- 1Neonatal Research Institute, Sharp Mary Birch Hospital for Women & Newborns, San Diego, United States
- 2Divisions of Neonatology, Baskent University Faculty of Medicine, Ankara, Türkiye
The most common methods for providing additional placental blood to a newborn are delayed cord clamping (DCC) and umbilical cord milking (UCM). However, DCC carries the potential risk of hypothermia due to extended exposure to the cold environment in the operating room or delivery room, as well as a delay in performing resuscitation. As an alternative, umbilical cord milking (UCM) and delayed cord clamping with resuscitation (DCC-R) have been studied, as they allow for immediate resuscitation after birth. Given the relative ease of performing UCM compared to DCC-R, UCM is being strongly considered as a practical option in non-vigorous term and near-term neonates, as well as preterm neonates requiring immediate respiratory support. However, the safety profile of UCM, particularly in premature newborns, remains a concern. This review will highlight the currently known benefits and risks of umbilical cord milking and explore ongoing studies.
Introduction
An estimated 140 million neonates born worldwide each year are treated with various umbilical cord management techniques (1). The importance of providing additional blood during the birth transition cannot be overstated. During pregnancy, fetal blood volume is approximately 110–115 ml/kg of fetal weight (2). Only 10% of the fetal cardiac output goes to the pulmonary circulation, whereas 30% to 50% is directed toward the placenta for gas exchange (3). After birth, as the lungs expand and the capillary beds around each alveolus are filled with blood, there is a significant increase in cardiac output to the lungs (4, 5). The placenta serves as an ideal blood reserve intended to supply this sudden shift in blood volume. Blood flow through the umbilical cord typically lasts several minutes after delivery which help maintain hemodynamic stability and red cell volume (6). Early observational studies demonstrated that the infant to placenta blood volume ratio increased from 67%/33% at birth to 87%/13% at the end of placental transfusion (7). The amount of placental transfusion that occurs may be influenced by various factors, such as uterine contractions, delivery method, length of time from birth to cord clamp, and possibly gravity and the newborn's spontaneous respirations (8). Placental transfusion is an essential part of the neonatal transition as the increased blood volume affects all organ systems.
Delayed cord clamping (DCC), delayed cord clamping with resuscitation (DCC-R) and umbilical cord milking (UCM) are different approaches to provide placental transfusion after delivery. DCC may be challenging to apply in infants with poor respiratory effort who may require resuscitation or are at risk for hypothermia. Conversely, DCC-R and UCM allow for immediate resuscitation and warming after birth. Compared to DCC-R, UCM poses fewer logistical challenges (equipment, personnel training) and can be more broadly applicable to all neonates worldwide. UCM enhances overall blood volume by 20%–30%, but the limited information on the physiological and hemodynamic impacts of UCM is concerning (4). Gaps in knowledge render UCM difficult to recommend universally. We will discuss the advantages and limitations of UCM in this review.
Cord management strategies
Overview of cord management strategies
Cord management plays a vital role in blood volume transfer as well as in hemodynamic stability during transition (7, 9–11). DCC is now widely adapted as the standard of care and is the recommended practice for vigorous neonates by multiple national and international governing bodies (ILCOR (12), WHO (13), SOGC (14), EAPM (15), AAP (16)). DCC can provide up to one-third of a term neonate's total blood volume without increasing the risk of maternal hemorrhage in either vaginal or cesarean section deliveries (17). Animal models have shown negative impacts of early cord clamping (ECC) such as higher pulmonary artery pressure and reduced cardiac output, while demonstrating DCC after lung aeration is established can reduce pulmonary vascular resistance and improve oxygen delivery (18).
In preterm neonates, animal models suggested a potential reduction in the risk of intraventricular hemorrhage since DCC allows for smoother hemodynamic transition, better cardiac output, and less carotid artery pressure fluctuations (19). A randomized control trial of 1,566 preterm neonates randomized to DCC vs. ECC showed a reduction in hospital death following DCC (20). However, multiple meta-analysis did not show differences in the rates of major morbidities including severe IVH, retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC) or late onset sepsis (LOS) (21, 22). Potential barriers to optimizing placental transfusion in preterm neonates is their need for immediate resuscitation and need for respiratory support to establish lung aeration prior to cord clamping (23).
DCC with resuscitation with an intact cord (DCC-R) has been shown to be feasible for providing placental transfusion in preterm neonates in need of respiratory assistance or in non-vigorous term and near-term neonates (24). Studies comparing DCC alone to DCC-R in preterm neonates showed no differences in the rates of severe IVH, NEC, ROP, or hemodynamic markers on echocardiography (25). The benefits of DCC-R in preterm neonates may be limited by inadequate lung recruitment when relying on non-invasive modes of respiratory support during resuscitation with intact cord. In contrast to preterm animal models that are sedated and invasively ventilated, preterm neonates in the delivery room are resuscitated with non-invasive respiratory modalities. Preterm rabbit models showed that resuscitation with non-invasive interfaces such as a face mask is hindered by a larynx and epiglottis that is closed three-quarters of the time during resuscitation (26). Ongoing studies exploring DCC-R vs. DCC in preterm neonates include the VentFirst trial (NCT02742454), the Baby-DUCC trial (ACTRN12617000610336), the ABC3 Trial (NCT03808051). There is a need for additional studies to elucidate the effectiveness of DCC-R for improving immediate and long-term outcomes.
