- 1Department of Pediatric Heart Surgery, Faculty of Medicine, Atakent Hospital, Acibadem Mehmet Ali Aydinlar University, istanbul, Turkey
- 2Department of Cardiovascular Surgery, Faculty of Medicine, Atakent Hospital, Acibadem Mehmet Ali Aydinlar University, istanbul, Turkey
- 3School of Medicine, Acibadem Mehmet Ali Aydinlar University, istanbul, Turkey
- 4Department of Pediatric Cardiology, Faculty of Medicine, Atakent Hospital, Acibadem Mehmet Ali Aydinlar University, istanbul, Turkey
- 5Department of Anesthesiology, Faculty of Medicine, Atakent Hospital, Acibadem Mehmet Ali Aydinlar University, istanbul, Turkey
- 6Department of Radiology, Faculty of Medicine, Atakent Hospital, Acibadem Mehmet Ali Aydinlar University, istanbul, Turkey
Background: Extracorporeal membrane oxygenation (ECMO) is widely used after congenital heart surgery. The purpose of this study is to analyze the neurodevelopmental (ND) outcomes in patients who receivedECMO support after congenital cardiac surgery.
Methods: Between January 2014 and January 2021, 111 patients (5.8%) receivedECMO support after congenital heart operations, and 29 (26,1%) of these patients were discharged. Fifteen patients who met the inclusion criteria were included. A propensity score matching (PSM) analysis model was established using eight variables (age, weight, sex, Modified Aristotle Comprehensive Complexityscores, seizures, cardiopulmonary bypass duration, number of operations, and repair method) with 1:1 matching. According to the PSM model, 15 patients who underwent congenital heart operations were selected as the non-ECMO group. The Ages & Stages Questionnaire Third Edition (ASQ-3) was used for ND screening;it includes communication, physical skills (gross and fine motor), problem-solving, and personal–social skills domains.
Results: There were no statistically significant differences between the patients' preoperative and postoperative characteristics. All patients were followed up for a median of 29 months (9–56 months). The ASQ-3 results revealed that communication, fine motor, and personal–social skills assessments were not statistically different between the groups. Gross motor skills (40 vs. 60), problem-solving skills (40 vs. 50), and overall scores (200 vs. 250) were better in the non-ECMO patients (P = 0.01, P = 0.03, and P = 0.03, respectively). Nine patients (%60) in the ECMO group and 3 patients (%20) in the non-ECMO group were with neurodevelopmental delay (P = 0,03).
Conclusion: ND delay may occur in congenital heart surgery patients who receivedECMO support. We recommend ND screening in all patients with congenital heart disease, especially those who receivedECMO support.
Introduction
Congenital heart disease (CHD) is the most common congenital defect at birth (1). Recent developments in cardiopulmonary facilities and increasing surgical experiences allow patients to reach adulthood. However, increased survival rates lead to long-term morbidity (2). Patients may face several comorbidities after a successful congenital heart operation. One of the most critical and hidden comorbidities is neurodevelopmental delay (NDD).
Neurodevelopmental (ND) disorders can affect the quality of personal and school success. Although many variables can impact brain development, extracorporeal membrane oxygenation (ECMO) support is possibly one of the most challenging. Around 2%–5% of children undergoing congenital heart operationsreceivedECMO support (3). Although ECMO can be a lifesaving choice in selected patients who are refractory to conventional medical treatments, it carries a high risk of cranial-nervous system (CNS) complications (4–6). It may cause NDD without evidence of anatomic injury in radiological screening (7, 8). Therefore, close screening, early detection, and appropriate reinforcement of delayed function may improve the results (9, 10).
We conducted a retrospective and comparative study of children who underwent congenital heart surgery. The primary outcome was the effect of ECMO support on NDD.
