
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 21 February 2023
Sec. Pediatric Oncology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1145941
This article is part of the Research TopicAcute Toxicities and Late Effects of Childhood Cancer TreatmentView all 6 articles
 Elisabetta Bigagli1*
Elisabetta Bigagli1* Sara Agostiniani1
Sara Agostiniani1 Alessandra Pugi2*
Alessandra Pugi2* Barbara Rombi3
Barbara Rombi3 Elena Eve Tornaboni2
Elena Eve Tornaboni2 Maria Luigia Censullo4
Maria Luigia Censullo4 Carlotta Gemma Gori4
Carlotta Gemma Gori4 Rossana Pavone4
Rossana Pavone4 Iacopo Sardi4,†
Iacopo Sardi4,†
In immunocompetent individuals, cytomegalovirus (CMV) infection is usually mild but may cause severe complications such as retinitis, pneumonitis, and encephalitis in immunocompromised individuals. So far, cases of CMV retinitis in patients with medulloblastoma undergoing chemotherapy and radiotherapy, have not been reported. We herein report the case of a pediatric patient with high-risk medulloblastoma who experienced an unexpected CMV retinopathy and leukoencephalopathy following high dose thiotepa and proton irradiation. The patient underwent a four-course induction therapy (1st cycle: methotrexate and vinorelbine; 2nd cycle: etoposide and hematopoietic stem cells apheresis; 3rd cycle: cyclophosphamide and vinorelbine; 4th cycle: carboplatin and vinorelbine) and then a consolidation phase consisting in high dose thiotepa followed by autologous HSC transplant and proton cranio-spinal irradiation plus boost to the primary tumor site and pituitary site with concomitant vinorelbine. After two months of maintenance treatment with lomustine and vinorelbine, the patient showed complete blindness and leukoencephalopathy. A diagnosis of CMV retinopathy was made and oral valganciclovir was administered. CMV retinopathy was judged to be possibly related to the use of high dose thiotepa worsened by radiotherapy. This case report suggests that in pediatric patients undergoing immunosuppressive chemo-radiotherapy, CMV reactivation should be carefully monitored to prevent serious complications such as retinopathy and visual loss.
The vast majority of CMV infections are mild or asymptomatic in healthy children and adults, however, in individuals with a suppressed immune system, CMV might cause a high burden of diseases such as pneumonia, encephalopathy and retinitis (1).
CMV retinitis is a potentially blinding disease that has been extensively reported in patients who have deficient T-cell responses such as solid and hematopoietic cell transplant recipients or those with acquired immunodeficiency syndrome (2, 3). Increasing cases of CMV retinitis have been also described during chemotherapy for acute lymphoblastic leukemia in children (4–10).
In contrast, only very few cases of CMV retinitis have been reported so far in children with medulloblastoma and retinoblastoma (11–13).
This report describes the clinical presentation and outcomes of CMV retinopathy in a child enrolled in an open-label, phase II clinical trial aimed at evaluating the safety and efficacy of standard and high-dose chemotherapy associated with proton cranio-spinal irradiation in metastatic medulloblastoma and other high risk embryonal tumors.
In September 2018, a 5-year-old female was referred to our hospital with a history of headache and vomiting. A Computed Tomography (CT) scan showed a voluminous posterior fossa tumor with several supratentorial metastases, associated with ventricular dilatation. The gadolinium-enhanced MRI confirmed the presence of a cerebellar tumor with a voluminous metastasis in the hypothalamic site (Figure 1). She underwent endoscopic septostomy and biopsy of the hypothalamic lesion and received a ventriculoperitoneal shunt. Pathology showed medulloblastoma, classic variant (WHO-grade IV; MYC/MYCN non-amplified, synaptophysin+, CD56+, beta-catenin+, and INI1+) subgroup non-wingless-activated (WNT)/non-sonic hedgehog-activated (SHH), subgroup 4. The diagnosis was confirmed by the CNS national pathology panel. The patient was enrolled in the clinical trial MBMET_MEYER2017 (Eudract Number 2017-000801-19).