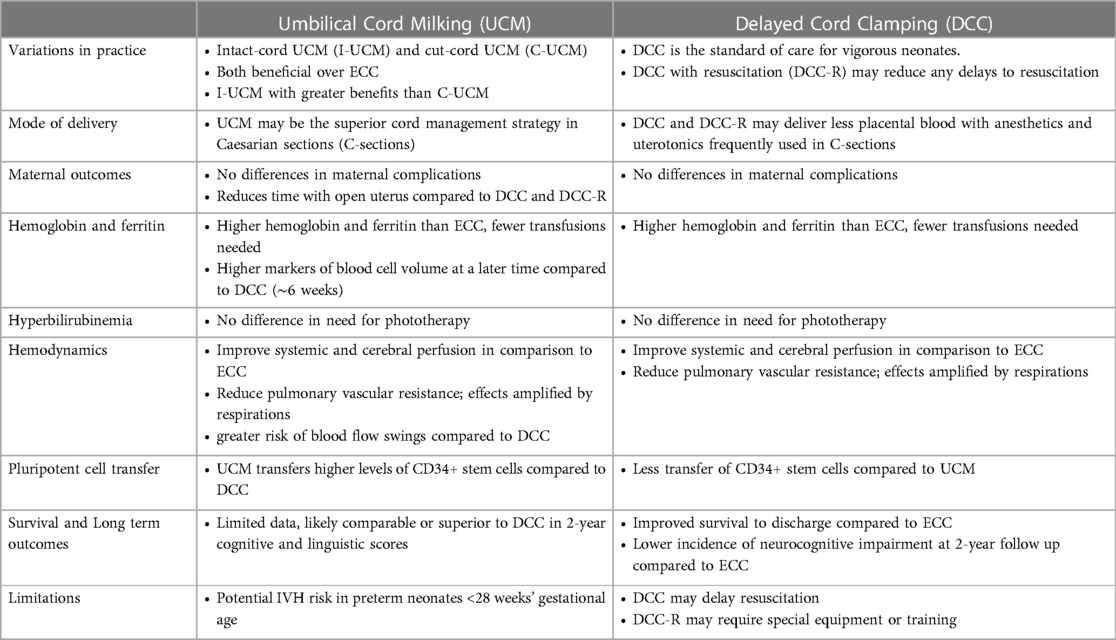
Resuscitation with an intact cord can be done using simple equipment for providing respiratory support while stabilizing temperature, as is the case in developing countries (27). However, a survey has shown that clinicians view DCC-R using a resuscitation trolley as the “same”, “better”, or “much better” than conventional equipment (28). Whether standard resuscitation equipment is used, or if specialized resuscitation trolleys are employed, DCC-R warrants special training as well as the need to consider the sterility of the field in the case of C-section deliveries (29) Resuscitation trolleys with the ability to provide heat and respiratory support are often utilized, and the personnel tasked with the resuscitation must be trained. Multiple trolleys have been developed, with one being FDA-approved and several others actively being tested (30). Due to a number of logistical challenges and requirements, UCM has been explored as an alternative to DCC as a means to provide placental transfusion without delaying resuscitation. Moreover, preterm lamb models showed that UCM with placental refill offered the most transfer of blood volume (median net transfer 8.8 ml/kg) compared to UCM without placental refill or DCC (31). Table 1 summarizes some key features, benefits, and limitations of UCM and DCC.
Cord management strategies during different modes of delivery and maternal outcomes
The mode of delivery may impact the efficacy of placental transfusion. A meta-analysis suggests that anesthesia and surgical procedures interfered with the uterine muscles' ability to contract actively and thereby limiting placental transfusion at birth. Term neonates born by cesarean section (C-section) received less placental transfusion compared to infants born by vaginal delivery, and thereby have lower hematocrit, hemoglobin, and erythrocyte levels (32).
Several randomized controlled trials have demonstrated preterm infants delivered by C-section undergoing UCM vs. DCC had better systemic blood flow, blood pressure, hemoglobin levels, and urine output in the first 72 h of life (33–35). UCM has been found to be feasible in term infants born by C-section and did not result in higher incidence of phototherapy, symptomatic polycythemia, NICU hospitalizations, or readmissions for phototherapy (36). UCM may be a good alternative in elective C-section to optimize placental transfusion (37).
Maternal outcomes do not differ with UCM vs. DCC (38, 39). Primary post-partum hemorrhage (PPH) was present in 3.1% of women in the DCC arm and 2.3% of mothers in the UCM arm. (p = 0.719) (38). UCM results in a shorter time with an open uterus at cesarean section and thereby unlikely to increase maternal adverse events.
Approaches to umbilical cord milking: intact vs. cut cord milking
There is presently no agreement on the ideal method for cord milking (Figure 1). Intact umbilical cord milking is performed by manually expressing umbilical cord blood three to four times at a rate of 10 cm per second down a 20–30 cm section of the umbilical cord (40). Cut cord milking (C-UCM) is performed by clamping the cord 10–30 cm away from the infant so that the infant is moved to a resuscitation bed before the cut-cord is milked (41). The clamped umbilical cord is milked once towards the infant while receiving respiratory support. Both cut and intact cords have been used for umbilical cord milking with varied results (42, 43).
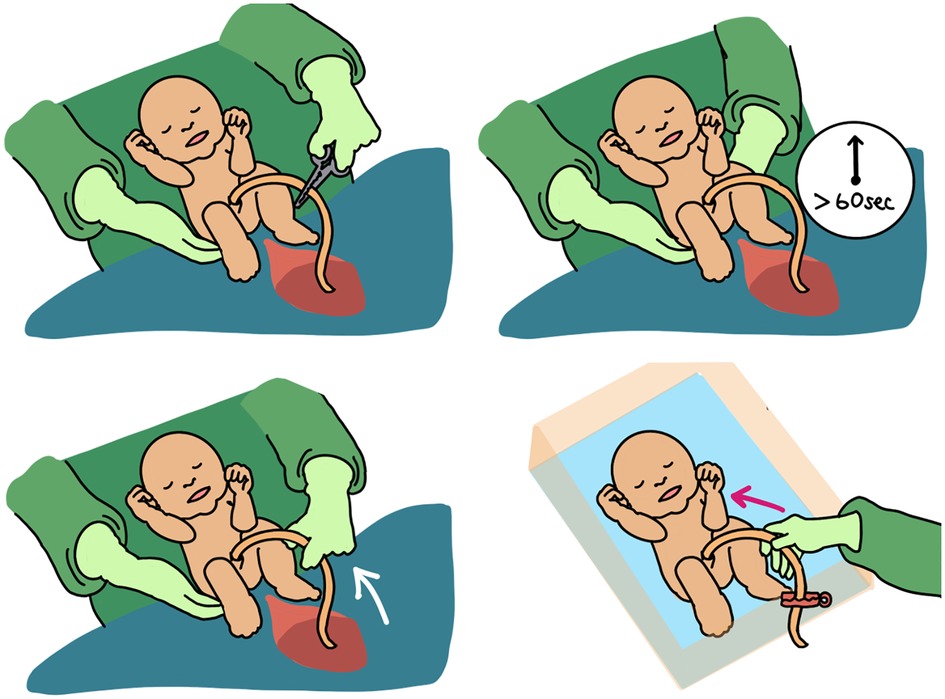
Figure 1. Early cord clamping (ECC) vs delayed cord clamping (DCC) vs. intact cord umbilical cord milking (I-UCM) vs. cut-cord umbilical cord milking (C-UCM). Different methods of cord management are depicted here.
Until recently it has not been clear whether milking the cut vs. intact cord provides a different amount of transfusion (40). McAdams et al. demonstrated that milking the intact cord (I-UCM) in term infants provided a greater volume of blood compared to milking a cut cord (40). Moreover, longer cord lengths provided greater blood volumes transferred to the neonate. C-UCM did not reduce newborn morbidities or enhance hemoglobin levels in a sample of 106 preterm infants born at 35 weeks of gestation when compared to historical controls who underwent ECC (41). A large randomized controlled trial demonstrated I-UCM resulted in higher hemoglobin and hematocrit levels at 48 h of age compared with the ECC group (44).
