- 1The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
- 2Department of Neonatology, Senior Department of Pediatrics, The Seventh Medical Center of PLA General Hospital, Beijing, China
Background: Premature rupture of membranes (PROM) is a common cause of extremely premature infants (EPIs) and also leads to adverse preterm complications. However, the effect of PROM on EPIs remains contradictory. This study used propensity score matching (PSM) to adjust the baseline characteristics to explore the impact of PROM on clinical outcomes of extremely premature infants (EPIs).
Methods: Medical data of 470 EPIs at gestational age < 28weeks who received prenatal examination in our hospital between January 1, 2015 and December 31, 2020 were analyzed retrospectively. According to the presence or absence of PROM, they were divided into a PROM group and a non-PROM group. Ten covariates including birth weight, male sex, artificial conception, cesarean delivery, 5-min Apgar score ≤ 7, oligohydramnios, gestational hypertension, preeclampsia, antenatal steroid use, and complete steroid treatment were matched 1:1 by PSM. The major complication occurrence and mortality during hospitalization were compared between the two groups by t-test, nonparametric test or test.
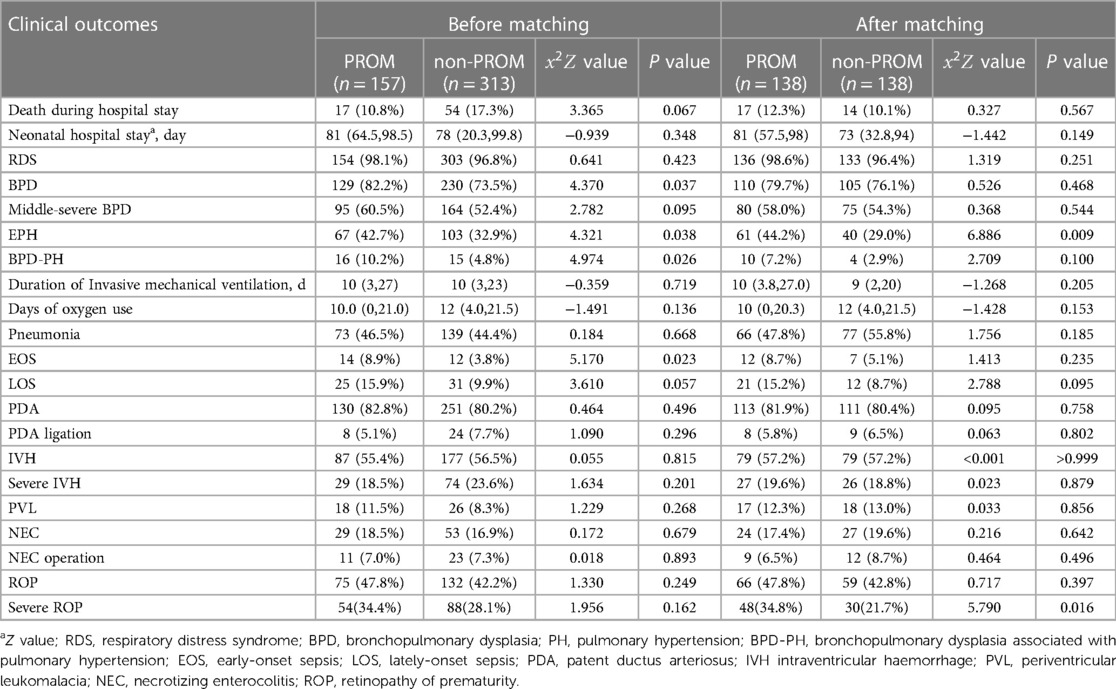
Results: Among the 470 infants enrolled, 157 (33.4%) were in the PROM group and 313 in the no-PROM group. After matching the ten confounding factors,276 cases were successfully enrolled. The incidence of early pulmonary hypertension (EPH) and severe retinopathy of prematurity (ROP) in the PROM group were higher than those in the no-PROM group [44.2% (61/138) vs. 29.0% (40/138); 34.8% (48/138) vs. 21.7% (30/138), x2 = 6.886 and 5.790, both P < 0.05]. However, there was no significant difference in the in-hospital mortality and the incidence of other major complications between the two groups (all P > 0.05).
