- 1Pediatric Intensive Care Unit, Children’s Hospital of Fudan University, National Center for Children’s Health, Shanghai, China
- 2Dermatological Department, Children’s Hospital of Fudan University, National Center for Children’s Health, Shanghai, China
- 3Surgical Oncology Department, Children’s Hospital of Fudan University, National Center for Children’s Health, Shanghai, China
- 4Institute of Antibiotics, Huashan Hospital, Fudan University, Shanghai, China
- 5Lab. of Mycology, Department of Dermatology, Huashan Hospital, Fudan University, Shanghai, China
- 6Radiology Department, Children’s Hospital of Fudan University, National Center for Children’s Health, Shanghai, China
- 7Medical Department, Nanjing Dinfectome Technology Inc., Nanjing, China
Lichtheimia ramosa (L. ramosa) is an opportunistic fungal pathogen of the order Mucorales that may result in a rare but serious mucormycosis infection. Mucormycosis could be angioinvasive, causing thrombosis and necrosis in the nose, brain, digestive tract, and respiratory tract. The infection is highly lethal, especially in immunocompromised hosts, and the incidence has been on the rise. However, due to its relatively low incidence in pediatric population and the challenges with diagnosis, the awareness and management experience for pediatric mucormycosis are extremely limited, which might lead to poor outcomes. In this study, we comprehensively reviewed the course of a fatal rhinocerebral mucormycosis case in a pediatric neuroblastoma patient receiving chemotherapy. Due to a lack of awareness of the infection, the standard care of amphotericin B treatment was delayed and not administered until the identification of L. ramosa by metagenomic next-generation sequencing (mNGS)-based pan-pathogen detection of the patient's peripheral blood sample. We also reviewed the literature on L. ramosa infection cases reported worldwide between 2010 and 2022, with an analysis of clinical manifestation, prognosis, and epidemiological data. Our study not only highlighted the clinical value of comprehensive mNGS in rapid pathogen detection but also raised awareness of recognizing lethal fungal infection early in immunocompromised hosts including pediatric cancer patients.
Introduction
Mucormycosis is a highly lethal infection caused by the opportunistic fungal pathogens of the Mucorales order, usually in patients with malignancies, transplantation, and diabetes (1). It is the world's third most common invasive mycosis after candidiasis and aspergillosis (2). The clinical management of mucormycosis has drawn increasing attention, accompanying the growing number of invasive mucormycosis reported during the present coronavirus disease (COVID-19) pandemic (3). Based on the infection manifestations, it can be classified into rhinocerebral, pulmonary, gastrointestinal, cutaneous, disseminated, and uncommon types (1). Rhinocerebral mucormycosis refers to mucormycosis that enters the nasal cavity through the junction of skin and mucosa, then spreads to the palate, sinus, and orbit, and finally causes intracranial infection due to vascular invasion or bone destruction. The most common pathogens of mucormycosis are Rhizopus spp., Mucor spp., and Lichtheimia spp. (4). Rhizopus spp. is currently the most common pathogen of mucormycosis worldwide, especially in rhinocerebral mucormycosis.
The nomenclature and taxonomy of subphylum Mucoromycotina have been evolving in recent years. In the subphylum of Mucoromycotina, Absidia corymbifera was a common clinical pathogen, especially in the secondary infection of skin lesions after severe trauma or burn. In 2007, Hoffman et al. (5) classified thermotolerant Absidia as Mycocladus, which then comprises the three species of M. corymbifer, M. blakesleeanus, and M. hyalospora based on their morphological, physiological, and phylogenetic characteristics. In 2009, the genus Mycocladus was renamed Lichtheimia and divided into two species, L. corymbifera and L. ramosa, and the former was found to be more resistant to amphotericin B (6). In 2010, the genus Lichtheimia was further classified into five species: L. corymbifera, L. ornata, L. ramosa, L. hyalospora, and L. sphaerocystis, by their molecular markers, mating tests, morphology, and growth rate (7). The first three species have been reported to be clinically relevant.