A systematic review and meta-analysis included nine RCTs (n = 1,632) that compared I-UCM, C-UCM and DCC in term and late-preterm babies. C-UCM increased hemoglobin levels in term and late-preterm babies at 30 min when compared to DCC. Both UCM methods improved hemoglobin levels at 48–72 h when compared to DCC. Only I-UCM was associated with higher hemoglobin at a later time point (6 to 8 weeks postnatally) when compared to DCC. There was no variation in serum ferritin levels (45). Another study found that C-UCM was safe in term and near-term newborns and produced greater hemoglobin levels at 6 weeks of life than ECC (46). To date, literature suggests superiority of I-UCM over C-UCM, but any method of UCM has clinical benefits over ECC. For that reason, and given the safety profile of C-UCM, it is an acceptable variation of UCM in term and near-term newborns.
Benefits of umbilical cord milking
Transfusion, anemia, polycythemia, and jaundice
Children with iron deficiency have delayed cognitive development, behavioral problems, and decreased motor development (47–49). Iron deficiency may be decreased by cord management and placental transfusion at delivery. The outcomes in premature newborns receiving UCM are mixed; some report increased ferritin and hemoglobin levels, while other studies report no change (50–55). The need for blood transfusion was significantly lower in the I-UCM group when compared to ECC in a systematic review, with a number needed to treat (NNT) of four to prevent one packed red blood cell (pRBC) transfusion (56). Another systematic review of six trials (n = 587 preterm infants) also reported fewer pRBC transfusions with UCM compared to ECC (57). Lastly, a systematic review from three studies that compared UCM and DCC in 650 term babies delivered vaginally or by cesarean section had similar hemoglobin and ferritin levels in the short term period. However, UCM was found to have a higher hemoglobin level at six weeks of age (58).
There are conflicting data on the association between UCM and polycythemia and hyperbilirubinemia. While some small studies suggest higher rates or longer duration of phototherapy, many contemporary observational and randomized control trials have refuted these concerns (50, 59, 60). The most recent Cochrane analysis found no difference for the need for phototherapy with DCC or UCM (8 studies, 495 infants) (21). A systematic review of UCM and DCC in preterm infants found two studies with blood bilirubin levels at 48 h of life were reported in 385 newborns and found no difference (58). Another RCT of 280 infants between 28 and 37 weeks found a higher incidence of neonatal jaundice with DCC (15.6%) compared to the UCM (6.8%) (38).
In a 3-arm randomized controlled trial of 300 term babies, Yadav et al. compared the effects of DCC with C-UCM or I-UCM. The number of patients receiving phototherapy for jaundice and the serum bilirubin level at 48 h were comparable among all three groups (61). A cluster crossover trial comparing early cord clamping to cord milking in non-vigorous infants (N = 1,730) demonstrated a higher serum bilirubin with cord milking but no increase in the need for phototherapy (62). Bilirubin is a the most abundant antioxidant in the newborn, and may be associated with improved neurodevelopmental outcomes in infants with hypoxic ischemic encephalopathy, so higher levels without increased need for therapy may even be neuroprotective (63). No large studies or systematic reviews to date have demonstrated an increase in the need for phototherapy treatment or exchange transfusion with umbilical cord milking compared to delayed or early cord clamping.
Hemodynamic parameters, cerebral oxygenation, and breathing
UCM and DCC enhance systemic and cerebral perfusion in comparison to ECC, both of which may be neuroprotective. Both UCM and DCC have shown some modest promise in increasing brain oxygenation and hemodynamic parameters on echocardiography (60, 64–67).
Cord management and spontaneous or assisted ventilation concertedly impact the degree of placental transfusion to the newborn (68). It is now understood that the onset of spontaneous respirations and the opening of the pulmonary vascular bed are key factors in placental blood transport. In a small observational study, spontaneous breathing was associated with collapse of the inferior vena cava (IVC) and increased anterograde flow in the ductus venosus and hepatic vein during inspiration. When breathing begins, lung expansion increases pulmonary blood flow, which makes pulmonary venous return the main source of preload for the left ventricle in place of umbilical venous return. The heart rate stabilizes when cardiac output rapidly rises (Figure 2) (69). In contrast, ECC lowers cardiac output in newborns who are apneic and hypoxic. Limiting the rise in cardiac output exposes the newborn to the combination of ischemia and hypoxia since higher cardiac output mitigates the consequences of hypoxemia (70).
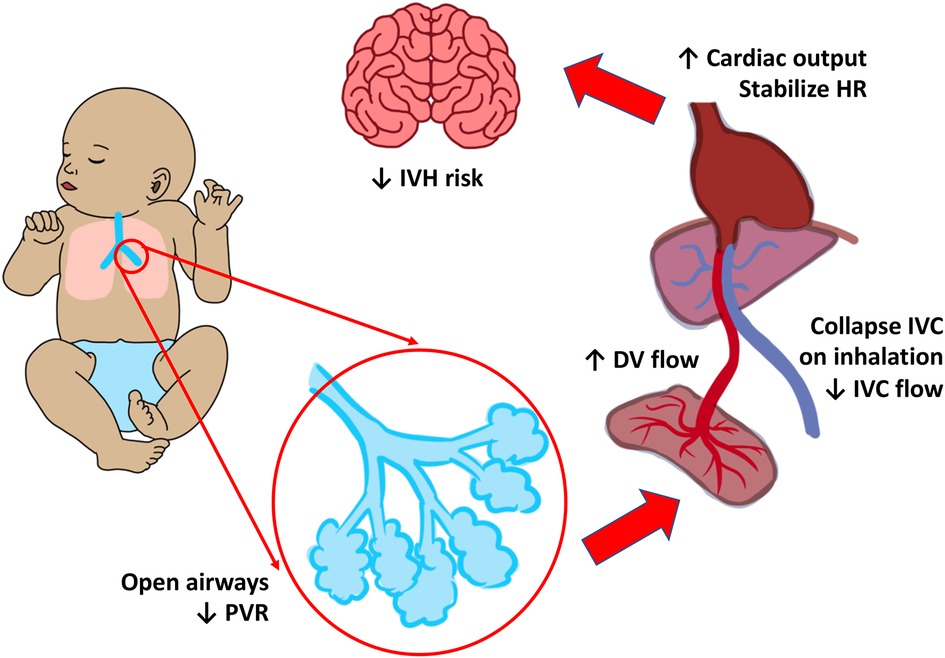
Figure 2. Cardiorespiratory changes during respiration before cord clamping. Respiration and lung recruitment drops the pulmonary vascular resistance (PVR), collapses the inferior vena cava (IVC), and thereby increases the flow of blood from the ductus venosus (DV). This increases cardiac output (CO) which in turn stabilizes the heart rate (HR), blood pressure (BP), and reduce the risk of intraventricular hemorrhage (IVH).
As noted above, animal models suggest that adequate ventilation is protective against abnormal fluctuations in lambs receiving UCM (71). However, it is possible that UCM may stimulate spontaneous breathing in human neonates as contrasted to anesthetized lamb models. Preterm newborns receiving ECC at delivery had lower heart rates and oxygen saturation than preterm infants getting UCM, and required more oxygen and ventilation in the first five minutes of life suggesting the UCM may play a role in respiratory adaptation (72).
