
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 27 June 2023
Sec. Pediatric Infectious Diseases
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1140556
 Ji Young Lee1,†
Ji Young Lee1,† Joonsik Park2,†
Joonsik Park2,† Myeongjee Lee3,†
Myeongjee Lee3,† Minkyung Han3
Minkyung Han3 Inkyung Jung4
Inkyung Jung4 Sung Min Lim1
Sung Min Lim1 Jee Yeon Baek1
Jee Yeon Baek1 Ji-Man Kang1,5
Ji-Man Kang1,5 Min Soo Park1,2,6,7,‡
Min Soo Park1,2,6,7,‡ Jong Gyun Ahn1,5,†*
Jong Gyun Ahn1,5,†*
Background: Non-pharmaceutical interventions (NPIs), such as social distancing and hand washing, have been associated with a decline in the preterm birth rate worldwide. We aimed to evaluate whether the preterm birth rate in Korea during the coronavirus disease 2019 lockdown has changed compared to that in previous years.
Method: A birth registry from the Korea Statistical Information Service, which is a nationwide official database, was used to include all births claimed to have occurred between 2011 and 2020. Newborns with gestational age (GA) less than 22 weeks and birth weight less than 220 g were excluded. The pre-NPI period was designated as January 2011 to January 2020, and the NPI period was defined as February 2020 to December 2020. We assessed the effect of NPI on the incidence of prematurity per 100 births using an interrupted time-series quasi-experimental design and implementing an autoregressive integrated moving average (ARIMA) model.
Results: From 2011 to 2020, a total of 3,931,974 live births were registered, among which 11,416 were excluded. Consequently, the final study population included 3,920,558 live births (both singleton and multiple births) among which 275,009 (7.0%) were preterm. The preterm birth rate was significantly higher during the NPI period (8.68%) compared to that in the pre-NPI period (6.92%) (P < 0.001). The ARIMA model showed that in all singleton and multiple births, except those in July (observed 9.24, expected 8.54, [95% prediction interval {PI} 8.13–8.96], percent difference 7.81%), September (observed 7.89, expected 8.35, [95% PI 7.93–8.76], percent difference −5.66%), and December (observed 9.90, expected 9.40, [95% PI 8.98–9.82], percent difference 5.2%), most observed values were within the 95% PI of the expected values and showed an increasing trend.
Conclusion: In this nationwide observational study, the trend in premature birth rate did not significantly change due to NPI implementation in Korea, as it had been increasing since 2011. The trend of Korea's birth rate appears to be unaffected by the implementation of NPIs; however, further studies with a longer follow-up period are needed.
The coronavirus disease 2019 (COVID-19) pandemic has brought about unexpected changes in the global society. After the first quarter of 2020, most countries decided to implement non-pharmaceutical interventions (NPIs) to prevent the spread of COVID-19 (1). NPIs consist of frequent hand washing, isolating infected individuals, wearing masks, closing schools and public facilities, and canceling or postponing large gatherings (2). These interventions provided effective measures to control the contagious disease; however, they also led to unwanted and often unexpected public health consequences (3).
Preterm labor is defined as labor that starts before 37 weeks of pregnancy. The estimated global preterm birth rate in 2014 was 10.6%, and this was similar in Asia, where a 10.4% preterm birth rate was reported (4). In recent decades, there has been a trend of increasing preterm birth rates in developed countries (5). Several reports have noted the severe adverse effects of maternal COVID-19 and its associated negative perinatal outcomes for newborns; however, there have also been findings of a potentially decreased rate of preterm births during the pandemic (6).
Investigators in Tennessee first identified an association between NPI and preterm birth after the COVID-19 pandemic (7). Birth records in Tennessee showed a decline in preterm births after the state's stay-at-home order was put into place in 2020. A single center study from South Korea also suggested the possible preventative effects of NPI on preterm birth rates due to the mitigation effects of NPI. Similar phenomena were observed in three Scandinavian countries; however, statistically significant impact of NPI on preterm birth rates was not observed in these studies (8). However, recent studies investigating the effect of NPI on preterm birth rates have demonstrated inconsistent findings. An increased focus on hygiene, strict physical distancing, and home confinement have been suggested as possible reasons for the potential effects of NPI on preterm birth, which may have influenced the overall inflammatory state of pregnant women (9). Therefore, the objective of this study was to establish whether a correlation exists between preterm birth rates and the NPI period in Korea in 2020.