Conclusions: PROM increased the incidence of EPH and severe ROP in EPI, but had no significant impact on in-hospital mortality, length of hospital stay, and the incidence of other complications.
Introduction
Premature rupture of membranes (PROM) is a common complication during pregnancy, and its incidence is about 5%–10% (1). According to the time of onset, PROM can be classified as full-term PROM and preterm premature rupture of membranes (PPROM). The earlier the time of occurrence, the greater the potential harm. About one-third of premature births are related to PROM (1, 2). In particular, PPROM, which usually occurs before the gestational age of 28 weeks, increases the incidence of extremely premature infants (EPIs) (3). Due to the immaturity of various organs, the prognosis of these infants is relatively poor, and the incidence of complications such as severe bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), and intraventricular haemorrhage (IVH) is high. However, the extent of adverse impact of PROM in EPIs remains uncertain. Some studies reported that the occurrence of PPROM increased the incidence and even mortality of diseases such as respiratory distress syndrome (RDS), sepsis, BPD, severe retinopathy of prematurity (ROP), IVH and NEC, among others (4, 5), while others held different views, believing that survival rates and serious complications in these infants were similar to those of preterm infants without PROM (4, 6, 7). These different results may be due to differences in study participants and baseline characteristics. In addition, there are few research data on EPIs. In this retrospective study, we analyzed the clinical data of EPIs who were admitted to the Department of Pediatrics of the 7th Medical Center of the PLA General Hospital (Beijing, China), compared the baseline characteristics of the patients in the PROM group and the non-PROM group by the propensity score matching (PSM) method after eliminating the influence of individualized factors, and explored the relationship of PROM with complications and mortality in EPIs during hospitalization for the sake of providing clinical basis for better prenatal consultation about neonatal care and management of EPIs born from mothers with PROM.
Material and methods
Study design
In this retrospective study, we selected EPIs at a gestational age < 28 weeks who were admitted to the Department of Pediatrics of the 7th Medical Center of the PLA General Hospital between January 1, 2015 and December 31, 2020. Exclusion Criteria (1) postnatal time >72 h; (2) incomplete birth or hospitalization information; (3) serious genetic or inherited metabolic diseases; and (4) abandonment of treatment due to social factors. According to the presence or absence of PROM before birth, they were divided into a PROM group and a non-PROM group.
Methods
Data collection: Perinatal and neonatal clinical data of the newborns were collected by reviewing the inpatient medical records. Perinatal data included the amniotic fluid contamination, oligohydramnios, fetal distress, gestational hypertension, gestational diabetes, preeclampsia, placental factors, prenatal infection, antenatal steroid use, and complete steroid use. Clinical data of the neonates included gestational age, birth weight, male sex, multiple births, artificial conception, cesarean delivery, 1 min Apgar score ≤ 7, 5 min Apgar score ≤ 7, surfactant therapy, death during hospital stay, neonatal hospital stay, RDS, BPD, moderate and severe BPD, early pulmonary hypertension (EPH), bronchopulmonary dysplasia associated with pulmonary hypertension (BPD-PH), duration of invasive mechanical ventilation, days of oxygen use, pneumonia, early-onset sepsis (EOS), late-onset sepsis (LOS), patent ductus arteriosus (PDA), PDA ligation, IVH, severe IVH, periventricular leukomalacia (PVL), NEC, ROP, and severe ROP.