In term infants who were randomly assigned to receive C-UCM or DCC, Jaiswal et al. evaluated the blood flow velocity and Doppler indices of the middle cerebral artery (MCA) between 24 and 48 h after birth, as well as hemodynamic parameters (such as blood pressure, heart rate, and respiratory rate) at 30 min, 24 h, and 48 h of life. They noted that both the C-UCM and the DCC groups had similar cerebral blood velocities and cranial Doppler indices. Similarly, there was no discernible difference between the two groups in terms of the MCA cerebral blood flow velocity, pulsatility index, and mean resistive index (73).
The higher systemic pressure combined with high pulmonary vascular resistance in non-breathing infants may prevent a rise in pulmonary blood flow during milking. Without a pulmonary pop-off, the rise in aortic pressure may result in fluctuations in cerebral blood flow (8). Understanding how UCM influences hemodynamic parameters is essential. UCM's impact on hemodynamic parameters must be further investigated in different gestational ages and in breathing and non-breathing infants.
Stem cell transfer
Reports from over 80 years ago have suggested that although DCC and UCM lead to increased hematocrit, umbilical cord milking is associated with a sustained increase in red blood cell count compared to DCC. A bedside to bench model studied umbilical cord blood contents in term infants randomly assigned to receive UCM or DCC. UCM blood exhibited higher levels of hematopoietic stem cells (CD34) and improved survival in irradiated mice when compared to DCC blood (74). In an observational study, preterm infants with placental insufficiency had higher CD34 counts following UCM as compared to historical controls who underwent ECC (75). Although not yet proven, stem cell transfusion during UCM has promising applications towards preterm and term infants with organ injury or hematopoietic cell line deficiencies (Figure 3) (76).
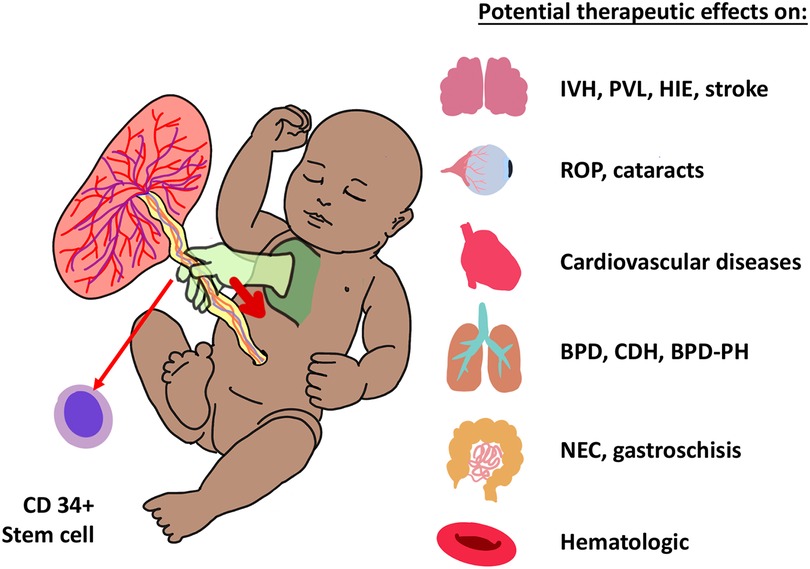
Figure 3. Umbilical cord milking (UCM) increases CD34 + pluripotent stem cells in babies, which have potential benefits as summarized in the figure. IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; HIE, hypoxic ischemic encephalopathy. ROP, retinopathy of prematurity; BPD, bronchopulmonary dysplasia; CDH, congenital diaphragmatic hernia; PH, pulmonary hypertension; NEC, necrotizing enterocolitis.
Long-term outcomes
Data on the long-term results of UCM in infants is scarce. Studies to date have described a positive association between neurodevelopmental outcomes and adequate iron storage (77). Katheria et al. demonstrated higher language and cognitive scores at two years of age in patients randomized to UCM compared to DCC, but no difference in rates of neurodevelopmental impairment (78). Rabe et al. observed that preterm children who received UCM at birth had higher or comparable cognitive and linguistic scores to those who received DCC (79). One study compared 56 neonates born between 24 and 27 weeks' gestation and found no differences in neurodevelopmental outcomes between the UCM and ECC groups (54). Larger studies evaluating long term outcomes are needed.
Cord milking for non-vigorous babies
An important stage in newborn stabilization may be optimum cord management to improve placental transfusion (80). Randomized controlled trials have excluded these infants due to the need for rapid resuscitation. While the difficulties of a high-pressure environment accompany the delivery of a depressed newborn, providing these newborns with an appropriate placental transfusion may be the first and most crucial step in assuring the best prognosis (81). Infants who are at risk of hypoxia may be at risk of not getting this transfusion right away. Asphyxiated infants lose 30% of their final blood volume and 60% of their RBC volume due to ECC (82). The use of ECC in these vulnerable newborns may aggravate hypoxia-ischemia and have negative short- and long-term consequences (83).
In the past, UCM has been used instead of DCC in neonates who were asphyxiated, required urgent resuscitation, or were delivered through C-section (84). UCM makes it possible for a neonate to get a faster placental transfusion, which makes it easier to move the baby from the mother to a warmer bed for more life-saving measures (85). Methods of placental transfusion may aid in the cardiovascular transition and be neuroprotective in newborns who are not in good health. This extra blood improves blood volume and boosts cardiac output, stabilizing pulmonary and cerebral circulation, increasing cardiac preload before the placenta is removed from the circulation, perhaps reducing further ischemia in a newborn already suffering from poor health (81, 86).
However, there may be risks of provide rapid volume in asphyxiated infants. Concerns about volume overload in newborns who have had hypoxic-ischemic events during delivery and increased endothelial activation products entering the infant's circulation with milking of the umbilical arteries have been raised (87).
There have been few studies on UCM in infants with depression. Ram Mohan et al. performed a pilot RCT of 60 non-vigorous preterm infants to either C-UCM or ECC and demonstrated similar clinical outcomes, but infants randomized to C-UCM infants had higher hemoglobin and ferritin levels at six weeks of age (88). Girish et al. performed a quasi-randomized trial of 100 near term and term non-vigorous newborns to either UCM or ECC and demonstrated similar outcomes of HIE death and need for resuscitation (89). However, there is inadequate research to support DCC or umbilical cord milking (UCM) in cases of prenatal distress (80). In a cluster-randomized crossover study (MINVI trial) proposed that UCM, as opposed to early cord clamping (ECC), would decrease admission to the neonatal intensive care unit (NICU) in non-vigorous newborns delivered between 35 and 42 weeks of gestation. While UCM did not result in a decrease in NICU admissions when compared to ECC, it did result in fewer occurrences of moderate to severe hypoxic-ischemic encephalopathy, less therapeutic hypothermia usage, and higher hemoglobin levels. When compared to ECC, there was no evidence that UCM caused any harm. These findings may represent the first randomized controlled trial proof that UCM is possible, safe, and preferable to ECC in non-vigorous newborns (62). An ongoing trial in India aimed to reduce HIE may help determine whether UCM can be efficacious in non-vigorous newborns (NCT03657394).