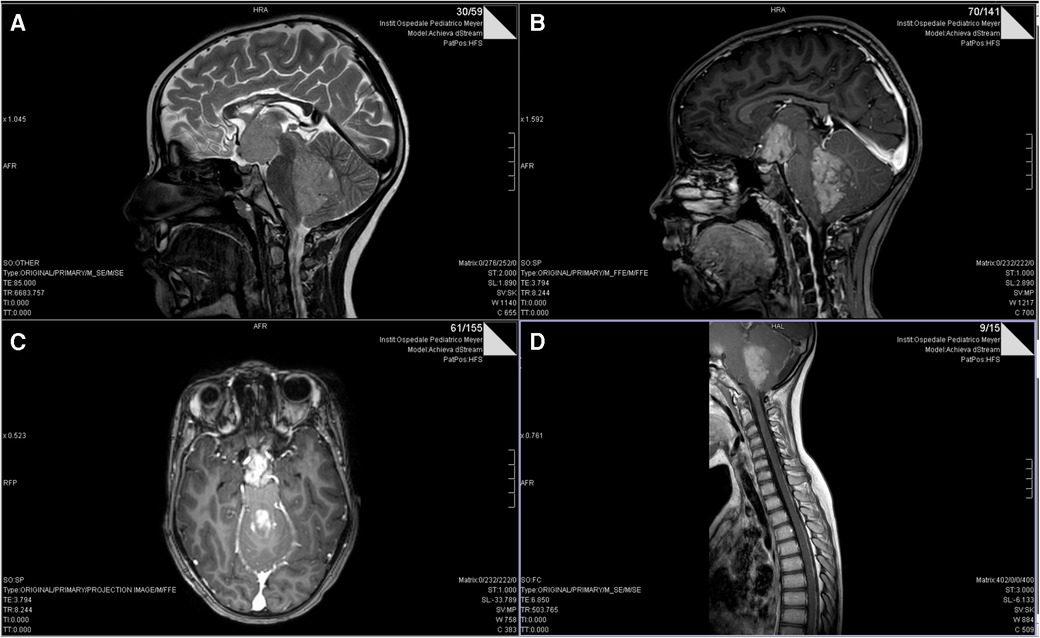
Figure 1. Pre-operative T2 (A) and post-gadolinum (B) sagittal and axial (C) MRI scans of our patient showing the extensive metastasis to hypothalamus. (D) post-gadolinium-enhanced T1-weighted sagittal spine MRI image showing leptomeningeal dissemination of medulloblastoma.
The induction phase of the protocol included the following four courses: 1st cycle with methotrexate (8 g/m2) plus vinorelbine (20 mg/m2); 2nd cycle with etoposide (2.4 g/m2) followed by hematopoietic stem cells (HSC) collection via apheresis; 3rd cycle with cyclophosphamide (4 g/m2) plus vinorelbine (20 mg/m2); 4th cycle with carboplatin (800 mg/m2) plus vinorelbine (20 mg/m2).
After induction chemotherapy, the patient showed stable disease (SD) and she continued the consolidation phase with high dose thiotepa (300 mg/m2) for 3 consecutive days followed by autologous HSC transplant.
Chemotherapy was followed by proton cranio-spinal irradiation (total dose of 39.6 CGE in 22 fractions, 1.8 RBE daily) and an additional dose to the primary tumor site and to the hypothalamic metastatic site up to 14.4 RBE and to the spine (D1–D2) and to cauda equina up to 10.8 RBE. She received concomitant vinorelbine (20 mg/m2 every 2 weeks). The proton irradiation was well tolerated and no significant toxicities (i.e., neurological symptoms) were observed except for alopecia and one blood transfusion. After proton irradiation we continued to observe a SD at Magnetic Resonance Imaging (MRI).
In MBMET_MEYER2017 protocol, the maintenance phase in SD arm consists of lomustine 80 mg/m2 repeated every 9 weeks and vinorelbine 20 mg/m2 every 3 weeks, for overall 6 months.