Definitions
Placental factors included placental abruption, placenta previa, and placenta accrete. Death during the hospital stay refers to the failure of rescue efforts during hospitalization, or the abandonment of treatment due to the terminal stage of the disease. The diagnosis and classification criteria of BPD are as follows: (1) oxygen inhalation for at least 28 days; and (2) infants who did not need oxygen and were evaluated at 36 weeks of postmenstrual age (PMA) or at discharge. Those who needed oxygen but with FiO2 < 0.3 were considered moderate cases, and who needed oxygen but with FiO2 ≥ 0.3 or needed positive pressure ventilation were considered severe cases (8). EPH and BPD-PH: According to echocardiography, the specific diagnostic criteria refer to the diagnosis of pulmonary hypertension in premature infants (9), and the PH diagnosed within 3–14 days after delivery of EPH. The presence of PH in children with BPD after 36 PMA was referred to as BPD-PH. EOS was defined as septicemia when the clinical manifestations were observed within 72 h after birth, while LOS was defined as sepsis when the clinical manifestations were observed 72 h after birth (10). IVH was graded according to Papile's criteria (11). Grade III IVH referred to bilateral ventricular enlargement with ventricular hemorrhage, and grade IV referred to intraventricular hemorrhage with periventricular hemorrhagic infarction, both of which were considered severe forms of IVH. NEC referred to Bell stage ≥ II (12). ROP was diagnosed and staged according to the international diagnostic and staging standards of ROP (13). Severe ROP referred to stage ≥ III and required laser or Ranibizumab treatment.
Statistical analysis
All statistical analyses were performed using SPSS 26.0 statistical software. Measurement data of normal distribution are represented as ±s, and two-sample t-test was used for the between-group comparison. Measurement data of abnormal distribution are represented by M (P25, P75), and the Mann-Whitney U test was used for nonparametric test for intergroup comparison. The number of use cases and percentage (%) of the counting data indicate that the test was used for the between-group comparison. The PSM method was used to match 1:1, and the caliper value was 0.01. Variable selection for the PSM model: baseline parameters with statistically significant differences between groups were selected as covariates for score matching, knowing that these covariates may affect the clinical outcomes of EPIs. P < 0.05 was considered statistically significant.
Results
Comparison of the two sets of basic conditions before and after matching
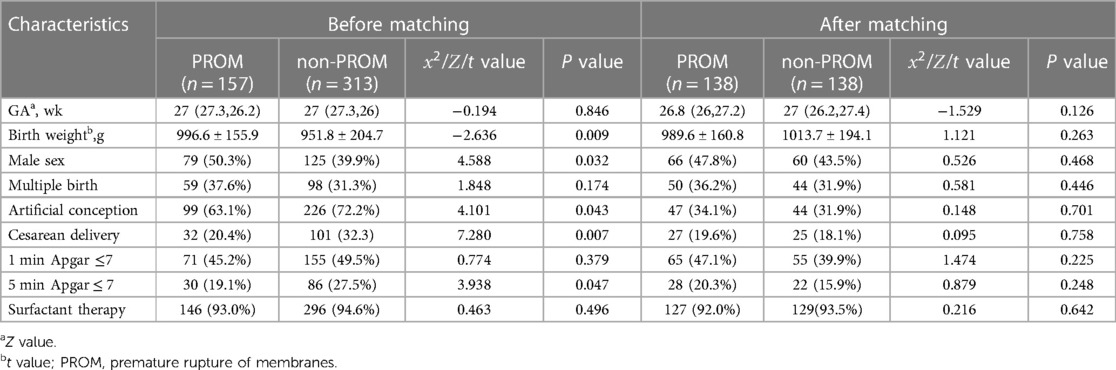
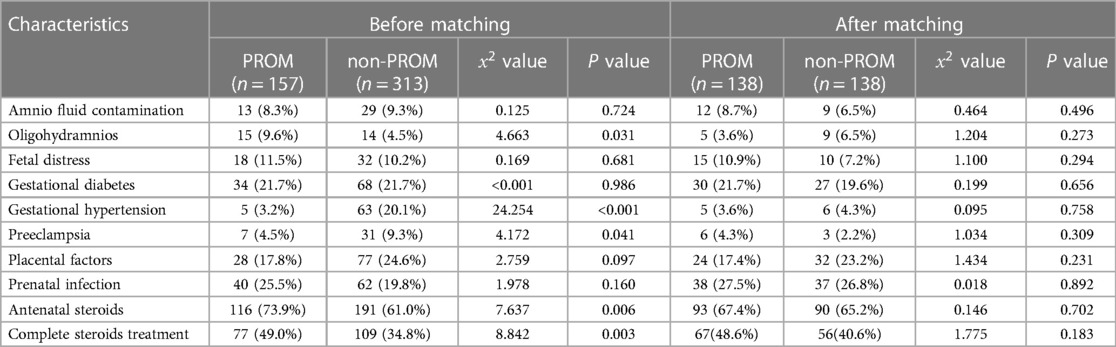