Birth registry data from the Korean Statistical Information Service (an official national database) was used to include information on all births between 2011 and 2020 (10). Parents are required to register a newborn's birth with the Korean government within 1 month of birth, and the data includes not only information about the newborn but also parents’ personal information such as age, location of delivery, and educational level. Among preterm live births, both singleton and multiple births were included for analysis.
This was a retrospective, observational study that evaluated the change in preterm birth rates after the implementation of NPI. After the first confirmed case of COVID-19 in Korea on January 20, 2020, the government imposed mitigation measures, such as social distancing and restricted overseas travel, in February 2020. In this study, the NPI period was defined as January 2011 to January 2020 and the pre-NPI period as February 2020 to December 2020. Although only live births are meant to be registered, we observed 11,416 cases in the categories of gestational age (GA) less than 22 weeks and birth weight less than 220 g (Figure 1). These cases, generally regarded as medically nonviable, were excluded (11, 12).
As per the World Health Organization's definition, in this study, preterm birth was defined as births with a GA < 37 weeks (13). Subgroup analysis was conducted by further categorizing the data into the following GA groups: extremely preterm (22 to <28 weeks), very preterm (28 to <32 weeks), and moderate to late preterm (32 to <37 weeks). Small for gestational age (SGA) was defined as a birth weight of less than the 10th percentile for GA and large for gestational age (LGA) as a birth weight of more than the 90th percentile for GA (14).
Categorical variables were expressed as frequencies (%) and compared using the Chi-square test. Odds ratios of preterm births by birth types, such as singletons and multiples, and GA subgroups were calculated using multivariable logistic regression analyses between the defined pre-NPI and NPI periods after adjusting for maternal age, sex, maternal education, and birth order.
We assessed the effect of NPI on preterm birth rates and prematurity incidence per 100 births using an interrupted time-series quasi-experimental design (15). To account for seasonality and autocorrelation in preterm rates over time, an autoregressive integrated moving average (ARIMA) model was implemented in the data from the pre-NPI period. The optimal ARIMA parameters were identified based on an automated algorithm, specifically, auto.arima() in the forecast package in R (16). Using the optimal model selected for each outcome, we derived the expected preterm birth rates after NPI implementation and the percentage difference between the observed and expected preterm birth rates to evaluate the prediction effect. An “unexpected” outcome was defined as an observed value that was outside the expected 95% prediction interval (PI). As a sensitivity analysis, we implemented a standard interrupted time analysis with a segmented regression model allowing level and slope change to evaluate whether the trend in monthly prematurity birth rate incidence changed in the NPI period when compared to the pre-NPI period. A P-value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R Statistical Software (version 4.1.2; R Core Team 2021).
Additionally, to compare the results using different statistical methods, segmented regression analysis was performed for statistical modeling of interrupted time series to evaluate whether the trend changed during the NPI period (17, 18). The results of this analysis are included in the supplementary material.
This research was conducted ethically, in accordance with the World Medical Association and Declaration of Helsinki, and the institutional review board (No. 4-2021-0416) approved the study.
The final study population included 3,920,558 births, of which 275,009 (7.0%) were preterm (Figure 1). A slight male predominance was observed (51.3%). The proportion of preterm births was significantly higher during the NPI period (8.48%) compared to that in the pre-NPI period (6.92%) (P < 0.001). Additionally, the proportion of low-birth-weight neonates, with birth weight under 2,500 g, especially in the subcategory of 1,500 g to less than 2,500 g (5.09% vs. 5.98%, respectively, P < 0.001), was higher in the NPI period compared to the pre-NPI period. Detailed demographic features of the study populations for both periods are shown in Table 1.