Risks with umbilical cord milking
UCM can provide placental transfusion without significantly delaying resuscitation. Despite clinical trials demonstrating its advantages for the short-term outcomes of preterm infants, UCM has not been adopted as an alternative to DCC. There remain concerns about rapid changes in blood flow in extremely preterm infants that may increase the risk for intraventricular hemorrhage (64, 90, 91).
Anesthetized preterm lamb models delivered by C-section have demonstrated oscillations in the systemic arterial pressure and cerebral blood flow with UCM (12, 31, 92). However, asphyxiated preterm lamb models receiving UCM concurrently with ventilation exhibited a decrease in fluctuations of carotid and pulmonary blood flow as compared to UCM alone (71).
The safety of providing UCM to extremely premature infants remain unclear. To date, there is only one randomized controlled trial that has demonstrated an association with UCM and severe IVH compared to delayed cord clamping (93). However, the trial enrolled substantial numbers (N = 182) of infants 23–27 weeks' gestation and demonstrated an increase in severe IVH at each of the 10 centers that participated. Whether prior animal data is translatable in these infants is unclear, however there is biological plausibility that the additional blood volume provided by UCM may not be as well tolerated in the extremely preterm infant who lack cerebral autoregulation and have a very fragile germinal matrix which may lead to hemorrhage (8). Unfortunately, most of the literature on preterm infants comes from small studies or mostly mature infants (34, 90, 94, 95). Recent meta-analyses of these studies may also only provide false assurance of safety (56). Retrospective studies have additional problems of bias since sicker infants that receive UCM may go on to develop IVH and die irrespective of their cord management. A 3-arm study comparing cut cord milking, intact cord milking and delayed cord clamping in China may help support or refute current concerns regarding UCM in preterm infants (96).
Research directions
There is an urgent need for larger randomized controlled trials of cord management powered to detect signals of safety and harm. Studies need to include multiples and assess the safety of delayed cord clamping or milking on neonatal outcomes. Monochorionic placentation also needs to be included if there is no risk for twin-to-twin transfusion. Moreover, variations of the milking practice, including how many times, over how long, allowing for placental refill or not, and whether cut cord milking provides similar or greater benefit than milking the intact cord. The impact of UCM on different populations needs to be further delineated, including neonates of different gestational ages, vigorous neonates with spontaneous respirations, and depressed, non-breathing neonates requiring positive pressure ventilation.
Translational studies are paramount for elucidating the physiologic changes that take place during umbilical cord milking in order to identify additional risks and benefits of this practice.
The study and application of cord milking in low-middle income countries where the incidence of HIE is 10-fold higher is urgently needed.
Conclusions
Understanding the benefits and risks of UCM can be challenging, even when considering systematic reviews and meta-analyses. UCM may be safe for many infants, but exercise caution in subgroups such as the extremely preterm infant. Cord milking may be of particular benefit in conditions where DCC is contraindicated such as abruptions or maternal instability. Future studies will hopefully provide additional guidance as to which populations may benefit from UCM.
Author contributions
Review was designed and written by HK, JK and AK. All figures were illustrated by JK. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ritchie H, Mathieu E. 12/27/2022]; Available at: https://ourworldindata.org/births-and-deaths
2. Linderkamp O. Placental transfusion: determinants and effects. Clin Perinatol. (1982) 9(3):559–92. doi: 10.1016/S0095-5108(18)31013-3
3. Kiserud T. Physiology of the fetal circulation. Semin Fetal Neonatal Med. (2005) 10(6):493–503. doi: 10.1016/j.siny.2005.08.007
4. Erickson-Owens DA, Mercer JS, Oh W. Umbilical cord milking in term infants delivered by cesarean section: a randomized controlled trial. J Perinatol. (2012) 32(8):580–4. doi: 10.1038/jp.2011.159
5. Mercer JS, Skovgaard RL. Neonatal transitional physiology: a new paradigm. J Perinat Neonatal Nurs. (2002) 15(4):56–75. doi: 10.1097/00005237-200203000-00007
6. Billman GE. Homeostasis: the underappreciated and far too often ignored central organizing principle of physiology. Front Physiol. (2020) 11:200. doi: 10.3389/fphys.2020.00200
7. Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet. (1969) 2(7626):871–3. doi: 10.1016/S0140-6736(69)92328-9
8. McAdams RM, Lakshminrusimha S. Management of placental transfusion to neonates after delivery. Obstet Gynecol. (2022) 139(1):121–37. doi: 10.1097/AOG.0000000000004625
9. Colozzi AE. Clamping of the umbilical cord; its effect on the placental transfusion. N Engl J Med. (1954) 250(15):629–32. doi: 10.1056/NEJM195404152501502
10. Whipple GA, Sisson TR, Lund CJ. Delayed ligation of the umbilical cord; its influence on the blood volume of the newborn. Obstet Gynecol. (1957) 10(6):603–10. doi: 10.1097/00006250-195712000-00002
11. Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol. (2013) 591(Pt 8):2113–26. doi: 10.1113/jphysiol.2012.250084
12. Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, et al. Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. (2015) 132(16 suppl 1):S204–41. doi: 10.1161/cir.0000000000000276
13. WHO Guidelines Approved by the Guidelines Review Committee. Guidelines on Basic Newborn Resuscitation. Geneva: World Health Organization (2012).