Materials and methods
Patients' selection
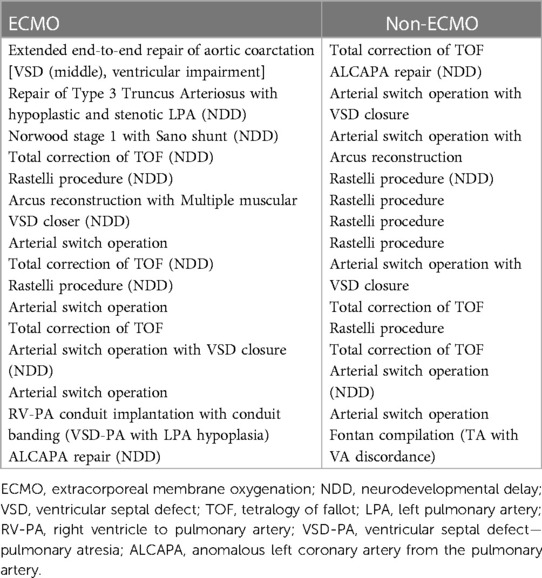
This is a single-center retrospective study. Approval from the institutional ethical committee was obtained on 19.11.2021 before establishing the study with the number 2021/22-22. The primary outcome is the long-term assessment of the ND progress of children with congenital heart defects requiring ECMO support after surgery. We compared patients who receivedECMO support after cardiac surgery with those who didn't receiveECMO support. Between January 2014 and January 2021, 1,884 patients who underwent CHD repair were retrospectively scanned. Data were collected from the institution's medical records. A total of 111 (5.8%) patients receivedECMO support after a heart operation, and 29 (26.1%) were discharged. All patients had veno-arterial support. Veno-venous ECMO support patients and patients who received ECMO support due to noncardiac indications were not included in the study. In addition, patients with CHD who did not undergocardiac surgery or who only underwent cardiac catheterization were omitted. Patients with syndromes that can cause intellectually disabled, patients older than 66 months, and patients who were lost to follow-up were excluded. Also, patients suffered from significant ischemic or hemorrhagic cranial events were excluded, because these cerebral events already result with NDD regardless of ECMO support or cardiac surgery. Patients who had abnormal findings on HUS were evaluated further with MRI and examined by a pediatric neurologist. Patients who underwent magnetic resonance imaging (MRI) screening were evaluated according to a previous study by Radhakrishnan et al. (11). Fifteen patients who agreed to join the study were included in the ECMO group. None of these patients underwent ECMO support with extracorporeal cardiopulmonary resuscitation (ECPR), and all underwent ECMO support with transthoracic cannulation. We identified the diagnosis of ECMO patients and defined 700 patients among 1,804 non-ECMO patients who had diagnoses similar to ECMO patients. The patients' diagnoses are listed in Table 1. Among these 700 patients, we found 346 patients who were still alive and reachable.
We sorted these 346 patients according to their protocol number and randomly selected 116 patients. Finally, we defined the patients' age, weight, sex, Modified Aristotle Comprehensive Complexity (MACC) score (12), biventricular repair, seizures, cardiopulmonary bypass (CPB) time, cross-clamping time, number of operations, and septicemia in the total cohort. A propensity score matching (PSM) analysis model was established using these variables with 1:1 matching and without sampling replacement. Fifteen patients were matched for the non-ECMO group. We would like to exclude patients with low birth weight(LBW) and prematurity. However, these patients were not excluded since excluding these patients would further reduce the patient population.Since the MACC scoring system includedLBW and prematurity, they were not included in the PSM model separately. LBW was identified in patients less than 2,500grams of birth weight, and prematurity was identified in patients born before 35 weeks of gestational age. The corrected age of premature patients was calculated using an age calculator on the official site of the Ages & Stages Questionnaire Third Edition (ASQ-3). After approval from the ethical committee, informed consent was obtained from the parents.
Neurodevelopmental screening
The ASQ-3 was used to screen NDD at random time. The ASQ was designed and developed by J. Squires and D. Bricker. It is an age-specific developmental screening questionnaire assessing communication, physical skills (gross and fine motor), problem-solving skills, and personal–social skills based on parental reports (13–15). The test was performed by two researchers who were trained in the ASQ-3. Since some of our patients live outside the province, it was necessary to reach them by phone. Although we had the opportunity to evaluate some patients during hospital visits, it would have been more accurate to continue the study with a single method. Therefore, the test was performed via phone interviewswith all patients. Patients were ordered by protocol ID and assigned to the researchers with the ordinal numbers “odd” and “even”.
The test includes five domains with six closed-ended questions and one part with open-ended questions. The closed-ended questions have three answer options. The answer is “yes” when the behavior is present (10 points), “sometimes” when the behavior is emerging (5 points), or “not yet” when the behavior is absent (0 points). The sixth domain has 7–10 open-ended questions. Response options for an open-ended question are “yes” when there is a parental concern related to the child's development or health status present and “no” when there is no concern. This part of the test is not included in the scoring but is discussed separately. Open-ended questions help us to get a more clear idea of the child's development and the parents' related concerns.
Since there is no Turkish version of the ASQ-3 test, some questions were modified according to the article published by Kapci et al. (16). In addition, NDD was defined in patients below the 25% interquartile range (IQR) of the median overall scores (first definition) and in patients who were below the 25% IQR in at least two domains (second definition). The second definition of NDD was also defined according to Kapci et al. (16). Patients with NDD, according to both definitions, were specified separately. However, true NDD is accepted in patients with NDD included within either of the two definitions.Except this, all patients are followed at regular intervals (at most 6 months intervals) according to their general condition.