A total of 470 patients were enrolled, including 157 in the PROM group and 313 in the non-PROM group. The time of premature rupture of membranes was 48(18.5,96.0) hours before propensity matching. After matching, it was 48(18.3,96.0) hours. The basic situation before and after matching of the two groups is shown in Tables 1, 2. Before matching, there were 10 covariate differences between the two groups (all P < 0.05), including birth weight, male sex, artificial conception, cesarean delivery, 5 min Apgar score ≤ 7, oligohydramnios, gestational hypertension, preeclampsia, antenatal steroid use, and complete steroid treatment. After matching, 276 EPIs were successfully matched, and all covariates matched well, and the differences were not statistically significant (all P > 0.05).
Comparison of clinical outcomes between the two groups
Before matching: The incidence of BPD, EPH, BPD-PH and early-onset sepsis during hospitalization in the PROM group was higher than that in the non-PROM group (P < 0.05), and there was no significant difference in the incidence of mortality, neonatal hospital stay, RDS, BPD, duration of Invasive mechanical ventilation, days of oxygen use, pneumonia, LOS, PDA, IVH, severe IVH, PVL, NEC, ROP and severe ROP between the two groups (P > 0.05) (Table 3).
After matching: The incidence of EPH and severe ROP during hospitalization in the PROM group was higher than that in the non-PROM group (P < 0.05), and there was no significant difference in the incidence of other complications between the two groups (P > 0.05), and there was no significant difference in mortality and length of hospital stay between the two groups (P > 0.05) (Table 3).
Discussion
In this review article, we explored the impact of PROM on clinical outcomes of EPIs by PSM analysis, and found that the incidence of EPH and severe ROP increased in the PROM group as compared with that in the non-PROM group, while there was no clear difference in mortality and length of hospital stay between the two groups.
Current findings on PROM for EPI survival and length of hospital stay reported in the literature are inconsistent (14, 15). A previous study (14) reported that PROM increased the mortality in preterm infants compared with other settings. Gezer et al. (16) believed that the mortality rate of children with PROM was mainly related to preterm birth, and the younger the gestational age at birth, the higher the mortality rate. Newman et al. (17) reported that PROM increased the infant mortality at 23–24 weeks, but they did not find a significant relationship between the infant mortality and PROM after adjusting for gestational age and sex. Two other studies (18, 19) suggested that birth weight was the strongest risk factor for survival and length of hospital stay. In our study, we did not find significant differences in in-hospital mortality and length of hospital stay between the two groups before and after matching, which is consistent with the finding of Yair et al. (15) and Hanke et al. (7), who reported that PROM did not increase the EPI in-hospital mortality in mothers of the same gestational age and birth weight.