The incidence of mucormycosis has been studied globally showing a general trend of increase and geographic differences (8–10). Though lacking statistics on the exact burden of mucormycosis, the prevalence has been estimated based on population or hospital-based studies worldwide and the differences between developed and developing countries were observed, resulted from multiple factors including risk groups, healthcare accessibility, clinical management and intervention (11). Generally speaking, the incidence of mucormycosis in pediatric population is relatively lower than that in adults, which lead to less awareness of the disease regarding the clinical diagnosis and limited experience in disease management (12). Similar to adult patients, the common risk factors in children include hematologic malignancy, stem cell/solid organ transplant, autoimmune diabetes mellitus, premature infants, and so on (13). However, diagnosing pediatric mucormycosis is very challenging due to the lack of straightforward testing approaches and complicated clinical manifestations and symptoms (14).
At present, the treatment mainly relies on amphotericin B. However, the renal and hepatic toxicity of amphotericin B formulations should be taken into account, especially for immunodeficient patients, and therefore, a modified dose should be considered in intolerant cases (15, 16). Early diagnosis, timely treatment with prescribed drugs, and surgical operations are therefore essential. In this study, we reported the disease course of a lethal L.ramosa infection in a pediatric cancer patient receiving chemotherapy. The empirical diagnosis and treatment were initially suboptimal due to a lack of awareness of the infection until the identification of L.ramosa by metagenomic next-generation sequencing (mNGS). The patient died two days later due to multiple organ failures. We also performed the first comprehensive literature review of L. ramosa infection cases documented between 2010 and 2022 to augment the understanding of the clinical manifestation, prognosis, and risk factors of mucormycosis.
Methods
To comprehensively study the world-wide reported mucormycosis cases, we searched for literatures in PubMed, Ovid MEDLINE, Embase, WANFANG, and CNKI Database. Due to the redefinition and classification of Lichtheimia in 2010 (7), we decided to focus on the publications between January 2010 and February 2022 with any of the following key words: Lichtheimia ramosa, ramosa, Lichtheimia, mucormycosis, Absidia corymbifera, Mycocladus corymbifera, L. corymbifera, L. ornata, L. ramosa, Absidia ramosa, Rhizopus ramosus, Mucor ramosus, L. hyalospora, L. blakesleeaana, and L. sphaerocystis. Only the cases with confirmed L. ramosa infection were included, whose clinical and epidemiological data were summarized in Table 1.
Results
Case presentation
A 6-year-old male patient developed a fever accompanied by nasal bleeding and a small amount of vomiting. He was admitted to a local hospital on October 6, 2021 (Day 0 after symptom onset, DAO 0). Before this hospital admission, the patient was admitted to the hospital in July 2019 due to a six-month poor appetite and significant weight loss, as well as right leg pain for 20 days. He was then diagnosed of neuroblastoma accompanied by intracranial and multiple bone metastases after a complete bone marrow biopsy. The patient completed three cycles of maintenance chemotherapy for high-risk neuroblastoma and radical resection of the retroperitoneal tumor under general anesthesia. Starting from DAO 0, routine peripheral blood testings were performed regularly to monitor the patient's conditions, which showed constantly low levels of white blood cells, neutrophils, platelets, and Hemoglobin (Figure 1A). Up till DAO13, the patient was empirically suspected of having a bacterial infection post-chemotherapy and treated as follows (Figure 1A): piperacillin/tazobactam (45 mg/kg, q8h), cefazoxime (50 mg/kg, bid), cefoperazone sulbactam (50 mg/kg, q12h), meropenem (20 mg/kg, q8h), vancomycin (10 mg/kg, q6h), and metronidazole (7.5 mg/kg, q8h) treatment. The patient also received the infusion of platelet (1 U) on DAOs of 3, 7, and 12, hemoglobin (1.5 U) on DAOs of 8 and 11, and recombinant human granulocyte colony-stimulating factor (rhGCSF, 150 µg, qd) on DAOs of 2–13. After the whole antibacterial treatment, the patient still experienced intermittent fever, nasal bleeding, and mouth pain (Figures 1A,B). The C-Reactive protein (CRP) level increased to 103.5 mg/L (DAO 10) and 181.0 mg/L (DAO 13). The conventional culture with blood samples at the local hospital failed to detect the presence of any possible causative pathogens.