Statistical analyses
In this study, the distribution of the variables was classified, and descriptive results were obtained using the SPSS version 23 (Statistical Package for the Social Sciences for Windows) program. The normality of the data was analyzed using the Kolmogorov–Smirnov test. Continuous variables were presented as medians with ranges, and categorical variables were presented as frequencies and percentages of the total. Continues variables were compared using the Mann–Whitney U test, and categorical variables were compared using the chi-square test. A statistically significant difference was accepted with a P-value of <0.05. The effects of covariates on the possibility of NDD in multivariate analysis are reported as odds ratios (OR) with a 95% confidence interval (CI).
Results
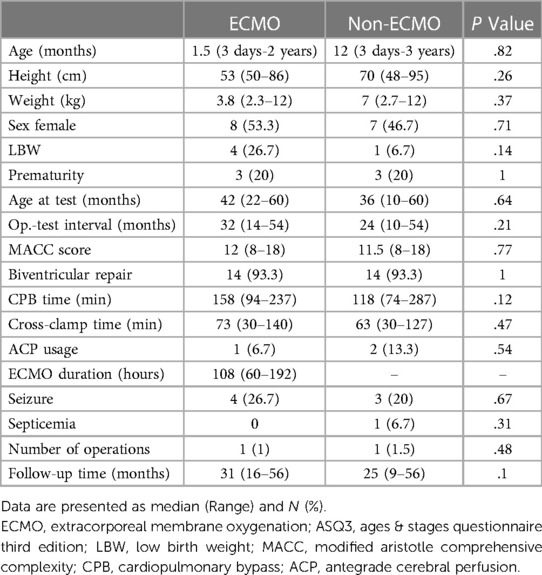
There were 30 patients in the cohort. Thirteen patients (43.3%) were neonates at the time of surgery. The median age of the total cohort was 3.5 months at the time of surgery. The ECMO patients tended to be younger than the non-ECMO patients (1.5 months and 12 months, respectively). Therefore, the ECMO patients were smaller (3.8 kg vs. 7 kg). Gender distributionswere similar between the groups. The median ECMO duration was 108 h (range 60–192 h) in the ECMO group. Preoperative risk factors, operative characteristics, and postoperative complications were also not statistically significant between the groups (Table 2).
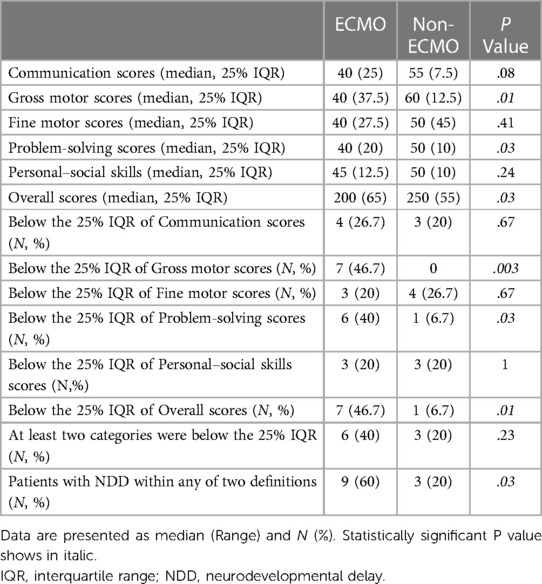
All patients followed-up with a median of 29 months (9–56 months). The median age at the test was 36 months. The non-ECMO patients gained better points in all five categories of the ASQ-3 test. However, there were statistically significant differences only in the assessment of gross motor and problem-solving categories (P = 0.01, and P = 0.03, respectively). The median overall score was 200 in the ECMO patients and 250 in the non-ECMO patients (P = 0.03). Seven patients (46.7%) were below the 25% IQR of the total score in the ECMO patients. Only one (6.7%) patient was below 25% IQR of the total score in the non-ECMO patients. In addition, the number of patients below the 25% IQR of gross motor, problem-solving, and overall scores was higher and statistically significant in the ECMO group (P = 0.003, P = 0.03, and P = 0.01, respectively). Although the number of patients who were below the 25% IQR in at least two categories was higher in the ECMO group (6 [40%] and 3 [20%]), it wasn't statistically significant (P = 0.23). Three patients with NDD, according to the first definition, were not neurodevelopmentally delayed according to the second definition in the ECMO group. While two patients didn't have NDD according to the first definition, NDD was found according to the second definition. In the non-ECMO group, the patient with NDD, according to the first definition, was also found to be neurodevelopmentally delayed according to the second definition. While two patients were not neurodevelopmentally delayed according to the first definition, NDD was found according to the second definition in the non-ECMO group. When we took into consideration any of the two definitions, the number of patients with NDD was nine (60%) in the ECMO group and three (20%) in the non-ECMO group. The median ECMO duration was 120 h (range 60–132 h) in nine NDD patients in the ECMO group. The median ECMO duration of six patients who were not neurodevelopmentally delayed was 102 h (range 60–192 h). The ASQ-3 results are presented in Table 3. Additionally, the median “yes” answer expressing parental concern about the child was two in the open-ended questions in the ECMO group and one in the non-ECMO group. Antegrade cerebral perfusion was used in one patient with NDD in the ECMO group (40 min) and in one patient in the non-ECMO group (11 min).