The observed and expected preterm birth rates were plotted in Figure 2, by either birth type or GA subgroup. Overall, the preterm birth rates, including both singleton and multiple births, showed an increasing trend in all GA subgroups. (Figure 2, Supplementary Figure S1) However, based on the ARIMA model, NPI implementation was not associated with an immediate or further change in the preterm birth rate. For all singleton and multiple births, most observed values were within the 95% PI of the expected values, with an increasing trend (Figure 2A), except for those in July (observed 9.24, expected 8.54, [95% PI 8.13–8.96], percent difference 7.81%), September (observed 7.89, expected 8.35, [95% PI 7.93–8.76], percent difference −5.66%), and December (observed 9.90, expected 9.40, [95% PI 8.98–9.82], percent difference 5.2%) (Supplementary Table S1A). When preterm singleton births were separately analyzed, all the observed values were within the 95% PI (Supplementary Table S1B). For multiple births alone, most observed values were within the 95% PI, except for those in July (observed 3.39, expected 3.01 [2.68–3.35], percent difference 11.69%) and December (observed 3.61, expected 3.20 [95% PI 2.84–3.57], percent difference 11.94%) (Supplementary Table S1C).
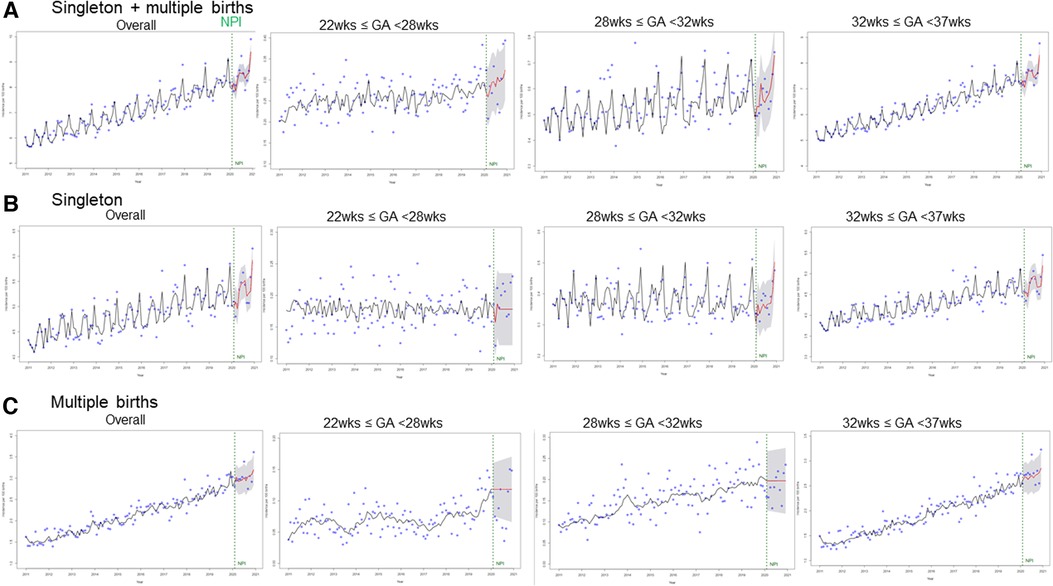
Figure 2. The observed values and expected monthly trend of the preterm birth rate (per 100 births) in the pre-NPI and NPI periods. (A) All births including singleton and multiple birhts, (B) Singleton births, (C) Multiple births The blue dots are observed values. The vertical green line denotes the period of NPI implementation. The black and red lines are trends for before and after NPI implementation, respectively. x-axis: incidence per 100 births, y-axis: year 2011–2021. NPI, non-pharmaceutical interventions.
Preterm births (including both singletons and multiple births) increased in the NPI period when compared to the pre-NPI period (odds ratio (OR) = 1.25, 95% CI, 1.23–1.27, P < 0.001) and a similar trend was observed in the multivariate analysis (OR = 1.18, 95% CI, 16–1.20, P < 0.001). (Table 2) When preterm births were separated into singleton and multiple birth groups and analyzed according to GA, both types of births showed similar trends with regards to preterm birth rates.