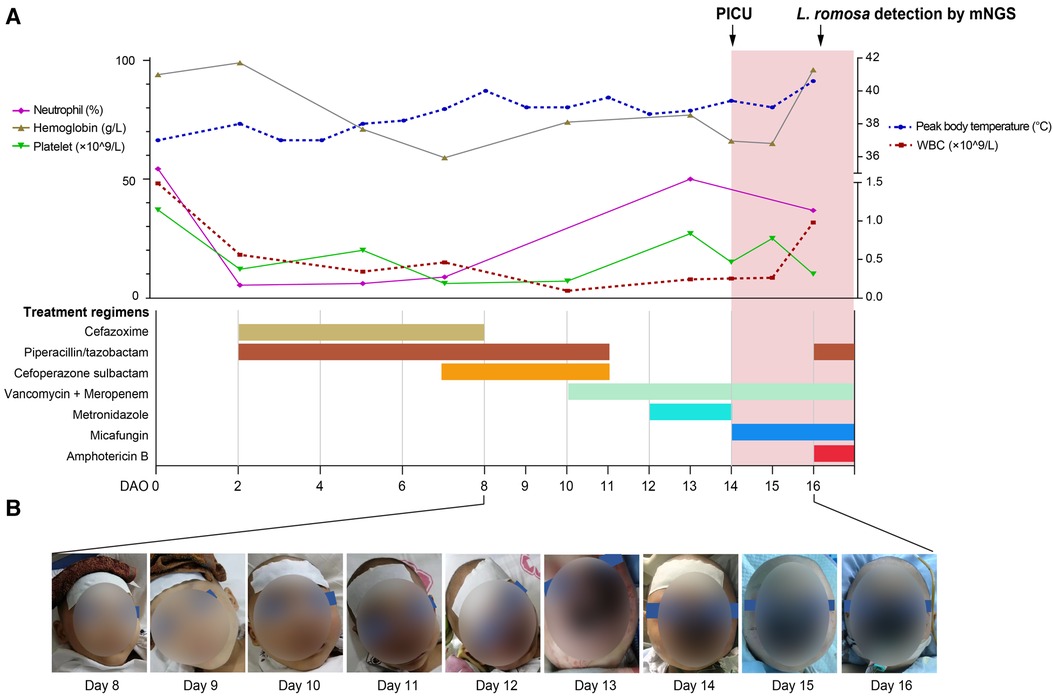
Figure 1. Timeline of the patient's disease course. (A) The patient's treatment course and clinical testing data. Routine blood workup results were shown in the top panel, including neutrophil, hemoglobin, and platelet on the left and white blood cells (WBCs) on the right. The patient's treatment course was shown in the bottom panel. (B) Images showing the development of the rhinocerebral L. ramosa infection from DAO 8 to DAO 16.
On DAO 14, the patient was admitted to Pediatric Intensive Care Unit (PICU), Children's Hospital of Fudan University, due to severe conditions, including a high fever up to 39.2°C, a small amount of blood oozing from both eyes, and progressive gangrene. The standard cultivate experiments were performed with blood, throat swab, and anal swab samples, but all returned negative results. (1–3)-β-D-Glucan (G) and glactomannan (GM) tests and lipopolysaccharides (LPS) were within the normal range but the evaluated levels of procalcitonin (PCT, 4.27 ng/ml) and Interleukin 6 (IL-6, 3.57 ng/ml) were reported. Computed tomography (CT) showed scalp abscess (Figures 2A,B), pulmonary infarction (Figures 2C,D), and evidence of osteomyelitis (Figures 2E,F). The following treatments were given to relieve the severe symptoms (Figure 1A): meropenem (40 mg/kg, q8h, three days), vancomycin (15 mg/kg, q6h, three days), piperacillin/tazobactam (112.5 mg/kg, q8h, one day), micafungin (2 mg/kg, qd, three days), infusion of platelet (1 U, DAO 14), hemoglobin (1 U, DAO 15), and albumin (10 g, DAOs 14–16), and rhGCSF (150 µg, qd, DAOs 2–13). However, the patient showed no noticeable improvement (Figures 1A,B), and his CRP remained above 160 mg/l. In the meantime, the peripheral blood sample was subject to metagenome next-generation sequencing (mNGS) to comprehensively search for possible pathogens (DAO 14). On DAO 15, the patient received rescue, endotracheal intubation, and vasoactive drug therapy due to worsened conditions. On DAO 16, L. ramosa was identified by mNGS with unique DNA sequences covering 8.11% of the L. ramosa genome (Figure 3A), supporting the diagnosis of mucormycosis by L. ramosa infection. Amphotericin B deoxycholate (5 mg/kg, ivgtt) was immediately administered, and tissue samples were collected from the nasal gangrene region for microscopic examination, which confirmed the diagnosis of L. ramosa infection shortly thereafter (Figures 3B–E). Despite the immediate treatment by amphotericin B after the detection of L. ramosa, the patient died of severe multiple organ failure on DAO 16.