Some studies (5, 20, 21) suggested that PROM increased the occurrence of EOS and NEC, but the baseline data on oligohydramnios and antenatal steroid treatment in these studies were inconsistent. In addition, cesarean section and 5 min Apgar scores have also been shown to be associated with the occurrence of NEC and EOS (22–24). We did not find significant differences in the incidence of EOS and NEC between the two groups after matching these factors, but we found that the incidence of EPH in the PROM group was higher than that in the non-PROM group both before and after matching, which is similar to previous studies (6). PH was found to be closely related to the mortality of preterm infants, BPD and long-term cardiopulmonary diseases, and a recent study (6) reported that EPH after long-term PROM increased the mortality and IVH in preterms. Data concerning EPH in preterm infants are limited, and it is usually associated with oligohydramnios secondary to PROM because the swelling pressure formed by the lung fluid in the airways is the main physical force that stimulates normal lung development, and oligohydramnios can reduce the size of the thoracic cage and interfere with the normal growth of the fetal lung (25). This may explain our finding why the incidence of EPH in the PROM group was higher than that in non-PROM group before matching, but the incidence of EPH in the PROM group was still higher than that in non-PROM group after adjusting for factors such as oligohydramnios, indicating that PROM increased the incidence of EPH, which we believe may be related to pulmonary dysplasia complicated by PROM. At present, it is known that PROM can participate in the pathogenesis of pulmonary dysplasia via various mechanisms such as inflammatory factors, abnormal expression of pulmonary surfactant protein, vascular endothelial growth factor (VEGF) gene polymorphisms and oxidative stress (26). Early injury to the developing lung impairs angiogenesis and alveolar formation, leading to simplified distal lung spaces and resulting in PH (27). In addition, PROM has also been shown to accelerate fetal lung maturation, increase the degree of pulmonary artery musculosis, and increase incidence of EPH in preterm neonates (28).
ROP is a common complication of EPIs and a leading cause of blindness in children (29). Preterm birth and postpartum oxygen therapy have been reported to be closely related to the occurrence of ROP, but many studies have proposed that the pathogenesis of ROP may exist in utero, and the prenatal intrauterine environment may be related to the occurrence of severe ROP (30, 31), including maternal preeclampsia (32), chorioamnionitis (33), and smoking (34). In addition, gender has also been shown to be associated with the occurrence of severe ROP (35). At present, there are conflicting reports about the relationship between PROM and ROP. Lynch et al. (36) and Badeeb et al. (37) showed that PROM was a protective factor for severe ROP, possibly because pregnant mothers who develop PROM are more likely to receive glucocorticoids, which plays an important role in promoting the maturation of the cerebrovascular system by downregulating VEGF to improve angiogenesis and systemic circulation (38). But when we matched these factors, we found that the incidence of severe ROP was higher in the PROM group than that in the non-PROM group. Another study (39) also concluded that newborns with PROM longer than 18 h had an increased risk of severe ROP. We think that this may be because the inflammatory-oxidative stress axis involved in PROM is also involved in ROP formation (40).
To clarify the impact of PROM on mortality and complications during EPI hospitalization, we used the PSM method to match the baseline data of EPIs between the two groups and found that PROM increased the incidence of EPH and severe ROP during EPI hospitalization. Nevertheless, the study also has some limitations. First, this is a non-randomized retrospective study, which may miss some unobserved differences such as some treatment measures and data on chorioamnionitis, antenatal magnesium use and delayed cord clamping due to imperfect obstetric pathological data. That could have an impact on the results. Second, this study only discussed the short-term prognosis, and did not assess the long-term prognosis, especially the occurrence of BPD-PH after discharge. Finally, this study is a single-center study with a relatively small sample size. More data from multiple centers are required to further validate the conclusions of this study.