After two months of maintenance treatment, clinical (major asthenia) and biological (hypocortisolism and hypothyroidism) features of post actinic hypophysitis appeared, managed with steroids (dexamethasone 4 mg/day) and hormonal supplementation. Five days after dexamethasone introduction, the patient showed complete blindness, without significant or new alteration in CT cerebral scan. The MRI instead suggested radiological patterns of leukoencephalopathy (Figure 2). She presented elevated liver enzymes and persistent thrombocytopenia. Interestingly, the ophthalmologist suspected on sequential optical coherence tomography (OCT) scans a retinal neurodegenerative process resembling CMV retinopathy. CMV infection was diagnosed with quantitative and qualitative blood PCR analysis which showed elevated values of viral DNA. Due to the precarious clinical conditions, it was not possible to analyze the CSF. In September 2018, at disease onset, the serological examination showed elevated levels of CMV IgG (77.4 U/ml; normal value <14 U/ml), CMV IgG avidity (Index: 0.54; high values >0.25) and CMV IgM (10 U/ml; normal value <10 U/ml). The patient had never previously received immunoglobulin treatments. The patient had corticosteroid therapy only in the first few days due to hydrocephalus. Therefore, oral valganciclovir (1200 mg/day) was began, with persisting bilateral blindness but progressive normalization of liver function indices and negativization of blood CMV DNA. She continued valganciclovir for a total of 22 days. Steroids were stopped after 41 days. The young girl required monitoring of electrolytes especially at the beginning of the hospitalisation (severe hyponatremia). The clinical course of the patient is depicted in Figure 3. Due to CMV retinopathy and leukoencephalopathy, chemotherapy was interrupted after only two maintenance cycles.
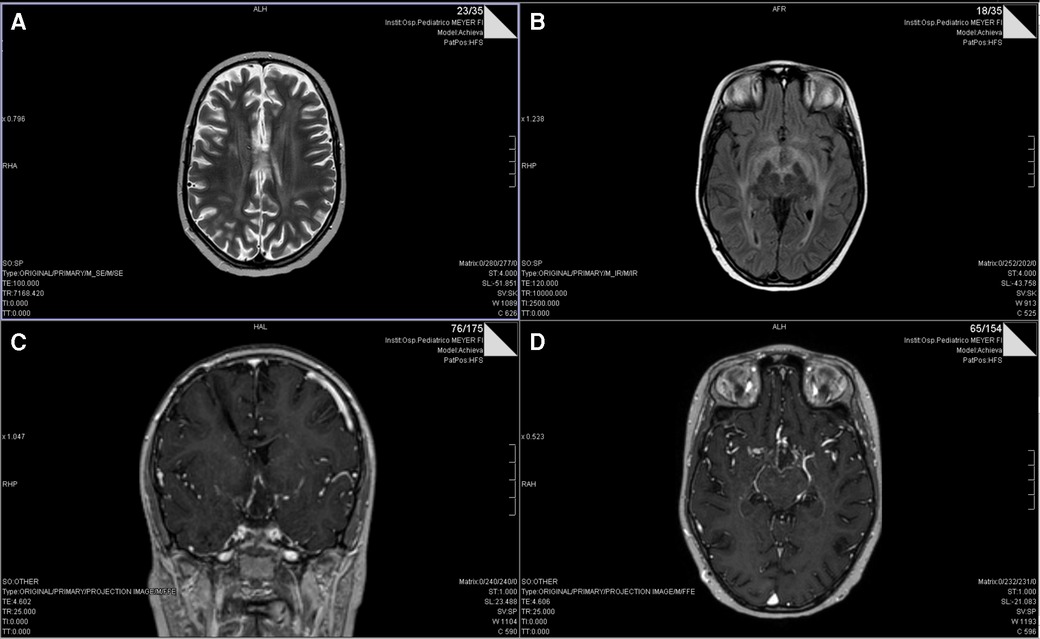
Figure 2. T2 (A) and FLAIR (B) axial MRI scans showing multifocal leukoencephalopathy after two months of maintenance treatment. Gadolinium-enhanced T1-weighted MRI (C, coronal section; D, axial section) shows a ring enhancement of the hypothalamic lesion compressing the chiasm and the optic tracts.