14. Leduc D, Senikas V, Lalonde AB, Ballerman C, Biringer A, Delaney M, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. (2009) 31(10):980–93. doi: 10.1016/S1701-2163(16)34329-8
15. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European Consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants—2010 update. Neonatology. (2010) 97(4):402–17. doi: 10.1159/000297773
17. Farrar D, Airey R, Law GR, Tuffnell D, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with cord intact. BJOG. (2011) 118(1):70–5. doi: 10.1111/j.1471-0528.2010.02781.x
18. Bhatt S, Polglase GR, Wallace EM, Te Pas AB, Hooper SB. Ventilation before umbilical cord clamping improves the physiological transition at birth. Front Pediatr. (2014) 2:113. doi: 10.3389/fped.2014.00113
19. Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol. (2014) 41(1):47–67. doi: 10.1016/j.clp.2013.09.007
20. Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, et al. Delayed versus immediate cord clamping in preterm infants. N Engl J Med. (2017) 377(25):2445–55. doi: 10.1056/NEJMoa1711281
21. Rabe H, Gyte GML, Díaz-Rossello JL, Duley L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. (2019) 9. doi: 10.1002/14651858.CD003248.pub4
22. Fogarty M, Osborn DA, Askie L, Seidler AL, Hunter K, Lui K, et al. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol. (2018) 218(1):1–18. doi: 10.1016/j.ajog.2017.10.231
23. Polglase GR, Dawson JA, Kluckow M, Gill AW, Davis PG, Te Pas AB, et al. Ventilation onset prior to umbilical cord clamping (physiological-based cord clamping) improves systemic and cerebral oxygenation in preterm lambs. PLoS One. (2015) 10(2):e0117504. doi: 10.1371/journal.pone.0117504
24. Katheria AC, Brown MK, Faksh A, Hassen KO, Rich W, Lazarus D, et al. Delayed cord clamping in newborns born at term at risk for resuscitation: a feasibility randomized clinical trial. J Pediatr. (2017) 187:313–317.e1. doi: 10.1016/j.jpeds.2017.04.033
25. Katheria A, Poeltler D, Durham J, Steen J, Rich W, Arnell K, et al. Neonatal resuscitation with an intact cord: a randomized clinical trial. J Pediatr. (2016) 178:75–80.e3. doi: 10.1016/j.jpeds.2016.07.053
26. Crawshaw JR, Kitchen MJ, Binder-Heschl C, Thio M, Wallace MJ, Kerr LT, et al. Laryngeal closure impedes non-invasive ventilation at birth. Arch Dis Child Fetal Neonatal Ed. (2018) 103(2):F112–f119. doi: 10.1136/archdischild-2017-312681
27. Andersson O, Rana N, Ewald U, Målqvist M, Stripple G, Basnet O, et al. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 minutes of birth (Nepcord III)—a randomized clinical trial. Matern Health Neonatol Perinatol. (2019) 5:15. doi: 10.1186/s40748-019-0110-z
28. Thomas MR, Yoxall CW, Weeks AD, Duley L. Providing newborn resuscitation at the mother's bedside: assessing the safety, usability and acceptability of a mobile trolley. BMC Pediatr. (2014) 14:135. doi: 10.1186/1471-2431-14-135
29. Koo J, Katheria A. Cardiopulmonary resuscitation with an intact umbilical cord. Neoreviews. (2022) 23(6):e388–99. doi: 10.1542/neo.23-6-e388
30. Katheria A, Lee HC, Knol R, Irvine L, Thomas S. A review of different resuscitation platforms during delayed cord clamping. J Perinatol. (2021) 41(7):1540–8. doi: 10.1038/s41372-021-01052-3
31. Blank DA, Polglase GR, Kluckow M, Gill AW, Crossley KJ, Moxham A, et al. Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition. Arch Dis Child Fetal Neonatal Ed. (2018) 103(6):F539-F546.29208663
32. Zhou YB, Li HT, Zhu LP, Liu JM. Impact of cesarean section on placental transfusion and iron-related hematological indices in term neonates: a systematic review and meta-analysis. Placenta. (2014) 35(1):1–8. doi: 10.1016/j.placenta.2013.10.011
33. Katheria AC, Truong G, Cousins L, Oshiro B, Finer NN. Umbilical cord milking versus delayed cord clamping in preterm infants. Pediatrics. (2015) 136(1):61–9. doi: 10.1542/peds.2015-0368
34. Rabe H, Jewison A, Fernandez Alvarez R, Crook D, Stilton D, Bradley R, et al. Milking compared with delayed cord clamping to increase placental transfusion in preterm neonates: a randomized controlled trial. Obstet Gynecol. (2011) 117–2(Pt 1):205–11. doi: 10.1097/AOG.0b013e3181fe46ff
35. Katheria AC, Lakshminrusimha S, Rabe H, McAdams R, Mercer JS. Placental transfusion: a review. J Perinatol. (2017) 37(2):105–11. doi: 10.1038/jp.2016.151
36. Chiruvolu A, Medders A, Daoud Y. Effects of umbilical cord milking on term infants delivered by cesarean section. Am J Perinatol. (2021) 38(10):1042–7. doi: 10.1055/s-0040-1701617
37. Consonni S, Vaglio Tessitore I, Conti C, Plevani C, Condo M, Torcasio F, et al. Umbilical cord management strategies at cesarean section. J Obstet Gynaecol Res. (2020). 10: 925656. doi: 10.1111/jog.14501
38. Sura M, Osoti A, Gachuno O, Musoke R, Kagema F, Gwako G, et al. Effect of umbilical cord milking versus delayed cord clamping on preterm neonates in Kenya: a randomized controlled trial. PLoS One. (2021) 16(1):e0246109. doi: 10.1371/journal.pone.0246109
39. Wyckoff MH, Singletary EM, Soar J, Olasveengen TM, Greif R, Liley HG, et al. 2021 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations: summary from the basic life support; advanced life support; neonatal life support; education, implementation, and teams; first aid task forces; and the COVID-19 working group. Resuscitation. (2021) 169:229–311. doi: 10.1016/j.resuscitation.2021.10.040
40. McAdams RM, Fay E, Delaney S. Whole blood volumes associated with milking intact and cut umbilical cords in term newborns. J Perinatol. (2018) 38(3):245–50. doi: 10.1038/s41372-017-0002-x
41. Simonin A, Safarulla A, Farmer Z, Coleman J, Sutton D, Wheeler K, et al. Cut umbilical cord milking: an ineffective method of placental transfusion in preterm infants? J Matern Fetal Neonatal Med. (2020) 33(18):3132–5. doi: 10.1080/14767058.2019.1569616
42. Basile S, Pinelli S, Micelli E, Caretto M, Benedetti Panici P. Milking of the umbilical cord in term and late preterm infants. Biomed Res Int. (2019) 2019:9185059. doi: 10.1155/2019/9185059
43. Upadhyay A, Gothwal S, Parihar R, Garg A, Gupta A, Chawla D, et al. Effect of umbilical cord milking in term and near term infants. Obstet Gynecol Surv. (2013) 68(5):333–4. doi: 10.1097/01.ogx.0000430374.84251.54