In summary, antenatal PROM increased the incidence of EPH and severe ROP during EPI hospitalization, but had no significant impact on mortality, length of hospital stay, and other morbidities during hospitalization. For EPIs appearing in the second trimester of pregnancy, more and special attention should be paid to the screening of postnatal PH and ROP, and timely prevention and treatment measures should be given to help improve the prognosis of EPIs. Our data may provide some information about the quality and outcomes of EPI treatment in mothers with PROM, and provide clinical guidance to help improve the quality of treatment for EPIs and future research.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
J-KC, DW and Q-PL: wrote the paper. J-KC and C-GL: contributed to the collection and analysis of the data. Q-PL: contributed to the design and preparation of the paper. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Supported by the National Key R&D Program of China (Grant No. 2021YFC2701702).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Waters T, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynaecol. (2011) 54(2):307–12. doi: 10.1097/GRF.0b013e318217d4d3
2. Günes A, Kiyak H, Yüksel S, Bolluk G, Erbiyik R, Gedikbasi A. Predicting previable preterm premature rupture of membranes (pPPROM) before 24 weeks: maternal and fetal/neonatal risk factors for survival. J Obstet Gynaecol. (2022) 42(4):597–606. doi: 10.1080/01443615.2021.1935818
3. Zhu Z, Yuan L, Wang J, Li Q, Yang C, Gao X, et al. Mortality and morbidity of infants born extremely preterm at tertiary medical centers in China from 2010 to 2019. JAMA Network Open. (2021) 4(5):e219382. doi: 10.1001/jamanetworkopen.2021.9382
4. Soylu H, Jefferies A, Diambomba Y, Windrim R, Shah P. Rupture of membranes before the age of viability and birth after the age of viability: comparison of outcomes in a matched cohort study. J Perinatol. (2010) 30(10):645–9. doi: 10.1038/jp.2010.11
5. Rodrigo FGM, Henríquez GG, Aloy JF, Pérez AGA, Network S. Outcomes of very-low-birth-weight infants exposed to maternal clinical chorioamnionitis: a multicentre study. Neonatology. (2014) 106(3):229–34. doi: 10.1159/000363127
6. Park G, Park W, Sung S, Kim M, Lee M, Jeon G, et al. Neonatal outcome comparisons between preterm infants with or without early pulmonary hypertension following prolonged preterm premature rupture of membranes before 25 gestational weeks in Korean neonatal network. J Matern Fetal Neonatal Med. (2022) 35(7):1286–94. doi: 10.1080/14767058.2020.1749590
7. Hanke K, Hartz A, Manz M, Bendiks M, Heitmann F, Orlikowsky T, et al. Preterm prelabor rupture of membranes and outcome of very-low-birth-weight infants in the German neonatal network. PLoS One. (2015) 10(4):e0122564. doi: 10.1371/journal.pone.0122564
8. Jobe A, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163(7):1723–9. doi: 10.1164/ajrccm.163.7.2011060
9. Kumar V. Diagnostic approach to pulmonary hypertension in premature neonates. Children. (2017) 4(9):1–24. doi: 10.3390/children4090075.