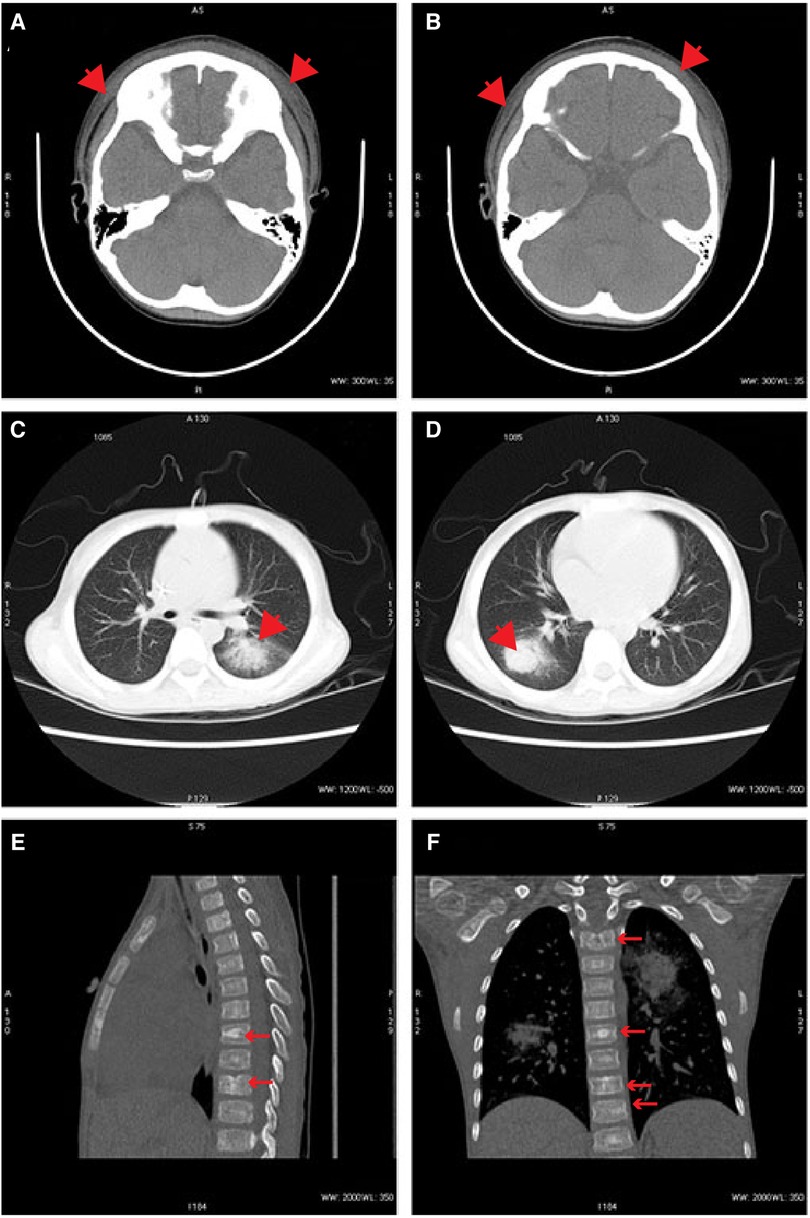
Figure 2. Representative radiographs. (A,B) CT images demonstrating scalp abscess on DAO 14. (C,D) CT images demonstrating pulmonary infarctions on DAO 14. (E,F) CT images demonstrating evidence of osteomyelitis on DAO 14.
