Luu TM. Impact of prenatal exposure to opioids, cocaine, and Cannabis on eye disorders in children. J Addict Med. (2020) 14(6):459–66. doi: 10.1097/ADM.0000000000000621
29. Day NL, Richardson GA, Geva D, Robles N. Alcohol, marijuana, and tobacco: effects of prenatal exposure on offspring growth and morphology at age six. Alcohol Clin Exp Res. (1994) 18(4):786–94. doi: 10.1111/j.1530-0277.1994.tb00041.x
30. Reece AS, Hulse GK. Cannabis teratology explains current patterns of coloradan congenital defects: the contribution of increased cannabinoid exposure to rising teratological trends. Clin Pediatr (Phila). (2019) 58(10):1085–123. doi: 10.1177/0009922819861281
31. Reece AS, Hulse GK. Contemporary epidemiology of rising atrial septal defect trends across USA 1991–2016: a combined ecological geospatiotemporal and causal inferential study. BMC Pediatr. (2020) 20(1):539. doi: 10.1186/s12887-020-02431-z
32. Reece AS, Hulse GK. Canadian Cannabis consumption and patterns of congenital anomalies: an ecological geospatial analysis. J Addict Med. (2020) 14(5):E195–210. doi: 10.1097/ADM.0000000000000638
33. Reece AS, Hulse GK. Geotemporospatial and causal inference epidemiological analysis of US survey and overview of cannabis, cannabidiol and cannabinoid genotoxicity in relation to congenital anomalies 2001–2015. BMC Pediatr. (2022) 22(1):47. doi: 10.1186/s12887-021-02996-3
34. Peterson BS, Rosen T, Dingman S, Toth ZR, Sawardekar S, Hao X, et al. Associations of maternal prenatal drug abuse with measures of newborn brain structure, tissue organization, and metabolite concentrations. JAMA Pediatr. (2020) 174(9):831–42. doi: 10.1001/jamapediatrics.2020.1622
35. Coleman-Cowger VH, Oga EA, Peters EN, Mark K. Prevalence and associated birth outcomes of co-use of Cannabis and tobacco cigarettes during pregnancy. Neurotoxicol Teratol. (2018) 68:84–90. doi: 10.1016/j.ntt.2018.06.001
36. Cornelius MD, Taylor PM, Geva D, Day NL. Prenatal tobacco and marijuana use among adolescents: effects on offspring gestational age, growth, and morphology. Pediatrics. (1995) 95(5):738–43. doi: 10.1542/peds.95.5.738
37. Day N, Cornelius M, Goldschmidt L, Richardson G, Robles N, Taylor P. The effects of prenatal tobacco and marijuana use on offspring growth from birth through 3 years of age. Neurotoxicol Teratol. (1992) 14(6):407–14. doi: 10.1016/0892-0362(92)90051-B
38. Hingson R, Alpert JJ, Day N, Dooling E, Kayne H, Morelock S, et al. Effects of maternal drinking and marijuana use on fetal growth and development. Pediatrics. (1982) 70(4):539–46. doi: 10.1542/peds.70.4.539
39. Linn S, Schoenbaum SC, Monson RR, Rosner R, Stubblefield PC, Ryan KJ. The association of marijuana use with outcome of pregnancy. Am J Public Health. (1983) 73(10):1161–4. doi: 10.2105/AJPH.73.10.1161
40. O’Connell CM, Fried PA. An investigation of prenatal cannabis exposure and minor physical anomalies in a low risk population. Neurobehav Toxicol Teratol. (1984) 6(5):345–50.
41. Astley SJ, Clarren SK, Little RE, Sampson PD, Daling JR. Analysis of facial shape in children gestationally exposed to marijuana, alcohol, and/or cocaine. Pediatrics. (1992) 89(1):67–77. doi: 10.1542/peds.89.1.67
42. Gibson GT, Baghurst PA, Colley DP. Maternal alcohol, tobacco and cannabis consumption and the outcome of pregnancy. Aust N Z J Obstet Gynaecol. (1983) 23(1):15–9. doi: 10.1111/j.1479-828X.1983.tb00151.x
43. Bandoli G, Jelliffe-Pawlowski L, Schumacher B, Baer RJ, Felder JN, Fuchs JD, et al. Cannabis-related diagnosis in pregnancy and adverse maternal and infant outcomes. Drug Alcohol Depend. (2021) 225:108757. doi: 10.1016/j.drugalcdep.2021.108757
44. Bourque DK, Meng L, Dougan S, Momoli F, Riddell C, Walker M, et al. Gastroschisis in Ontario, Canada: 2012–2018. Birth Defects Res. (2021) 113(14):1044–51. doi: 10.1002/bdr2.1896
45. Downing KF, Riehle-Colarusso T, Gilboa SM, Lin AE, Oster ME, Tinker SC, et al. Potential risk factors for ebstein anomaly, national birth defects prevention study, 1997–2011. Cardiol Young. (2019) 29(6):819–27. doi: 10.1017/S1047951119000970
46. Forrester MB, Merz RD. Comparison of trends in gastroschisis and prenatal illicit drug use rates. J Toxicol Environ Health A. (2006) 69(13):1253–9. doi: 10.1080/15287390500361750