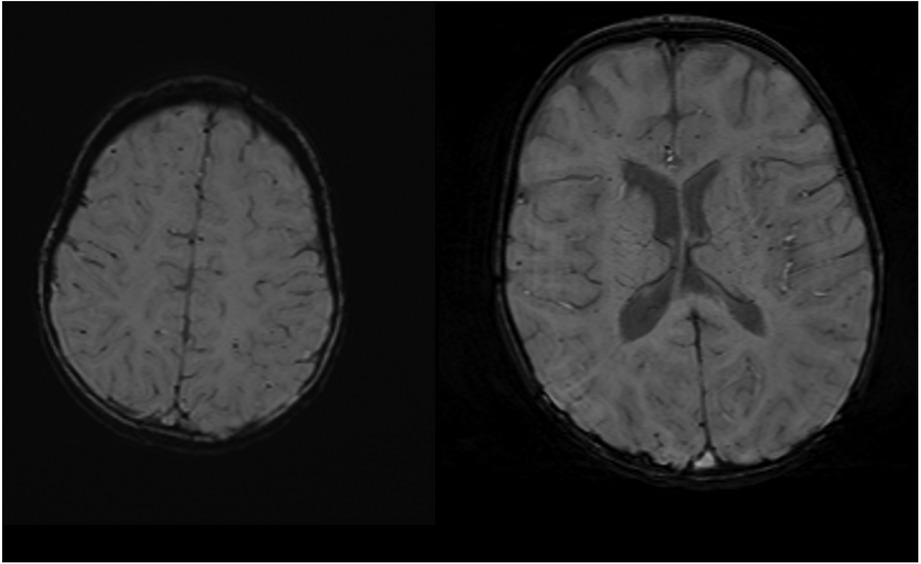
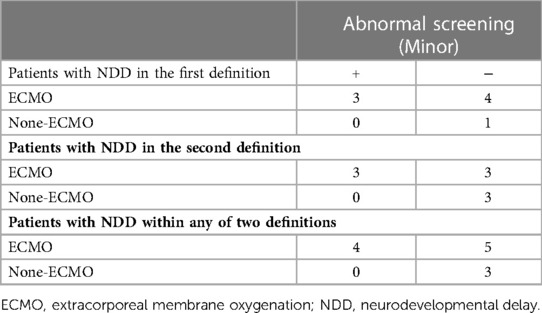
Twenty patients had neuroimaging with head ultrasound (HUS), MRI, or computed tomography (CT). All nine patients with NDD in the ECMO group and three patients with NDD in the non-ECMO group had at least one neuroimaging. Two patients with NDD had only HUS, and one patient had an MRI in the non-ECMO group. Four patients with NDD in the ECMO group had only HUS, four patients had MRI, and one patient had CT imaging in addition to HUS. We compared neuroimaging and patients with NDD. Of the nine ECMO patients, four had minor abnormalities in the MRI. Five of the ECMO patients with NDD had normal neuroimaging (four HUS and one CT scan). In addition, three non-ECMO patients with NDD had no anatomic lesions on neuroimaging (two HUS and one MRI). Table 4 outlines minor abnormalities in the neuroimaging of NDD patients according to group. Figure 1 shows images ofMRI brainwith no abnormal anatomic structure in a three-year-old male patient. This patient was placed on ECMO support after surgery at one month of age. This patient has no evidence of brain damage, but he has NDD. Figure 2 shows images of MRI brain with micro hemorrhagic foci of two patients. These patients are not neurodevelopmentally delayed, but they both have abnormal imaging.