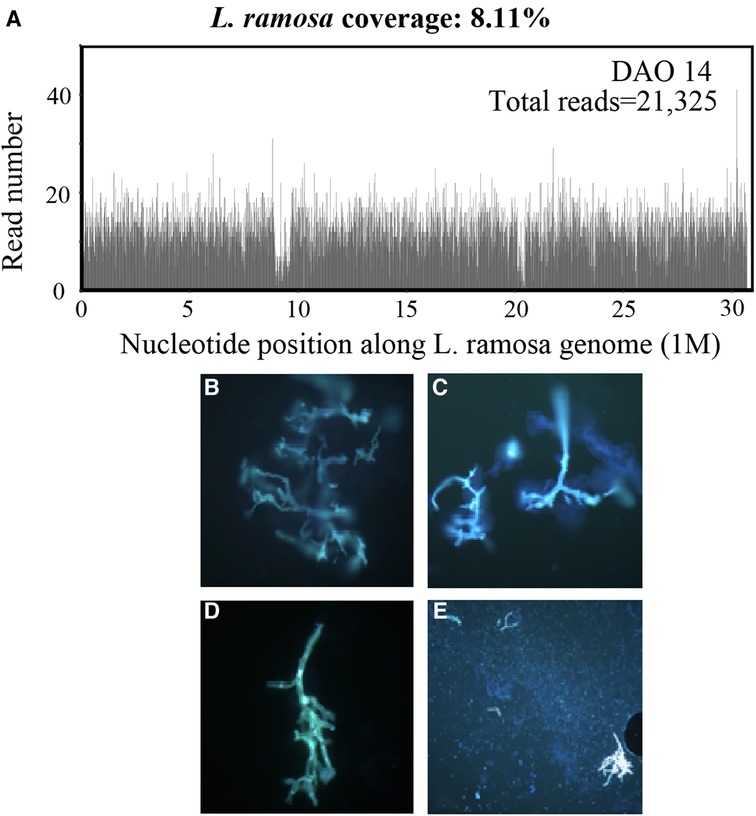
Figure 3. Laboratory investigations. (A) mNGS results showing the read depth throughout the L. ramosa genome derived from the patient's blood sample on DAO 14. (B–E) Fluorescence staining of the tissue samples collected from the nasal gangrene region. Microscopic examination showed direct evidence of presence ribbon-shaped broad sparsely septate hyphae (B–D, 400×) and clusters of sporangiospores (E, 200×), suggestive of the Mucorales order.
Literature review
We retrieved 25 publications with a total of 41 L. ramosa infectious cases (Table 1) where the majority were from Europe (22/41, 53.7%), followed by 12 from Asia (29.3%), 4 from America (9.8%), and 3 from Africa (7.3%). The most common sites of infection were cutaneous (N = 17) and pulmonary (N = 10). As expected, most cases were immunocompromised subjects with underlying risk factors such as cancer (9, 22.0%), diabetes (6, 14.6%), burn (6, 14.6%), and organ transplantation (5, 12.2%). Excluding eight cases with unknown age at diagnosis, the median age was 46 years old (range: 4–84) and six of them were under 10 years old. More specifically, five of the six pediatric cases were treated with liposomal amphotericin B (LAMB) and three were cured. While among all treatment-specified cases, 32 of them received the Amphotericin B treatment which successfully cured 72% (23/32) of the patients. Meanwhile, we observed a trend of adopting NGS-based methods in pathogen detection and more recently, a rising incidence in COVID-19 patients worldwide after 2020.
Discussion
In the past decades (42), mucormycosis incidence has increased rapidly, with the overall mortality rate as high as 90%. Mucormycosis mainly occurs in immunocompromised patients with conditions such as diabetes mellitus, malignancies, burns, autoimmune diseases, penetrating trauma or receiving corticosteroids etc (1). Notably, several studies have witnessed a resurgence of mucormycosis during the present global COVID-19 pandemic, which indicated that the accompanied immunocompromised state and steroid use in COVID-19 patients were the risk factors underlying mucormycosis (43, 44).
The prevalence of diagnosed infection shows a geographical difference, mainly distributed in Europe (68.2%), followed by Asia (16%) and Africa (9%) (45, 46). In developed countries, the number of Lichtheimia infection cases is high among hematopoietic stem cell transplantation recipients and hematological malignancies. In contrast, mucormycosis is more associated with diabetes mellitus and ketoacidosis in developing countries (46).
To our best knowledge, we reported the first case of mucormycosis by L. ramosa in a pediatric neuroblastoma patient, who had received chemotherapy prior to L. ramosa infection and neuroblastoma is the most common extra-cranial solid tumor in infants and children and represents 8%–10% of all childhood tumors (47). Based on our literature review, cancer is an important risk factor underlying L. ramosa infection in both pediatric and adult populations. A total of six pediatric L. ramosa infection cases were included in our literature review and both of the AML relapsed children died after infection (17, 30). In addition, early diagnosis and treatment are essential for the cure, as demonstrated by the cases collected in our study. For instance, Cases 23 and 24 are brothers who were both infected by L. ramosa (32). The older patient died, while the younger patient received early detection and timely treatment, leading to a favorable prognosis.