The patient is still alive 47 months after diagnosis, with persisting blindness and panhypopituitarism needing hormonal supplementation.
After primary infection, in immunocompetent individuals, CMV remains latent and asymptomatic, but reactivation may occur when the immune system is compromised leading to several clinical manifestations such as colitis, hepatitis, pneumonitis and encephalitis (1). The most severe manifestation of CMV infection in the eye, is CMV retinitis; uncontrolled virus replication in the retina may indeed lead to cell death, retinal detachment, blurred vision and ultimately blindness (2). Prior to highly active antiretroviral therapy, CMV retinitis was commonly observed in patients with acquired immunodeficiency syndrome, and to a lesser extent, in other conditions associated with deficient T-cell response such as solid organ transplantation (3, 14, 15), hematopoietic stem cell and bone marrow transplantation (16–18).
Conversely, CMV retinitis is rarer in patients undergoing only chemotherapy: in acute lymphoblastic leukaemia children, few cases, etiologically associated with profound immunosuppression and a delayed T-cell regeneration caused by the maintenance chemotherapy with 6-mercaptopurine, methotrexate, vincristine, and steroids, have been reported (6, 19, 20). The addition of dexamethasone and vincristine to methotrexate and 6-mercaptopurine was also suggested to increase the risk of CMV retinitis (8). A delayed immune reconstitution following completion of chemotherapy was also assumed to be the cause of CMV retinitis occurred in an adolescent with acute lymphoblastic leukaemia (7).
Other cases of CMV retinitis occurred in patients with acute lymphoblastic leukaemia and with Burkitt's lymphoma undergoing HyperCVAD chemotherapy or following ocular and systemic steroid administration (4, 21, 22).
As far as we know, this is the first report in medical literature of leukoencephalopathy and retinopathy secondary to CMV reactivation, associated with high dose thiotepa and radiotherapy in a pediatric patient. Although the underlying disease as well as other protocol medications, may have confounded this association, the co-causal role of the use of high dose thiotepa and cranio-spinal radiotherapy cannot be excluded. Despite being listed among the adverse effects of thiotepa (https://www.ema.europa.eu), the specificity and outcome of leukoencephalopathy and infection were considered unexpected.
Regarding temporal relationship, although these adverse reactions occurred during the maintenance therapy, 4 and 3 months after the last dose of thiotepa and radiotherapy, respectively, they were deemed as delayed side effects of the consolidation phase regimen. This is in line with a median time of around 100 days to the development of CMV disease in the allogeneic hematopoietic stem cell transplantation setting (23).
Regarding possible pathophysiological explanations, the myeloablative regimen with high dose thiotepa, was suspected to have caused CMV reactivation and subsequent leukoencephalopathy and retinopathy. The cranio-spinal irradiation plus a boost to the hypothalamic/pituitary metastatic region close to the chiasm and optic nerves, may have also worsened the clinical scenario: although the connection between CMV reactivation and radiotherapy of the brain has been little explored, Goerig et al. (2016) hypothesized that radiotherapy reactivated CMV contained in the tumor mass leading to encephalopathy and neurological decline in four patients with high-grade gliomas and cerebral metastases (24). The same authors confirmed their observation in a prospective trial demonstrating that CMV reactivation frequently causes encephalopathy during radio(chemo)therapy of the brain (25).
The severe immunosuppression following intensive chemotherapy and craniospinal radiotherapy, was also deemed the possible cause of three herpes virus infections, including CMV reactivation, observed in a child diagnosed with medulloblastoma (12). A recent retrospective study reported that most children who developed CMV ocular diseases, including a patient with medulloblastoma, were in an immunocompromised status post-stem cells/bone marrow transplantation (13). A high risk of risk of developing CMV disease among children with retinoblastoma undergoing chemotherapy was also documented in the study by Han and coworkers (11). Similarly, in young children with haemato-oncologic diseases receiving chemotherapy without hematopoietic stem cell transplantation, a significant morbidity of CMV infection was reported (26).