We summarized and compared the results of previous international studies on whether there was a change in the preterm birth rate due to NPI implementation (Table 3). Overall, there were nine international studies, and their defined lockdown periods ranged from 1 month to 12 months (19–22). A heterogeneity in outcomes was noted; however, the majority reported a decline in preterm birth rates in either the extremely preterm or late preterm groups (20, 23–27). There was also conflicting literature, that found no change in preterm births before and after NPI implementation in Spain, Sweden, and the United Kingdom (28–30). The results and design of these studies are described in Table 3.
In this study, we aimed to establish whether a correlation exists between preterm birth rates and the NPI period in Korea in 2020. Our findings showed that the preterm birth rate was increasing in the pre-NPI period, and this continued to increase after the implementation of such measures, suggesting an unclear impact of NPI implementation on the preterm birth rate.
Preterm births have been increasing in most developed countries, including South Korea. The percentage of preterm births in the United States increased from 9% in 1981 to 10.5% in 2021 (31, 32). Similar trends have also been observed in East Asian countries such as Taiwan and South Korea (33). Increased accessibility to medical services, increased number of survivors of chronic diseases which were previously fatal, and the development of assisted reproductive technologies have contributed to an overall increase in high-risk pregnancies such as twins or those of advanced maternal age (34).
The etiology of preterm delivery can be broadly categorized into three main groups: (1) spontaneous preterm labor; (2) maternal or fetal infections; and (3) premature preterm rupture of the membranes (9). Etiologies for preterm birth are generally unclear; however, environmental factors, such as viral infection and smoking exposure, are important risk factors for both ruptured and intact membrane preterm labor (35). NPI could change maternal environmental and habitual exposures, leading to a possible change in preterm birth rates. Bian et al. suggested that changes in the overall behavior of pregnant women during the pandemic might also have contributed to the above, as one-third of preterm births are iatrogenic (23, 36).
In contrast to our findings, many international data-based studies supported a negative association between NPI and preterm birth that were investigated up to the first quarter of 2020. In the United States, Gemmill et al. reported a decline in preterm birth rate during the pandemic, especially during the early and late 2020 (37). Oakley et al. observed a decrease in preterm births after NPI initiation until March 2020 in Norway, Sweden, and Denmark (8). Hedermann et al. also noted this phenomenon among the Danish population born before February 2020 (21). Bian et al. investigated births up to December 2020 in a single center study in Shanghai, China, and found a significant decrease in preterm birth rates (23). Kim et al., in a Korean single center study, reported a decrease in preterm labor after the NPI period (38). Serial works of published data that followed support similar associations of decreased preterm birth rates with the COVID-19 lockdown (Table 3).
Owing to the lack of confirmed etiologies for preterm births, the decline in preterm births during pandemic lockdowns could simply be a coincidence. On the other hand, further investigating this potential association could lead to more effective measures to prevent preterm births. Cohort data from other countries with lockdown periods during the pandemic showed a potential decrease in preterm births during the pandemic; however, they only investigated a short period of time after the NPI, mostly the first three months of 2020. This contrasts with our study, with data collection over a large period, in which we found no significant relationships between NPI implementation and preterm birth. Another limitation to our study is the lack of individual patient data in our database. For instance, the database does not include information on the cause or type of preterm birth, such as an underlying maternal or fetal condition (“indicated preterm birth”) or preterm rupture of membranes or cervical dilatation (“spontaneous preterm birth”). Consequently, it is difficult to correlate NPI with a specific type of preterm birth. However, because NPI could affect the maternal or fetal environment in multifaceted ways, differentiating the type of birth in the analysis may not have necessarily provided additional helpful information. However, to improve the quality of the database, further collection of National Health Insurance Data and the Korean Developmental Survey Data is required in the future. As a result of these limitations, we cannot definitively conclude that a causal relationship between COVID-19 mitigation measures and preterm birth rate exists.
In conclusion, we were unable to establish a significant relationship between the NPI and pre-NPI preterm birth rates in South Korea. The overall South Korean preterm birth rate continues to increase, and this contributes to the country's disease burden. Future research should ideally aim to investigate whether there are more significant variables that could affect preterm birth rates, as well as how clinicians can reduce these factors and ultimately decrease the rate of preterm births.