44. Kilicdag H, Karagun BS, Antmen AB, Candan E, Erbas H. Umbilical cord management in late preterm and term infants: a randomized controlled trial. Am J Perinatol. (2022) 39(12):1308–13. doi: 10.1055/s-0040-1722327
45. Jeevan A, Ananthan A, Bhuwan M, Balasubramanian H, Rao S, Kabra NS. Umbilical cord milking versus delayed cord clamping in term and late-preterm infants: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. (2022) 35(25):5478–88. doi: 10.1080/14767058.2021.1884676
46. Upadhyay A, Gothwal S, Parihar R, Garg A, Gupta A, Chawla D, et al. Effect of umbilical cord milking in term and near term infants: randomized control trial. Am J Obstet Gynecol. (2013) 208(2):120e1–6. doi: 10.1016/j.ajog.2012.10.884
47. Gunnarsson BS, Thorsdottir I, Palsson G, Gretarsson SJ. Iron status at 1 and 6 years versus developmental scores at 6 years in a well-nourished affluent population. Acta Paediatr. (2007) 96(3):391–5. doi: 10.1111/j.1651-2227.2007.00086.x
48. Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. (2001) 131(2s-2):649S-666S. discussion 666S–668S. doi: 10.1093/jn/131.2.649S
49. Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med. (2006) 160(11):1108–13. doi: 10.1001/archpedi.160.11.1108
50. Kumar B, Upadhyay A, Gothwal S, Jaiswal V, Joshi P, Dubey K. Umbilical cord milking and hematological parameters in moderate to late preterm neonates: a randomized controlled trial. Indian Pediatr. (2015) 52(9):753–7. doi: 10.1007/s13312-015-0711-1
51. Patel S, Clark EA, Rodriguez CE, Metz TD, Abbaszadeh M, Yoder BA. Effect of umbilical cord milking on morbidity and survival in extremely low gestational age neonates. Am J Obstet Gynecol. (2014) 211(5):519 e1–7. doi: 10.1016/j.ajog.2014.05.037
52. Hosono S, Mugishima H, Fujita H, Hosono A, Minato M, Okada T, et al. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born at less than 29 weeks’ gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. (2008) 93(1):F14–9. doi: 10.1136/adc.2006.108902
53. Alan S, Arsan S, Okulu E, Akin IM, Kilic A, Taskin S, et al. Effects of umbilical cord milking on the need for packed red blood cell transfusions and early neonatal hemodynamic adaptation in preterm infants born </=1,500 g: a prospective, randomized, controlled trial. J Pediatr Hematol Oncol. (2014) 36(8):e493–8. doi: 10.1097/MPH.0000000000000143
54. Josephsen JB, Potter S, Armbrecht ES, Al-Hosni M. Umbilical cord milking in extremely preterm infants: a randomized controlled trial comparing cord milking with immediate cord clamping. Am J Perinatol. (2022) 39(4):436–43. doi: 10.1055/s-0040-1716484
55. Hosono S, Mugishima H, Takahashi S, Takahashi S, Masaoka N, Yamamoto T, et al. One-time umbilical cord milking after cord cutting has same effectiveness as multiple-time umbilical cord milking in infants born at <29 weeks of gestation: a retrospective study. J Perinatol. (2015) 35(8):590–4. doi: 10.1038/jp.2015.15
56. Balasubramanian H, Ananthan A, Jain V, Rao SC, Kabra N. Umbilical cord milking in preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2020) 105(6):572–80. doi: 10.1136/archdischild-2019-318627
57. Dang D, Zhang C, Shi S, Mu X, Lv X, Wu H. Umbilical cord milking reduces need for red cell transfusions and improves neonatal adaptation in preterm infants: meta-analysis. J Obstet Gynaecol Res. (2015) 41(6):890–5. doi: 10.1111/jog.12657
58. Fuwa K, Tabata N, Ogawa R, Nagano N, Yamaji N, Ota E, et al. Umbilical cord milking versus delayed cord clamping in term infants: a systematic review and meta-analysis. J Perinatol. (2021) 41(7):1549–57. doi: 10.1038/s41372-020-00825-6
59. Xie YJ, Xiao JL, Zhu JJ, Wang YW, Wang B, Xie LJ. Effects of umbilical cord milking on anemia in preterm infants: a multicenter randomized controlled trial. Am J Perinatol. (2022) 39(1):31–6. doi: 10.1055/s-0040-1713350
60. March MI, Hacker MR, Parson AW, Modest AM, de Veciana M. The effects of umbilical cord milking in extremely preterm infants: a randomized controlled trial. J Perinatol. (2013) 33(10):763–7. doi: 10.1038/jp.2013.70
61. Yadav AK, Upadhyay A, Gothwal S, Dubey K, Mandal U, Yadav CP. Comparison of three types of intervention to enhance placental redistribution in term newborns: randomized control trial. J Perinatol. (2015) 228(2):217.e1–217.e14. doi: 10.1038/jp.2015.65
62. Katheria AC, Clark E, Yoder B, Schmölzer GM, Yan Law BH, El-Naggar W, et al. Umbilical cord milking in nonvigorous infants: a cluster-randomized crossover trial. Am J Obstet Gynecol. (2022) 35(11):1107–12. doi: 10.1016/j.ajog.2022.08.015
63. Bin-Nun A, Mimouni FB, Kasirer Y, Schors I, Schimmel MS, Kaplan M, et al. Might bilirubin serve as a natural antioxidant in response to neonatal encephalopathy? Am J Perinatol. (2018) 35(11):1107–12. doi: 10.1055/s-0038-1641746
64. Ghavam S, Batra D, Mercer J, Kugelman A, Hosono S, Oh W, et al. Effects of placental transfusion in extremely low birthweight infants: meta-analysis of long- and short-term outcomes. Transfusion. (2014) 54(4):1192–8. doi: 10.1111/trf.12469
65. Katheria AC, Leone TA, Woelkers D, Garey DM, Rich W, Finer NN. The effects of umbilical cord milking on hemodynamics and neonatal outcomes in premature neonates. J Pediatr. (2014) 164(5):1045–1050 e1. doi: 10.1016/j.jpeds.2014.01.024
66. Takami T, Suganami Y, Sunohara D, Kondo A, Mizukaki N, Fujioka T, et al. Umbilical cord milking stabilizes cerebral oxygenation and perfusion in infants born before 29 weeks of gestation. J Pediatr. (2012) 7(4):257–67. doi: 10.1038/jp.2013.70
67. Backes CH, Rivera B, Haque U, Copeland K, Hutchon D, Smith CV. Placental transfusion strategies in extremely preterm infants: the next piece of the puzzle. J Neonatal Perinatal Med. (2014) 7(4):257–67. doi: 10.3233/NPM-14814034
68. Gomersall J, Berber S, Middleton P, McDonald SJ, Niermeyer S, El-Naggar W, et al. Umbilical cord management at term and late preterm birth: a meta-analysis. Pediatrics. (2021) 147(3) e2020015404. doi: 10.1542/peds.2020-015404
69. Koo J, Katheria AC, Polglase G. A newborn's “life line”—a review of umbilical cord management strategies. Semin Perinatol. (2022) 46(6):151621. doi: 10.1016/j.semperi.2022.151621