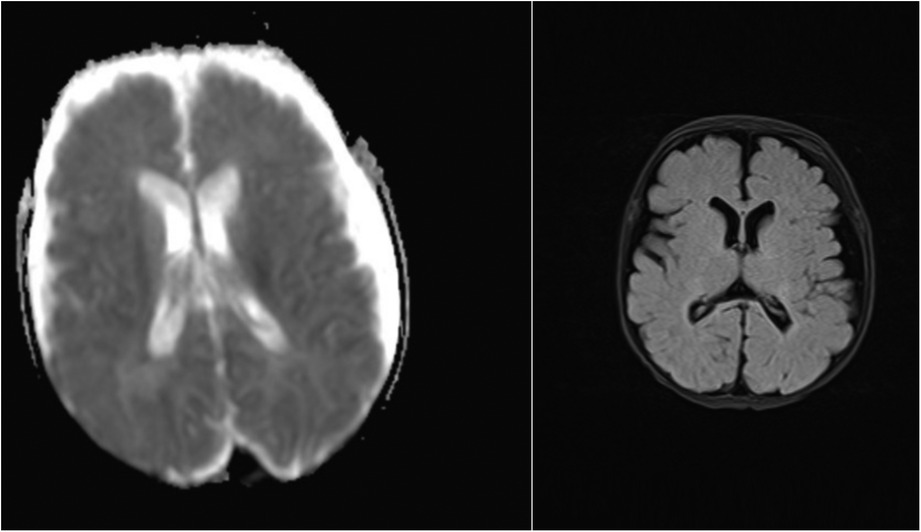
Figure 1. MRI screening with no abnormal anatomic structure in a three-year-old male patient with NDD.
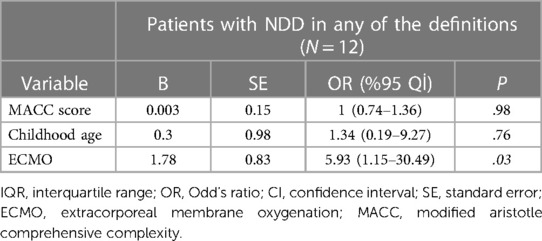
We established a multivariate logistic regression model to identify the independent variables that might cause NDD. The regression model was restricted to three variables because of the limited number of patients. The MACC score, childhood age, and ECMO support variables were analyzed in patients with NDD within any of the two definitions. Only the ECMO variable was statistically significant for NDD (P = 0.03, OR:5.93, CI:1.15–30.49). A multivariate regression model is presented in Table 5.
Discussion
To the best of our knowledge, this study is the first ND assessment of patients who underwent ECMO support after congenital heart surgery in Turkey. In this study, we sought to identify the effect of ECMO application on ND outcomes in patients who underwent congenital heart surgery. We found that NDD was more common in patients who received ECMO support after congenital heart surgery. Patients who didn't receiveECMO support gained higher points in all five categories of the ASQ-3 assessment. Another important finding was that most of these patients had no anatomic lesions on neuroimaging. This study emphasizes the importance of ND assessment and follow-up for all patients who undergo congenital heart surgery.
Although Bayley Scales of Infant Development, Denver Developmental Scale, Gessell Infant Scale, and Stanford–Binetwere the most used tests for ND assessment in publications and suggested by the Extracorporeal Life Support Organization (ELSO), these tests don't include a broad range of ages. In addition, these tests require educated health professionals with face-to-face examinations. Therefore, we chose the ASQ-3 test, which is much more feasible in Turkey because it can be applied via phone call interviews and doesnot require specific education. Another advantage of the ASQ-3 is that it can be conducted in around 15 min. Noeder et al. showed that the ASQ-3 corresponds well with the Bayley Scales of Infant and Toddler Development III (Bayley Scales III) as an ND screening test in patients with CHD (17). Most of the studies used the ASQ-3 and defined NDD in patients with astandard deviation (SD) of <1 (9, 10, 16). The ASQ-3 test has its own normative mean values in all versions of the test. However, we didn't have any normative mean and a defined SD because there was no Turkish version of the ASQ-3 test. Therefore, we defined NDD in patients below the 25% IQR of the total scores of all patients or in patients below the 25% IQR in at least two categories. In this way, we found that 60% of ECMO patients and 20% of non-ECMO patients were below the cut-off value. The proper assessment of NDD can be revealed only by comparing these patients with normal Turkish children. Considering the prevalence of NDD in patients with CHD, patients who aren't below the cut-off in our study may be behind in their age group. However, the primary outcome of this article is independent. We found that more patients with NDD were in the ECMO group, and this finding is consistent with previous reports (10, 18–20).