Notably, the presence of CMV in medulloblastoma is still a matter of debate since some reports have shown the presence of this virus in tumor samples (27, 28) while others have refuted these findings (29, 30). Despite these contradictory reports, accumulating evidence has suggested that, by activating immune-inflammatory and angiogenic signaling pathways, CMV has an onco-modulatory role in medulloblastoma and may be exploited as a potential therapeutic tool (31, 32).
Despite the limitations of being a context-specific, single case study, this report may contribute to drug safety surveillance and to medical awareness that in immunocompromised pediatric patients, such as those with medulloblastoma undergoing chemo-radiotherapy, the occurrence of CMV reactivation should be considered to allow prompt diagnosis and treatment as well as to prevent serious complications.
Guidelines for the management of CMV infection in transplant patients or in those with hematological malignancies have been published (33) and they may be useful also in the setting of radio (chemo) therapy of the brain.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by COMITATO ETICO PEDIATRICO—Meyer. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
JS, EB, RP and BR: took the lead in drafting the manuscript; SA, AP, EET, MLC and CCG: participated in data collection and in drafting the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from Fondazione Anna Meyer, Florence, Italy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. (2017) 64(1):87–91. doi: 10.1093/cid/ciw668
3. Munro M, Yadavalli T, Fonteh C, Arfeen S, Lobo-Chan AM. Cytomegalovirus retinitis in HIV and non-HIV individuals. Microorganisms. (2019) 8(1):55. doi: 10.3390/microorganisms8010055
4. Narayanan G, Haridas L, Soman LV. Cytomegalovirus retinitis occurring as a complication of HyperCVAD chemotherapy: report of two cases. Indian J Med Paediatr Oncol. (2018) 39:548–50. doi: 10.4103/ijmpo.ijmpo_48_17
5. Mandura RA, Talat K, Jastaniah W. Unilateral cytomegalovirus retinitis in a child with acute lymphoblastic leukemia while on maintenance chemotherapy. Cureus. (2021) 13(5):e15246. doi: 10.7759/cureus.15246
6. Rahbarimanesh A, Ehsani M, Karahroudi M, Rashidi A, Aghajani M, Meysami A, et al. Cytomegalovirus disease in children with acute lymphoblastic leukemia in the nontransplant setting: case series and review of the literature. J Pediatr Hematolwithol. (2015) 37(6):429–32. doi: 10.1097/MPH.0000000000000298
7. Han SB, Lee JH, Lee JW, Chung NG, Cho B, Kang JH, et al. Cytomegalovirus retinitis diagnosed after completion of chemotherapy for acute lymphoblastic leukemia in an adolescent. J Pediatr Hematol Oncol. (2015) 37(2):e128–30. doi: 10.1097/MPH.0000000000000252
8. Moritake H, Kamimura S, Kojima H, Shimonodan H, Harada M, Sugimoto T, et al. Cytomegalovirus retinitis as an adverse immunological effect of pulses of vincristine and dexamethasone in maintenance therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. (2013) 60(2):329–31. doi: 10.1002/pbc.24298
9. Samia L, Hamam R, Dbaibo G, Saab R, El-Solh H, Abboud M, et al. Cytomegalovirus retinitis in children and young adults with acute lymphoblastic leukemia in Lebanon. Leuk Lymphoma. (2014) 55(8):1918–21. doi: 10.3109/10428194.2013.854887