Birth registry data from the Korean Statistical Information Service (KOSIS) can be accessed from the KOSIS website. The raw data from the KOSIS is available at https://kosis.kr.
The studies involving human participants were reviewed and approved by Yonsei University College of Medicine. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
JA and IJ had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version before submission. JL, ML, JP, IJ, MH, and JA contributed to the study's concept and design. JL, JP, ML, SL, JB, JK, IJ, and JA were involved in data acquisition, analysis, or interpretation. JL, JP, and JA drafted the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content. JL, JP, ML, MH, IJ, and JA performed the statistical analysis. JA and MP supervised the study and are the guarantors of this study. All authors contributed to the article and approved the submitted version.
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1G1A100844111) and a faculty research grant from the Yonsei University College of Medicine (6-2021-0145). The study sponsor was not involved in the study design, analysis, and interpretation of data, the writing of the report, or the decision to submit the study results for publication.
The authors thank Medical Illustration & Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1140556/full#supplementary-material
1. Müller O, Razum O, Jahn A. Effects of non-pharmaceutical interventions against COVID-19 on the incidence of other diseases. The Lancet Regional Health—Europe. (2021) 6:100139. doi: 10.1016/j.lanepe.2021.100139
2. Lu G, Razum O, Jahn A, Zhang Y, Sutton B, Sridhar D, et al. COVID-19 in Germany and China: mitigation versus elimination strategy. Glob Health Action. (2021) 14(1):1875601. doi: 10.1080/16549716.2021.187560133472568
3. Kampf G, Kulldorff M. Calling for benefit–risk evaluations of COVID-19 control measures. Lancet. (2021) 397(10274):576–7. doi: 10.1016/S0140-6736(21)00193-833549169
4. Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7(1):e37–46. doi: 10.1016/s2214-109x(18)30451-030389451
5. Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. (2015) 64(1):1–65.
6. Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. (2020) 56(1):15–27. doi: 10.1002/uog.2208832430957
7. Harvey EM, McNeer E, McDonald MF, Shapiro-Mendoza CK, Dupont WD, Barfield W, et al. Association of preterm birth rate with COVID-19 statewide stay-at-home orders in Tennessee. JAMA Pediatr. (2021) 175(6):635–7. doi: 10.1001/jamapediatrics.2020.651233720307
8. Oakley LL, Ortqvist AK, Kinge J, Hansen AV, Petersen TG, Soderling J, et al. Preterm birth after the introduction of COVID-19 mitigation measures in Norway, Sweden, and Denmark: a registry-based difference-in-differences study. Am J Obstet Gynecol. (2022) 226(4):550e1–e22. doi: 10.1016/j.ajog.2021.11.034
9. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. (2008) 371(9606):75–84. doi: 10.1016/s0140-6736(08)60074-418177778
10. Microdata integrated service. Daejeon, Korea: MDIS (2022) (cited 2022 Jun 21). Available at: https://mdis.kostat.go.kr/index.do
11. Arzuaga BH, Lee BH. Limits of human viability in the United States: a medicolegal review. Pediatrics. (2011) 128(6):1047–52. doi: 10.1542/peds.2011-168922065266
12. Extremely Preterm Birth: FAQs. American College of Obstetericians and Gynecologists (cited 2022 Sep 2). Available at: https://www.acog.org/womens-health/faqs/extremely-preterm-birth
13. World Health Organization. Preterm birth. (2018) (updated Feb 19, 2018; cited 2022 Sep 21). Available at: https://www.who.int/news-room/fact-sheets/detail/preterm-birth
14. Lee JJ, Kim MH, Ko KO, Kim KA, Kim SM, Kim ER, et al. The study of growth measurements at different gestatioal ages of Korean newborn the survey and statistics. J Korean Soc Neonatol. (2006) 13(1):47–57.
15. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. (2017) 46(1):348–55. doi: 10.1093/ije/dyw09827283160
16. Hyndman RJ, Khandakar Y. Automatic time series forecasting: the forecast package for R. J Stat Softw. (2008) 27(3):1–22. doi: 10.18637/jss.v027.i03
17. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J. Clin Pharm Ther. (2002) 27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x12174032
18. Slavova S, Costich JF, Luu H, Fields J, Gabella BA, Tarima S, et al. Interrupted time series design to evaluate the effect of the ICD-9-CM to ICD-10-CM coding transition on injury hospitalization trends. Inj Epidemiol. (2018) 5(1):36. doi: 10.1186/s40621-018-0165-830270412
19. Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol-Urganci I, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. (2021) 9(6):e759–e72. doi: 10.1016/s2214-109x(21)00079-633811827
20. Philip RK, Purtill H, Reidy E, Daly M, Imcha M, McGrath D, et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: a “natural experiment” allowing analysis of data from the prior two decades. BMJ Glob Health. (2020) 9(2059-7908 (Print)):e003075. doi: 10.1136/bmjgh-2020-003075
21. Hedermann G, Hedley PL, Baekvad-Hansen M, Hjalgrim H, Rostgaard K, Poorisrisak P, et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed. (2021) 106(1):93–5. doi: 10.1136/archdischild-2020-31999032788391
22. Hawco S, Rolnik DL, Woolner A, Cameron NJ, Wyness V, Mol BW, et al. The impact of mitigation measures on perinatal outcomes during the first nine months of the COVID-19 pandemic: a systematic review with meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2022) 274:117–27. doi: 10.1016/j.ejogrb.2022.05.00735640440
23. Bian Z, Qu X, Ying H, Liu X. Are COVID-19 mitigation measures reducing preterm birth rate in China? BMJ Glob Health. (2021) 6(8):e006359. doi: 10.1136/bmjgh-2021-00635934385161
24. Been JV, Ochoa LB, Bertens LC, Schoenmakers S, Steegers EA, Reiss IK. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. (2020) 5(11):e604–e11. doi: 10.1016/s2468-2667(20)30223-133065022
25. De Curtis M, Villani L, Polo A. Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch Dis Child Fetal Neonatal ed. (2021) 106:456. doi: 10.1136/archdischild-2020-32068233127736
26. Meyer R, Bart Y, Tsur A, Yinon Y, Friedrich L, Maixner N, et al. A marked decrease in preterm deliveries during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol. (2021) 224(1097-6868 (Electronic)):234–7. doi: 10.1016/j.ajog.2020.10.01733069683
27. Huseynova R, Bin Mahmoud L, Abdelrahim A, Al Hemaid M, Almuhaini MS, Jaganathan PP, et al. Prevalence of preterm birth rate during COVID-19 lockdown in a tertiary care hospital, Riyadh. Cureus. (2021) 13:13634. doi: 10.7759/cureus.13634
28. Pasternak B, Neovius M, Soderling J, Ahlberg M, Norman M, Ludvigsson JF, et al. Preterm birth and stillbirth during the COVID-19 pandemic in Sweden: a nationwide cohort study. Ann Intern Med. (2021) 174(6):873–5. doi: 10.7326/M20-636733428442
29. Arnaez J, Ochoa-Sangrador C, Caserio S, Gutierrez EP, Jimenez MDP, Castanon L, et al. Lack of changes in preterm delivery and stillbirths during COVID-19 lockdown in a European region. Eur J Pediatr. (2021) 180(6):1997–2002. doi: 10.1007/s00431-021-03984-633580293
30. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. (2020) 324:705–6. doi: 10.1001/jama.2020.1274632648892
31. Preterm birth. Centers for Disease Control and Prevention (2021) (updated Nov 1, 2021; cited 2022 Oct 31). Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm
32. Martin JA, Kochanek KD, Strobino DM, Guyer B, MacDorman MF. Annual summary of vital statistics–2003. Pediatrics. (2005) 115(3):619–34. doi: 10.1542/peds.2004-269515741364