In our study, the median scores of the gross motor and problem-solving categories were clinically and statistically significantly worse for the ECMO patients compared to the non-ECMO patients. Sadhwani et al. reported that the gross motor subscale scores on Bayley-III were lowest in ECMO patients (18). Chorna et al. published a study that included 115 patients with CHD from the Cardiothoracic Surgery database (21). They found that NDD appeared significantly in gross motor and problem-solving skills among 96 patients with NDD who were evaluated using the ASQ-3. In most studies, gross motor delay was the most evident domain in NDD patients (9, 17, 18, 21).
Low birth weight and prematurity are one of the most important variables on NDD. Although LBW and prematurity variables were not statistically significant between groups, the proportion of patients with LBW was higher in the ECMO group. Previous studies have concluded that these two variables are associated with NDD in patients with complex CHD (22, 23). The higher proportion of patients with LBW in the ECMO group might have contributed to the results.
Neuroimaging was not performed on all patients, but all 12 patients with NDD had at least one neuroimaging session. Although six of these patients were examined with only HUS, eight had no evidence of injury. Although most previous articles concluded that patients with normal HUS might have evidence of injury by MRI or CT scan (7, 24), both HUS and MRI usually correlate with NDD (25). However, NDD may present with no evidence of anatomic lesions (7, 8).
Since there are a few publications from Turkey about NDD in patients with CHD (26–30), the other aim of the study is to raise awareness that NDD is a very common and masked comorbidity in patients who have undergone congenital heart surgery. Another subject we want to point out is that the ASQ-3 test is handy and feasible for ND assessment as a first step. Most congenital heart teams hesitate to evaluate the ND assessment because of the lack of educated health professionals and the difficulty of administering other tests.
There are several limitations to this study. Because of the lower survival rate of ECMO patients and because some patients lost to follow up, our study group was restricted to 15 patients.The reason for the lower survival rate in ECMO patients was a higher proportion of neonatal surgeries and patients with high MACC levels (31). Since we had no normative means for the ASQ-3 test in Turkish children, we need more publications with a large cohort to identify the normative means of the Turkish population. Because the primary outcome of this study was to assess the effect of ECMO application on NDD, we attempted to eliminate other variables that may affect ND progress using the PSM model. Although we couldn't compare the neuroimaging of all patients with their MRI, the occurrence of NDD may occur without abnormal screening. The socioeconomic status of the child's family and the child's nutritional status were not included in our analysis as contributing factors to NDD, which may also be considered a limitation of our study.
Conclusion
This article concluded that NDD is more common in patients who underwent ECMO support after congenital heart surgery. The ASQ-3 test can be used to evaluate ND progress as a first step. Every congenital heart clinic should be aware of NDD, and the assessment of ND progress should become widespread in Turkey.
Author's note
Presented at: 20th Congress of National Turkish Pediatric Cardiology and Pediatric Heart Surgery, March 10–13, 2028, Antalya and 17th Congress of Turkish Society of Cardiovascular Surgery, November17–20, 2022, Antalya.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Healthcare Institutions Medical Research Ethics Committee (ATADEK). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
SB and EE: contributed to conception and design of the study. ZSO, IG and OG: organized the database. SB and FG: performed the statistical analysis. SB and BT: wrote the first draft of the manuscript. SB, SA, and EE: wrote sections of the manuscript. AAK: evaluated radiologic imaging. All authors contributed to manuscript revision, read, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dolk H, Loane M, Garne E, European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation. (2011) 123(8):841–9. doi: 10.1161/CIRCULATIONAHA.110.958405