10. Jain R, Trehan A, Mishra B, Singh R, Saud B, Bansal D. Cytomegalovirus disease in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. (2016) 33(4):239–47. doi: 10.3109/08880018.2016.1173147
11. Han MS, Choi EH, Lee HJ, Yun KW, Kang HJ, Hong KT, et al. Cytomegalovirus disease in a retinoblastoma cohort: the role of preemptive screening. Pediatr Blood Cancer. (2020) 67(3):e28101. doi: 10.1002/pbc.28101
12. Ohta M, Taga T, Nomura A, Kato H, Takano T, Maruo Y, et al. Epstein-barr virus-related lymphoproliferative disorder, cytomegalovirus reactivation, and varicella zoster virus encephalitis during treatment of medulloblastoma. J Med Virol. (2011) 83(9):1582–4. doi: 10.1002/jmv.22136
13. Mercado CL, Froines CP, Gaier ED, Wang Q, Indaram M, Wan MJ, et al. Prevalence and characteristics of cytomegalovirus ocular disease in children: a multi-center study. Clin Ophthalmol. (2022) 16:2209–17. doi: 10.2147/OPTH.S364741
14. Hamouda M, Kahloun R, Jaballah L, Aloui S, Skhiri H, Jelliti B, et al. Cytomegalovirus ocular involvement in a kidney transplant recipient. Exp Clin Transplant. (2018) 16(4):495–8. doi: 10.6002/ect.2016.0022
15. Fu L, Santhanakrishnan K, Al-Aloul M, Jones NP, Steeples LR. Management of ganciclovir resistant cytomegalovirus retinitis in a solid organ transplant recipient: a review of current evidence and treatment approaches. Ocul Immunol Inflamm. (2020) 28(7):1152–8. doi: 10.1080/09273948.2019.1645188
16. Jeon S, Lee WK, Lee Y, Lee DG, Lee JW. Risk factors for cytomegalovirus retinitis in patients with cytomegalovirus viremia after hematopoietic stem cell transplantation. Ophthalmology. (2012) 119(9):1892–8. doi: 10.1016/j.ophtha.2012.03.032
17. Chen WB, Long Z, Hou J, Miao H, Zhao MW. Continuous high-dose (6 mg) vs. low-dose (3 mg) intravitreal ganciclovir for cytomegalovirus retinitis after haploidentical hematopoietic stem cell transplantation: a randomized controlled study. Front Med. (2021) 8:750760. doi: 10.3389/fmed.2021.750760
18. Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. (1996) 88(10):4063–71. doi: 10.1182/blood.V88.10.4063.bloodjournal88104063
19. Kobayashi R, Takanashi K, Suzuki D, Nasu T, Uetake K, Matsumoto Y. Retinitis from cytomegalovirus during maintenance treatment for acute lymphoblastic leukemia. Pediatr Int. (2012) 54(2):288–90. doi: 10.1111/j.1442-200X.2011.03429.x
20. El-Chennawi FA, Al-Tonbary YA, Mossad YM, Ahmed MA. Immune reconstitution during maintenance therapy in children with acute lymphoblastic leukemia, relation to co-existing infection. Hematology. (2008) 13(4):203–9. doi: 10.1179/102453308X316086
21. Tetsuju S, Iida T, Kaneko H, Saito M. Cytomegalovirus retinitis after intravitreal triamcinolone acetonide in an immunocompetent patient. Jpn J Ophthalmol. (2008) 52(5):414–6. doi: 10.1007/s10384-008-0576-0
22. Laws PM, Kingston TP, Walsh S, Shear NH. Cytomegalovirus retinitis: a rare but preventable cause of blindness in dermatology patients. J Cutan Med Surg. (2014) 18(4):287–90. doi: 10.2310/7750.2013.13149
23. Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. (2018) 7(1):1–16. doi: 10.1007/s40121-017-0180-z
24. Goerig N, Semrau S, Frey B, Korn K, Fleckenstein B, Überla K, et al. Clinically significant CMV (re)activation during or after radiotherapy/chemotherapy of the brain: correlation with neurological deterioration and improvement upon antiviral treatment. Strahlenther Onkol. (2016) 192(7):489–97. doi: 10.1007/s00066-016-0987-7