10. Hornik C, Fort P, Clark R, Watt K, Benjamin D, Smith P, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. (2012) 88(Suppl 2):S69–74. doi: 10.1016/S0378-3782(12)70019-1
11. Papile L, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92(4):529–34. doi: 10.1016/S0022-3476(78)80282-0
12. Bell M, Ternberg J, Feigin R, Keating J, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187(1):1–7. doi: 10.1097/00000658-197801000-00001
13. Prematurity I. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. (2005) 123(7):991–9. doi: 10.1001/archopht.123.7.991
14. Kamath-Rayne B, DeFranco E, Chung E, Chen A. Subtypes of preterm birth and the risk of postneonatal death. J Pediatr. (2013) 162(1):28–34.e2. doi: 10.1016/j.jpeds.2012.06.051
15. Blumenfeld Y, Lee H, Gould J, Langen E, Jafari A, El-Sayed Y. The effect of preterm premature rupture of membranes on neonatal mortality rates. Obstet Gynecol. (2010) 116(6):1381–6. doi: 10.1097/AOG.0b013e3181fe3d28
16. Gezer A, Parafit-Yalciner E, Guralp O, Yedigoz V, Altinok T, Madazli R. Neonatal morbidity mortality outcomes in pre-term premature rupture of membranes. J Obstet Gynaecol. (2013) 33(1):38–42. doi: 10.3109/01443615.2012.729620
17. Newman D, Paamoni-Keren O, Press F, Wiznitzer A, Mazor M, Sheiner E. Neonatal outcome in preterm deliveries between 23 and 27 weeks’ gestation with and without preterm premature rupture of membranes. Arch Gynecol Obstet. (2009) 280(1):7–11. doi: 10.1007/s00404-008-0836-8
18. Esteves J, de Sá R, de Carvalho P, Coca Velarde L. Neonatal outcome in women with preterm premature rupture of membranes (PPROM) between 18 and 26 weeks. J Matern Fetal Neonatal Med. (2016) 29(7):1108–12. doi: 10.3109/14767058.2015.1035643
19. Kurek Eken M, Tüten A, Özkaya E, Karatekin G, Karateke A. Major determinants of survival and length of stay in the neonatal intensive care unit of newborns from women with premature preterm rupture of membranes. J Matern Fetal Neonatal Med. (2017) 30(16):1972–5. doi: 10.1080/14767058.2016.1235696
20. Samuels N, van de Graaf R, de Jonge R, Reiss I, Vermeulen M. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr. (2017) 17(1):105. doi: 10.1186/s12887-017-0847-3
21. Dannapaneni N, Oleti T, Surapaneni T, Sharma D, Murki S. Immediate neonatal outcomes of preterm infants born to mothers with preterm pre-labour rupture of membranes. Indian J Med Res. (2017) 146(4):476–82. doi: 10.4103/ijmr.IJMR_219_15
22. Uauy R, Fanaroff A, Korones S, Phillips E, Phillips J, Wright L. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. National institute of child health and human development neonatal research network. J Pediatr. (1991) 119(4):630–8. doi: 10.1016/S0022-3476(05)82418-7
23. Duncan J, Sawangkum P, Hoover E, Aziz M, Vilchez G. Birthweight and apgar at 5 minutes of life for the prediction of severe neonatal outcomes in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. (2022) 35(23):4521–5. doi: 10.1080/14767058.2020.1854214
24. Bennett P, Brown R, MacIntyre D. Vaginal microbiome in preterm rupture of membranes. Obstet Gynecol Clin North Am. (2020) 47(4):503–21. doi: 10.1016/j.ogc.2020.08.001
25. Wu C, Chen C, Chou H. Pulmonary hypoplasia induced by oligohydramnios: findings from animal models and a population-based study. Pediatr Neonatol. (2017) 58(1):3–7. doi: 10.1016/j.pedneo.2016.04.001
26. Merritt T, Deming D, Boynton B. The ‘new’ bronchopulmonary dysplasia: challenges and commentary. Semin Fetal Neonatal Med. (2009) 14(6):345–57. doi: 10.1016/j.siny.2009.08.009
27. Mourani P, Sontag M, Younoszai A, Miller J, Kinsella J, Baker C, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2015) 191(1):87–95. doi: 10.1164/rccm.201409-1594OC