47. Forrester MB, Merz RD. Risk of selected birth defects with prenatal illicit drug use, Hawaii, 1986–2002. J Toxicol Environ Health A. (2007) 70(1):7–18. doi: 10.1080/15287390600748799
48. Kharbanda EO, Vazquez-Benitez G, Kunin-Batson A, Nordin JD, Olsen A, Romitti PA. Birth and early developmental screening outcomes associated with cannabis exposure during pregnancy. J Perinatol. (2020) 40(3):473–80. doi: 10.1038/s41372-019-0576-6
49. Lam PK, Torfs CP. Interaction between maternal smoking and malnutrition in infant risk of gastroschisis. Birth Defects Res A Clin Mol Teratol. (2006) 76(3):182–6. doi: 10.1002/bdra.20238
50. Shaw GM, Velie EM, Morland KB. Parental recreational drug use and risk for neural tube defects. Am J Epidemiol. (1996) 144(12):1155–60. doi: 10.1093/oxfordjournals.aje.a008894
51. Torfs CP, Velie EM, Oechsli FW, Bateson TF, Curry CJ. A population-based study of gastroschisis: demographic, pregnancy, and lifestyle risk factors. Teratology. (1994) 50(1):44–53. doi: 10.1002/tera.1420500107
52. van Gelder M, Donders ART, Devine O, Roeleveld N, Reefhuis J. Natl birth defects prevention S. Using Bayesian models to assess the effects of under-reporting of Cannabis use on the association with birth defects, national birth defects prevention study, 1997–2005. Paediatr Perinat Epidemiol. (2014) 28(5):424–33. doi: 10.1111/ppe.12140
53. Witter FR, Niebyl JR. Marijuana use in pregnancy and pregnancy outcome. Am J Perinatol. (1990) 7(1):36–8. doi: 10.1055/s-2007-999442
54. D’Onofrio BM, Class QA, Rickert ME, Sujan AC, Larsson H, Kuja-Halkola R, et al. Translational epidemiologic approaches to understanding the consequences of early-life exposures. Behav Genet. (2016) 46(3):315–28. doi: 10.1007/s10519-015-9769-8
55. Centers for Disease Control and Prevention & Office of Noncommunicable Diseases Injury and Environmental Health. What you need to know about marijuana and pregnancy. Atlanta, GA (2017).
56. ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in Cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol Psychiatry. (2016) 79(7):613–9. doi: 10.1016/j.biopsych.2016.01.004
57. American College of Obstetricians and Gynecologist. Marijuana use during pregnancy and lactation: committee opinion No. 722. Obstet Gynecol. (2017) 130:e205–9. doi: 10.1097/AOG.0000000000002354
58. Young-Wolff KC, Adams SR, Wi S, Weisner C, Conway A. Routes of cannabis administration among females in the year before and during pregnancy: results from a pilot project. Addict Behav. (2020) 100:106125. doi: 10.1016/j.addbeh.2019.106125
59. Young-Wolff KC, Tucker L-Y, Alexeeff S, Armstrong MA, Conway A, Weisner C, et al. Trends in self-reported and biochemically tested marijuana use among pregnant females in California from 2009 to 2016. JAMA. (2017) 318(24):2490–1. doi: 10.1001/jama.2017.17225
60. Young-Wolff KC, Sarovar V, Tucker LY, Goler N, Conway A, Weisner C, et al. Validity of self-reported Cannabis use among pregnant females in northern California. J Addict Med. (2020) 14(4):287–92. doi: 10.1097/ADM.0000000000000581
61. Bleyl SB, Schoenwolf GC. What is the timeline of important events during pregnancy that may be disrupted by a teratogenic exposure? Teratology primer. 3rd ed. Reston, VA: The Society for Birth Defects Research and Prevention (2018).
62. Goler N, Conway A, Young-Wolff KC. Data are needed on the potential adverse effects of marijuana use in pregnancy. Ann Intern Med. (2018) 169(7):492–3. doi: 10.7326/M18-1141
63. Ko JY, Farr SL, Tong VT, Creanga AA, Callaghan WM. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol. (2015) 213(2):201.e1–e10. doi: 10.1016/j.ajog.2015.03.021
Keywords: pregnancy, prenatal exposure, in utero exposure, cannabis, marijuana
Citation: Sujan AC, Pal A, Avalos LA and Young-Wolff KC (2023) A systematic review of in utero cannabis exposure and risk for structural birth defects. Front. Pediatr. 11:1149401. doi: 10.3389/fped.2023.1149401
Received: 21 January 2023; Accepted: 9 May 2023;
Published: 25 May 2023.
Edited by:
Gabrielle Lynn McLemore, Morgan State University, United StatesReviewed by:
Camille Fung, The University of Utah, United States© 2023 Sujan, Pal, Avalos and Young-Wolff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayesha C. Sujan YXN1amFuQHN0YW5mb3JkLmVkdQ==
†These authors contributed equally to this work and share senior authorship.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.