For now, the gold standard for diagnosis is histopathology testing, mainly with tissue samples. Traditional invasive procedures for etiologic diagnosis have limitations such as patient instability (37). In addition, the positive rate of histopathological and microbiological methods from cultivated clinical samples, is around 50% or even lower due to the morphological similarity between different Lichtheimia species (29–48). Therefore, molecular testing such as polymerase chain reaction (PCR) or NGS-based technologies using serum samples are essential supplemental tools for diagnosis. In our case, the local hospital lacked awareness of rare fungal infections and access to molecular tests such as mNGS, leading to the delay of diagnosis and treatment. The presented case also indicated the lethality of L. ramosa infection in pediatric cancer patients, and we believe a more timely etiologic diagnosis when the initial pan-antibiotics application was ineffective could result in a favorable outcome. Thus, mNGS is highly recommended for diagnostically challenging cases with undetermined infection and complex manifestations, especially for pediatric patients, which enables rapid and affordable pan-pathogen screening to guide targeted intervention against deadly infections. Admittedly, mNGS has limitations, especially the false-positive results caused by contamination. Also, the reference database selection and interpretation may affect the pathogen detection (49). Thus, alternative methods are needed to validate mNGS findings.
Amphotericin B is considered the first choice for mucormycosis (15). Indeed, 75.0% (24/32) of the patients that received the treatment of amphotericin B recovered from L. ramosa infection in our literature review. Furthermore, combining amphotericin B and surgical debridement of infected tissues was reported to be able to improve the cure rate. For example, Schneidawind et al. (50) reported three acute myeloid leukemia (AML) patients complicated with pulmonary mucormycosis who were successfully treated by combined LAMB and surgical resection before stem cell transplantation (SCT). Similarly, LAMB treatment effectively prevented infection recurrence despite immunosuppressive drugs in an SCT case with pulmonary mucormycosis after surgical resection (51). Alternatively, posaconazole has been reported as a salvage treatment for amphotericin B refractory patients, but it is less effective and more likely to cause resistance (52).
In conclusion, we reported a rhinocerebral mucormycosis pediatric case caused by L. ramosa with neuroblastoma and reviewed the L. ramosa infection cases published between 2010 and 2022. The limited number of clinical cases, lack of awareness, and technical challenges of detecting different infection sites may restrict the interpretation of the literature analysis. Our case demonstrates the importance of rapid pan-pathogen screening by mNGS to guide timely treatment selection against fast-developing, fatal infections for pediatric cancer patients.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
HS, XC, JL, WC, and GL contributed to the conceptualization; HS, XC, JL, YY, RD, JW, LL, QS, WC, and GL were responsible for data curation; HS, XC, YY, RD, and GL performed the formal analysis; GY, YM, QO, and MS validated the results; HS, XC, JL and WC wrote the original draft; GY, YM, QO, MS, WC, and GL revised the manuscript; GY and GL contributed to the funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China under Grant [2021YFC2701800] and [2021YFC2701805].
Acknowledgments
The authors thank the patient and the patient's guardian for providing consent for publication. We also thank all staff involved in this case study.