25. Goerig NL, Frey B, Korn K, Fleckenstein B, Überla K, Schmidt MA, et al. Frequent occurrence of therapeutically reversible CMV-associated encephalopathy during radiotherapy of the brain. Neuro Oncol. (2016) 18(12):1664–72. doi: 10.1093/neuonc/now120
26. Han MS, Lee HJ, Lee H, Choe YJ, Lee JW, Kang HJ, et al. Risk factors and clinical features of cytomegalovirus disease in children receiving anticancer chemotherapy. J Pediatr Hematol Oncol. (2016) 38(3):e113–9. doi: 10.1097/MPH.0000000000000459
27. Baryawno N, Rahbar A, Wolmer-Solberg N, Taher C, Odeberg J, Darabi A, et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J Clin Invest. (2011) 121(10):4043–55. doi: 10.1172/JCI57147
28. Bartek J Jr, Merchut-Maya JM, Maya-Mendoza A, Fornara O, Rahbar A, Bröchner CB, et al. Cancer cell stemness, responses to experimental genotoxic treatments, cytomegalovirus protein expression and DNA replication stress in pediatric medulloblastomas. Cell Cycle. (2020) 19(7):727–41. doi: 10.1080/15384101.2020.1728025
29. Sardi I, Lucchesi M, Becciani S, Facchini L, Guidi M, Buccoliero AM, et al. Absence of human cytomegalovirus infection in childhood brain tumors. Am J Cancer Res. (2015) 5(8):2476–83, eCollection 2015. PMID: 26396923; PMCID: PMC4568783
30. Vermeulen JF, van Hecke W, Jansen MK, Spliet WG, Broekhuizen R, Bovenschen N. No evidence for human cytomegalovirus infection in pediatric medulloblastomas. Neuro Oncol. (2016) 18(10):1461–2. doi: 10.1093/neuonc/now151
31. Hortal AM, Vermeulen JF, Van Hecke W, Bovenschen N. Oncogenic role of cytomegalovirus in medulloblastoma? Cancer Lett. (2017) 408:55–9. doi: 10.1016/j.canlet.2017.08.024
32. Athanasiou E, Gargalionis AN, Boufidou F, Tsakris A. The association of human herpesviruses with malignant brain tumor pathology and therapy: two sides of a coin. Int J Mol Sci. (2021) 22(5):2250. doi: 10.3390/ijms22052250
33. Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European conference on infections in leukaemia (ECIL 7). Lancet Infect Dis. (2019) 19(8):e260–72. doi: 10.1016/S1473-3099(19)30107-0
Keywords: retinopathy, leukoencephalopathy, cytomegalovirus, thiotepa, proton irradiation, medulloblastoma, pediatric, drug safety—clinical pharmacology
Citation: Bigagli E, Agostiniani S, Pugi A, Rombi B, Tornaboni EE, Censullo ML, Gori CG, Pavone R and Sardi I (2023) Unforeseen cytomegalovirus retinopathy following high dose thiotepa and proton irradiation in a pediatric patient with high-risk medulloblastoma: A case report. Front. Pediatr. 11:1145941. doi: 10.3389/fped.2023.1145941
Received: 16 January 2023; Accepted: 8 February 2023;
Published: 21 February 2023.
Edited by:
Katarzyna Muszynska-Roslan, Medical University of Bialystok, PolandReviewed by:
Barbara Spitzer, Memorial Sloan Kettering Cancer Center, United States© 2023 Bigagli, Agostiniani, Pugi, Rombi, Tornaboni, Censullo, Gori, Pavone and Sardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandra Pugi YWxlc3NhbmRyYS5wdWdpQG1leWVyLml0 Elisabetta Bigagli ZWxpc2FiZXR0YS5iaWdhZ2xpQHVuaWZpLml0
†The Principal Investigator of the clinical trial MBMET (Eudract Number 2017-000801-19) is Iacopo Sardi who had direct clinical responsibility of the patient.
Specialty Section: This article was submitted to Pediatric Oncology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.