33. Chen KH, Chen IC, Yang YC, Chen KT. The trends and associated factors of preterm deliveries from 2001 to 2011 in Taiwan. Medicine (Baltimore). (2019) 98(13):e15060. doi: 10.1097/MD.000000000001506030921237
34. Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. (2004) 103(3):551–63. doi: 10.1097/01.Aog.0000114989.84822.5114990421
35. Mercer BM, Goldenberg RL, Meis PJ, Moawad AH, Shellhaas C, Das A, et al. The preterm prediction study: prediction of preterm premature rupture of membranes through clinical findings and ancillary testing. The national institute of child health and human development maternal-fetal medicine units network. Am J Obstet Gynecol. (2000) 183(3):738–45. doi: 10.1067/mob.2000.10676610992202
36. Chen C, Zhang JW, Xia HW, Zhang HX, Betran AP, Zhang L, et al. Preterm birth in China between 2015 and 2016. Am J Public Health. (2019) 109(11):1597–604. doi: 10.2105/ajph.2019.30528731536409
37. Gemmill A, Casey JA, Catalano R, Karasek D, Margerison CE, Bruckner T. Changes in preterm birth and caesarean deliveries in the United States during the SARS-CoV-2 pandemic. Paediatr Perinat Epidemiol. (2022) 36(4):485–9. doi: 10.1111/ppe.1281134515360
38. Kim S, Kil K, Lee Y. Impact of COVID-19 mitigation policy in South Korea on the reduction of preterm or low birth weight birth rate: a single center experience. Children (Basel). (2021) 8(5):332. doi: 10.3390/children805033233922899
39. Caniglia EC, Magosi LE, Zash R, Diseko M, Mayondi G, Mabuta J, et al. Modest reduction in adverse birth outcomes following the COVID-19 lockdown. Am J Obstet Gynecol. (2021) 224(6):615e1–e12. doi: 10.1016/j.ajog.2020.12.1198
40. Matheson A, McGannon CJ, Malhotra A, Palmer KR, Stewart AE, Wallace EM, et al. Prematurity rates during the coronavirus disease 2019 (COVID-19) pandemic lockdown in Melbourne, Australia. Obstet Gynecol. (2021) 137:405–7. doi: 10.1097/AOG.000000000000423633543904
41. Handley SC, Mullin AM, Elovitz MA, Gerson KD, Montoya-Williams D, Lorch SA, et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 pandemic, march-June 2020. JAMA. (2020) 325:87–9. doi: 10.1001/jama.2020.20991
42. Kc A, Gurung R, Kinney MV, Sunny AK, Moinuddin M, Basnet O, et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob, Health. (2020) 8(10):e1273–281. doi: 10.1016/S2214-109X(20)30345-432791117
43. Kirchengast S, Hartmann B. Pregnancy outcome during the first COVID 19 lockdown in Vienna, Austria. Int, J Environ Res Public Health. (2021) 18(1660-4601):3782. doi: 10.3390/ijerph1807378233916365
44. Fresson J, Bruckner TA, Ray CL, Goffinet F, Rey S, Blondel B, et al. Decreases in preterm birth during the first COVID-19 lockdown in France by gestational age sub-groups and regional COVID-19 incidence. Ann Epidemiol. (2022) 72:74–81. doi: 10.1016/j.annepidem.2022.05.00435643288
Keywords: COVID-19, lockdown, non-pharmaceutical intervention, prematurity, preterm birth
Citation: Lee JY, Park J, Lee M, Han M, Jung I, Lim SM, Baek JY, Kang J-M, Park MS and Ahn JG (2023) The impact of non-pharmaceutical interventions on premature births during the COVID-19 pandemic: a nationwide observational study in Korea. Front. Pediatr. 11:1140556. doi: 10.3389/fped.2023.1140556
Received: 9 January 2023; Accepted: 12 June 2023;
Published: 27 June 2023.
Edited by:
Valeriane Leroy, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Balaji Govindaswami, Valley Medical Center Foundation, United States© 2023 Lee, Park, Lee, Han, Jung, Lim, Baek, Kang, Park and Ahn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jong Gyun Ahn amdhaG5AeXVocy5hYw==
†These authors have contributed equally to this work and share last authorship
‡These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.