70. Hooper SB, Polglase GR, te Pas AB. A physiological approach to the timing of umbilical cord clamping at birth. Arch Dis Child Fetal Neonatal Ed. (2015) 100(4):F355–60. doi: 10.1136/archdischild-2013-305703
71. Chandrasekharan P, et al. Placental transfusion during neonatal resuscitation in an asphyxiated preterm model. Pediatr Res. (2022) 92(3):678–84. doi: 10.1038/s41390-022-02086-9
72. Katheria A, Blank D, Rich W, Finer N. Umbilical cord milking improves transition in premature infants at birth. PLoS One. (2014) 9(4):e94085. doi: 10.1371/journal.pone.0094085
73. Jaiswal P, Upadhyay A, Gothwal S, Chaudhary H, Tandon A. Comparison of umbilical cord milking and delayed cord clamping on cerebral blood flow in term neonates. Indian J Pediatr. (2015) 82(10):890–5. doi: 10.1007/s12098-015-1734-2
74. Katheria AC, Amino R, Konop JM, Orona AJ, Kim E, Liu Y, et al. Stem cell composition of umbilical cord blood following milking compared with delayed clamping of the cord appears better suited for promoting hematopoiesis. J Pediatr. (2020) 216:222–6. doi: 10.1016/j.jpeds.2019.07.043
75. Nagy M, Nasef N, Gibreel A, Sarhan M, Aldomiaty H, Darwish M, et al. Impact of umbilical cord milking on hematological parameters in preterm neonates with placental insufficiency. Front Pediatr. (2022) 9:827219. doi: 10.3389/fped.2021.827219
76. Liao Y, Cotten M, Tan S, Kurtzberg J, Cairo MS. Rescuing the neonatal brain from hypoxic injury with autologous cord blood. Bone Marrow Trans. (2013) 48(7):890–900. doi: 10.1038/bmt.2012.169
77. Mercer JS, Erickson-Owens DA, Deoni SCL, Dean DC, Collins J, Parker AB, et al. Effects of delayed cord clamping on 4-month ferritin levels, brain myelin content, and neurodevelopment: a randomized controlled trial. J Pediatr. (2018) 203:266–272.e2. doi: 10.1016/j.jpeds.2018.06.006
78. Katheria A, Garey D, Truong G, Akshoomoff N, Steen J, Maldonado M, et al. A randomized clinical trial of umbilical cord milking vs delayed cord clamping in preterm infants: neurodevelopmental outcomes at 22-26 months of corrected age. J Pediatr. (2018) 194:76–80. doi: 10.1016/j.jpeds.2017.10.037
79. Rabe H, Sawyer A, Amess P, Ayers S. Neurodevelopmental outcomes at 2 and 3.5 years for very preterm babies enrolled in a randomized trial of milking the umbilical cord versus delayed cord clamping. Neonatology. (2016) 109(2):113–9. doi: 10.1159/000441891
80. Association, AAoPaAH. In: Weiner GM, Zaichkin J, editors. Textbook of neonatal resuscitation (NRP), 7th ed. Elk Grove Village, IL: American Academy of Pediatrics (2016). p. 326.
81. Katheria AC, et al. Placental transfusion for asphyxiated infants. Front Pediatr. (2019) 7:473. doi: 10.3389/fped.2019.00473
82. Philip AGS, Saigal S. When should we clamp the umbilical cord? NeoReviews. (2004) 5(4):e142–54. doi: 10.1542/neo.5-4-e142
83. Hutchon DJ. Immediate cord clamping may increase neonatal acidaemia. Bjog. (2008) 115(9):1190–1. doi: 10.1111/j.1471-0528.2008.01797.x
84. Siddall RS, Crissey RR, Knapp WL. Effect on cesarean section babies of stripping or milking of the umbilical cords. Am J Obstet Gynecol. (1952) 63(5):1059–64. doi: 10.1016/0002-9378(52)90546-2
85. Niermeyer S, Velaphi S. Promoting physiologic transition at birth: re-examining resuscitation and the timing of cord clamping. Semin Fetal Neonatal Med. (2013) 18(6):385–92. doi: 10.1016/j.siny.2013.08.008
86. Arcilla RA, et al. Pulmonary arterial pressures of newborn infants born with early and late clamping of the cord. Acta Paediatr Scand. (1966) 55(3):305–15. doi: 10.1111/j.1651-2227.1966.tb17659.x
87. Quan A, Leung SW, Lao TT, Man RY. 5-hydroxytryptamine And thromboxane A2 as physiologic mediators of human umbilical artery closure. J Soc Gynecol Investig. (2003) 10(8):490–5. doi: 10.1016/S1071-5576(03)00149-7
88. Ram Mohan G, Shashidhar A, Chandrakala BS, Nesargi S, Suman Rao PN. Umbilical cord milking in preterm neonates requiring resuscitation: a randomized controlled trial. Resuscitation. (2018) 130:88–91. doi: 10.1016/j.resuscitation.2018.07.003
89. Girish M, Jain V, Dhokane R, Gondhali SB, Vaidya A, Aghai ZH. Umbilical cord milking for neonates who are depressed at birth: a randomized trial of feasibility. J Perinatol. (2018) 38(9):1190–6. doi: 10.1038/s41372-018-0161-4
90. Al-Wassia H, Shah PS. Efficacy and safety of umbilical cord milking at birth: a systematic review and meta-analysis. JAMA Pediatr. (2015) 169(1):18–25. doi: 10.1001/jamapediatrics.2014.1906
91. Backes CH, Rivera BK, Haque U, Bridge JA, Smith CV, Hutchon DJ, et al. Placental transfusion strategies in very preterm neonates: a systematic review and meta-analysis. Obstet Gynecol. (2014) 124(1):47–56. doi: 10.1097/AOG.0000000000000324
92. Katheria A, Hosono S, El-Naggar W. A new wrinkle: umbilical cord management (how, when, who). Semin Fetal Neonatal Med. (2018) 23(5):321–26. doi: 10.1161/cir.0000000000000276
93. Katheria A, Reister F , Essers J , Mendler M, Hummler H, Subramaniam A, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. Jama. (2019) 322(19):1877–86. doi: 10.1001/jama.2019.16004
94. Shirk SK, Manolis SA, Lambers DS, Smith KL. Delayed clamping vs milking of umbilical cord in preterm infants: a randomized controlled trial. Am J Obstet Gynecol. (2019) 220(5):482.e1–e8. doi: 10.1016/j.ajog.2019.01.234
95. Nagano N, Saito M, Sugiura T, Miyahara F, Namba F, Ota E. Benefits of umbilical cord milking versus delayed cord clamping on neonatal outcomes in preterm infants: a systematic review and meta-analysis. PLoS One. (2018) 13(8):e0201528. doi: 10.1371/journal.pone.0201528
Keywords: umbilical cord milking, delayed cord clamping, placental transfusion, infants, newborn
Citation: Koo J, Kilicdag H and Katheria A (2023) Umbilical cord milking-benefits and risks. Front. Pediatr. 11:1146057. doi: 10.3389/fped.2023.1146057
Received: 16 January 2023; Accepted: 3 April 2023;
Published: 18 April 2023.
Edited by:
Simone Pratesi, Careggi University Hospital, ItalyReviewed by:
Praveen Chandrasekharan, University at Buffalo, United StatesDaniele Trevisanuto, University Hospital of Padua, Italy
© 2023 Koo, Kilicdag and Katheria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anup Katheria YW51cC5rYXRoZXJpYUBzaGFycC5jb20=
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Jenny Koo
Jenny Koo Hasan Kilicdag
Hasan Kilicdag Anup Katheria
Anup Katheria