2. Razzaghi H, Oster M, Reefhuis J. Long-term outcomes in children with congenital heart disease: national health interview survey. J Pediatr. (2015) 166(1):119–24. doi: 10.1016/j.jpeds.2014.09.006
3. Mascio CE, Austin EH 3rd, Jacobs JP, Jacobs ML, Wallace AS, He X, et al. Perioperative mechanical circulatory support in children: an analysis of the society of thoracic surgeons congenital heart surgery database. J Thorac Cardiovasc Surg. (2014) 147(2):658–64, discussion 664–5. doi: 10.1016/j.jtcvs.2013.09.075
4. Polito A, Barrett CS, Rycus PT, Favia I, Cogo PE, Thiagarajan RR. Neurologic injury in neonates with congenital heart disease during extracorporeal membrane oxygenation: an analysis of extracorporeal life support organization registry data. ASAIO J. (2015) 61(1):43–8. doi: 10.1097/MAT.0000000000000151
5. Polito A, Barrett CS, Wypij D, Rycus PT, Netto R, Cogo PE, et al. Neurologic complications in neonates supported with extracorporeal membrane oxygenation. An analysis of ELSO registry data. Intensive Care Med. (2013) 39(9):1594–601. doi: 10.1007/s00134-013-2985-x
6. Lin JJ, Banwell BL, Berg RA, Dlugos DJ, Ichord RN, Kilbaugh TJ, et al. Electrographic seizures in children and neonates undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. (2017) 18(3):249–57. doi: 10.1097/PCC.0000000000001067
7. Rollins MD, Yoder BA, Moore KR, Barnhart DC, Jones C, Null DM, et al. Utility of neuroradiographic imaging in predicting outcomes after neonatal extracorporeal membrane oxygenation. J Pediatr Surg. (2012) 47(1):76–80. doi: 10.1016/j.jpedsurg.2011.10.016
8. Lazar EL, Abramson SJ, Weinstein S, Stolar CJ. Neuroimaging of brain injury in neonates treated with extracorporeal membrane oxygenation: lessons learned from serial examinations. J Pediatr Surg. (1994) 29(2):186–90; discussion 190–1. doi: 10.1016/0022-3468(94)90315-8
9. Billotte M, Deken V, Joriot S, Vaksmann G, Richard A, Bouzguenda I, et al. Screening for neurodevelopmental disorders in children with congenital heart disease. Eur J Pediatr. (2021) 180(4):1157–67. doi: 10.1007/s00431-020-03850-x
10. Dhar AV, Scott S, Anton-Martin P, Tweed J, Morris MA, Modem V, et al. Neurodevelopmental outcomes in extracorporeal membrane oxygenation patients: a pilot study. ASAIO J. (2020) 66(4):447–53. doi: 10.1097/MAT.0000000000001035
11. Radhakrishnan R, Merhar S, Meinzen-Derr J, Haberman B, Lim FY, Burns P, et al. Correlation of MRI brain injury findings with neonatal clinical factors in infants with congenital diaphragmatic hernia. AJNR Am J Neuroradiol. (2016) 37(9):1745–51. doi: 10.3174/ajnr.A4787
12. Erek E, Yalçınbaş YK, Sarıosmanoğlu N, Özkan M, Yıldız O, Şenkaya I, et al. First harvest of pediatric and congenital heart surgery multicenter database in Turkey: novel application of real-time online reporting. World J PediatrCongenit Heart Surg. (2021) 12(3):377–86. doi: 10.1177/2150135121995474
13. Squires J, Bricker D. Ages & stages questionnaires®, third edition (ASQ- 3TM). A parent-completed child-monitoring system. Baltimore: Paul H. Brookes Publishing Co (2009).
14. Squires J, Bricker D, Potter L. Revision of a parent-completed developmental screening tool: ages and stages questionnaires. J Pediatr Psychol. (1997) 22:313–28. doi: 10.1093/jpepsy/22.3.313
15. Bricker DD, Squires J, Mounts L. Ages and stages questionnaires (ASQ): A parent-completed, child-monitoring system. Baltimore: Paul H. Brookes Publishing Co (1995).
16. Kapci EG, Kucuker S, Uslu RI. How applicable are ages and stages questionnaires for use with turkish children? Topics Early Child Spec Educ. (2010) 30(3):176–88. doi: 10.1177/0271121410373149
17. Noeder MM, Logan BA, Struemph KL, Condon N, Mueller I, Sands B, et al. Developmental screening in children with CHD: ages and stages questionnaires. Cardiol Young. (2017) 27(8):1447–54. doi: 10.1017/S1047951117000415
18. Sadhwani A, Cheng H, Stopp C, Rollins CK, Jolley MA, Dunbar-Masterson C, et al. Early neurodevelopmental outcomes in children supported with ECMO for cardiac indications. PediatrCardiol. (2019) 40(5):1072–83. doi: 10.1007/s00246-019-02115-1
19. International Cardiac Collaborative on Neurodevelopment (ICCON) Investigators. Impact of operative and postoperative factors on neurodevelopmental outcomes after cardiac operations. Ann Thorac Surg. (2016) 102(3):843–9. doi: 10.1016/j.athoracsur.2016.05.081
20. Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. American heart association congenital heart defects committee, council on cardiovascular disease in the young, council on cardiovascular nursing, and stroke council. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American heart association. Circulation. (2012) 126(9):1143–72. doi: 10.1161/CIR.0b013e318265ee8a
21. Chorna O, Baldwin HS, Neumaier J, Gogliotti S, Powers D, Mouvery A, et al. Feasibility of a team approach to complex congenital heart defect neurodevelopmental follow-up: early experience of a combined cardiology/neonatal intensive care unit follow-up program. Circ Cardiovasc Qual Outcomes. (2016) 9(4):432–40. doi: 10.1161/CIRCOUTCOMES.116.002614
22. Fuller S, Nord AS, Gerdes M, Wernovsky G, Jarvik GP, Bernbaum J, et al. Predictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur J Cardiothorac Surg. (2009) 36(1):40–7. doi: 10.1016/j.ejcts.2009.02.047
23. Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. (2007) 133(5):1344–53, 1353.e1–3. doi: 10.1016/j.jtcvs.2006.10.087
24. Gannon CM, Kornhauser MS, Gross GW, Wiswell TE, Baumgart S, Streletz LJ, et al. When combined, early bedside head ultrasound and electroencephalography predict abnormal computerized tomography or magnetic resonance brain images obtained after extracorporeal membrane oxygenation treatment. J Perinatol. (2001) 21(7):451–5. doi: 10.1038/sj.jp.7210593
25. Griffin MP, Minifee PK, Landry SH, Allison PL, Swischuk LE, Zwischenberger JB. Neurodevelopmental outcome in neonates after extracorporeal membrane oxygenation: cranial magnetic resonance imaging and ultrasonography correlation. J Pediatr Surg. (1992) 27(1):33–5. doi: 10.1016/0022-3468(92)90099-s
26. Elbayiyev S, Canpolat FE, KadıoğluŞimşek G, Işık S, Büyüktiryaki M, KanmazKutman HG. Long-term neurodevelopmental outcomes in very low birth weight infants with and without patent ductus arteriosus: a retrospective case control observational study. Child Care Health Dev. (2022) 48(5):862–8. doi: 10.1111/cch.12997
27. Yilmaz İZ, Erdur B, Ozbek E, Mese T, Karaarslan U, Genel F. Neurodevelopmental evaluation of children with cyanotic congenital heart disease. Minerva Pediatr. (2018) 70(4):365–70. doi: 10.23736/S0026-4946.17.04265-7
28. Piskin S, Unal G, Arnaz A, Sarioglu T, Pekkan K. Tetralogy of fallot surgical repair: shunt configurations, ductus arteriosus and the circle of willis. Cardiovasc Eng Technol. (2017) 8(2):107–19. doi: 10.1007/s13239-017-0302-5
29. Hallioglu O, Gurer G, Bozlu G, Karpuz D, Makharoblidze K, Okuyaz C. Evaluation of neurodevelopment using bayley-III in children with cyanotic or hemodynamically impaired congenital heart disease. Congenit Heart Dis. (2015) 10(6):537–41. doi: 10.1111/chd.12269
30. Polat S, Okuyaz C, Hallioğlu O, Mert E, Makharoblidze K. Evaluation of growth and neurodevelopment in children with congenital heart disease. Pediatr Int. (2011) 53(3):345–9. doi: 10.1111/j.1442-200X.2010.03230.x
Keywords: ECMO, neurodevelopment, congenital heart disease, child development disorders, assessment
Citation: Basgoze S, Temur B, Ozcan ZS, Gokce I, Guvenc O, Aydin S, Guzelmeric F, Altan Kus A and Erek E (2023) The effect of extracorporeal membrane oxygenation on neurodevelopmental outcomes in children after repair of congenital heart disease: A pilot study from Turkey. Front. Pediatr. 11:1131361. doi: 10.3389/fped.2023.1131361
Received: 24 December 2022; Accepted: 13 March 2023;
Published: 3 April 2023.
Edited by:
Francesco Morini, Meyer Children's Hospital, ItalyReviewed by:
Fabrizio De Rita, Newcastle upon Tyne Hospitals NHS Foundation Trust, United KingdomPeter Paul Roeleveld, Leiden University Medical Center (LUMC), Netherlands
© 2023 Basgoze, Temur, Ozcan, Gokce, Guvenc, Aydin, Guzelmeric, Altan Kus and Erek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serdar Basgoze YmFzZ296ZXNlcmRhckBnbWFpbC5jb20=
†ORCID Serdar Basgoze orcid.org/0000-0002-6146-2095
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
 Serdar Basgoze
Serdar Basgoze Bahar Temur
Bahar Temur Zeynep Sila Ozcan
Zeynep Sila Ozcan Ibrahim Gokce
Ibrahim Gokce Osman Guvenc
Osman Guvenc Selim Aydin
Selim Aydin Fusun Guzelmeric
Fusun Guzelmeric Aylin Altan Kus
Aylin Altan Kus Ersin Erek
Ersin Erek