28. Thibeault D, Kilbride H. Increased acinar arterial wall muscle in preterm infants with PROM and pulmonary hypoplasia. Am J Perinatol. (1997) 14(8):457–60. doi: 10.1055/s-2007-994179
29. Blencowe H, Lawn J, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. (2013) 74(Suppl 1):35–49. doi: 10.1038/pr.2013.205
30. Lee J, McElrath T, Chen M, Wallace D, Allred E, Leviton A, et al. Pregnancy disorders appear to modify the risk for retinopathy of prematurity associated with neonatal hyperoxemia and bacteremia. J Matern Fetal Neonatal Med. (2013) 26(8):811–8. doi: 10.3109/14767058.2013.764407
31. Woo S, Park J, Hong S, Kim Y, Park Y, Lee Y, et al. Inflammatory and angiogenic mediators in amniotic fluid are associated with the development of retinopathy of prematurity in preterm infants. Invest Ophthalmol Visual Sci. (2020) 61(5):42. doi: 10.1167/iovs.61.5.42
32. Shulman J, Weng C, Wilkes J, Greene T, Hartnett M. Association of maternal preeclampsia with infant risk of premature birth and retinopathy of prematurity. JAMA Ophthalmol. (2017) 135(9):947–53. doi: 10.1001/jamaophthalmol.2017.2697
33. Villamor-Martinez E, Cavallaro G, Raffaeli G, Mohammed Rahim O, Gulden S, Ghazi A, et al. Chorioamnionitis as a risk factor for retinopathy of prematurity: an updated systematic review and meta-analysis. PLoS One. (2018) 13(10):e0205838. doi: 10.1371/journal.pone.0205838
34. Hudalla H, Bruckner T, Pöschl J, Strowitzki T, Kuon R. Maternal smoking as an independent risk factor for the development of severe retinopathy of prematurity in very preterm infants. Eye. (2021) 35(3):799–804. doi: 10.1038/s41433-020-0963-4
35. Hoyek S, Peacker B, Acaba-Berrocal L, Al-Khersan H, Zhao Y, Hartnett M, et al. The male to female ratio in treatment-warranted retinopathy of prematurity: a systematic review and meta-analysis. JAMA Ophthalmol. (2022) 140(11):1110–20. doi: 10.1001/jamaophthalmol.2022.3988
36. Lynch A, Wagner B, Hodges J, Thevarajah T, McCourt E, Cerda A, et al. The relationship of the subtypes of preterm birth with retinopathy of prematurity. Am J Obstet Gynecol. (2017) 217(3):354.e1–e8. doi: 10.1016/j.ajog.2017.05.029
37. Badeeb N, Raffa L, AhmedHussain H, Bamefleh D, Mgharbil E, Alessa S, et al. Retinopathy of prematurity in Saudi Arabia: exploring maternal risk factors. Taiwan J Ophthalmol. (2021) 11(4):359–66. doi: 10.4103/tjo.tjo_72_20
38. Vinukonda G, Dummula K, Malik S, Hu F, Thompson C, Csiszar A, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. (2010) 41(8):1766–73. doi: 10.1161/STROKEAHA.110.588400
39. Ozdemır R, Sarı F, Tunay Z, Erdeve O, Canpolat F, Oguz S, et al. The association between respiratory tract Ureaplasma urealyticum colonization and severe retinopathy of prematurity in preterm infants ≤1250 g. Eye (London, England). (2012) 26(7):992–6. doi: 10.1038/eye.2012.77
Keywords: extremely premature infants, premature rupture of membranes (PROM), propensity score matching (PSM), disease prognosis, complications
Citation: Cao J-K, Liu C-G, Wang D and Li Q-P (2023) Impact of premature rupture of membranes on clinical outcomes of extremely premature infants: A propensity score matching study. Front. Pediatr. 11:1144373. doi: 10.3389/fped.2023.1144373
Received: 14 January 2023; Accepted: 13 March 2023;
Published: 30 March 2023.
Edited by:
Shi Yuan, Children's Hospital of Chongqing Medical University, ChinaReviewed by:
Balaji Govindaswami, Valley Medical Center Foundation, United StatesChristopher Luke Damron, Marshall University, United States
© 2023 Jingke, Changgeng, Wang and Qiuping. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu-Ping Li emhqaG9zcGl0YWxAMTYzLmNvbQ==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Jing-Ke Cao
Jing-Ke Cao Chang-Geng Liu
Chang-Geng Liu Dan Wang
Dan Wang Qiu-Ping Li1,2*
Qiu-Ping Li1,2*