Conflict of interest
YM, QO, and MS are employees of Nanjing Dinfectome Technology Inc., China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi (Basel). (2020) 6(4):265. doi: 10.3390/jof6040265
2. Kriengkauykiat J, Ito JI, Dadwal SS. Epidemiology and treatment approaches in management of invasive fungal infections. Clin Epidemiol. (2011) 3:175–91. doi: 10.2147/CLEP.S12502
3. Al-Tawfiq JA, Alhumaid S, Alshukairi AN, Temsah MH, Barry M, Al Mutair A, et al. COVID-19 and mucormycosis superinfection: the perfect storm. Infection. (2021) 49:833–53. doi: 10.1007/s15010-021-01670-1
4. Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European confederation of medical mycology (ECMM) working group on zygomycosis between 2005 and 2007. Clin Microbiol Infect. (2011) 17:1859–67. doi: 10.1111/j.1469-0691.2010.03456.x
5. Hoffmann K, Discher S, Voigt K. Revision of the genus Absidia (Mucorales, Zygomycetes) based on physiological, phylogenetic, and morphological characters; thermotolerant Absidia spp. form a coherent group, Mycocladiaceae fam. nov. Mycol Res. (2007) 111:1169–83. doi: 10.1016/j.mycres.2007.07.002
6. Hoffmann K, Voigt K. Absidia parricida plays a dominant role in biotrophic fusion parasitism among mucoralean fungi (Zygomycetes): Lentamyces, a new genus for A. parricida and A. zychae. Plant Biol (Stuttg). (2009) 11:537–54. doi: 10.1111/j.1438-8677.2008.00145.x
7. Alastruey-Izquierdo A, Hoffmann K, de Hoog GS, Rodriguez-Tudela JL, Voigt K, Bibashi E, et al. Species recognition and clinical relevance of the zygomycetous genus Lichtheimia (syn. Absidia pro parte, Mycocladus). J Clin Microbiol. (2010) 48:2154–70. doi: 10.1128/JCM.01744-09
8. Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. (2019) 25:26–34. doi: 10.1016/j.cmi.2018.07.011
9. Torres-Narbona M, Guinea J, Martinez-Alarcon J, Munoz P, Gadea I, Bouza E, et al. Impact of zygomycosis on microbiology workload: a survey study in Spain. J Clin Microbiol. (2007) 45:2051–3. doi: 10.1128/JCM.02473-06
10. Kontoyiannis DP, Yang H, Song J, Kelkar SS, Yang X, Azie N, et al. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: a retrospective study. BMC Infect Dis. (2016) 16:730. doi: 10.1186/s12879-016-2023-z
11. Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi (Basel). (2019) 5(1):26. doi: 10.3390/jof5010026
12. Steinbach WJ. Latest thoughts on treating pediatric mucormycosis. J Pediatric Infect Dis Soc. (2020) 9:640–4. doi: 10.1093/jpids/piaa106
13. Zaoutis TE, Roilides E, Chiou CC, Buchanan WL, Knudsen TA, Sarkisova TA, et al. Zygomycosis in children: a systematic review and analysis of reported cases. Pediatr Infect Dis J. (2007) 26:723–7. doi: 10.1097/INF.0b013e318062115c
14. Schuetz AN, Walsh TJ. Importance of fungal histopathology in immunocompromised pediatric patients: it’s not just “Aspergillus” anymore. Am J Clin Pathol. (2015) 144:185–7. doi: 10.1309/AJCPE3NSJ2RYLENS
15. Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. (2019) 19:e405–21. doi: 10.1016/S1473-3099(19)30312-3
16. Yoshida M, Tamura K, Masaoka T, Nakajo E. A real-world prospective observational study on the efficacy and safety of liposomal amphotericin B in 426 patients with persistent neutropenia and fever. J Infect Chemother. (2021) 27:277–83. doi: 10.1016/j.jiac.2020.10.005
17. Borrás R, Roselló P, Chilet M, Bravo D, de Lomas JG, Navarro D. Positive result of the Aspergillus galactomannan antigen assay using bronchoalveolar lavage fluid from a patient with an invasive infection due to Lichtheimia ramosa. J Clin Microbiol. (2010) 48:3035–6. doi: 10.1128/JCM.00902-10
18. Bibashi E, de Hoog GS, Pavlidis TE, Symeonidis N, Sakantamis A, Walther G. Wound infection caused by Lichtheimia ramosa due to a car accident. Med Mycol Case Rep. (2013) 2:7–10. doi: 10.1016/j.mmcr.2012.12.001
19. de Chaumont A, Pierret C, Janvier F, Goudard Y, de Kerangal X, Chapuis O. Mucormycosis: a rare complication of an amputation. Ann Vasc Surg. (2014) 28:1035.e15–9. doi: 10.1016/j.avsg.2013.10.008
20. Cateau E, Randriamalala E, Elsendoorn A, Giot JP, du Sorbier CM, Rodier MH. Fatal-mixed cutaneous zygomycosis-aspergillosis: a case report. Mycopathologia. (2013) 176:423–7. doi: 10.1007/s11046-013-9706-4
21. Poirier P, Nourrisson C, Gibold L, Chalus E, Guelon D, Descamp S, et al. Three cases of cutaneous mucormycosis with Lichtheimia spp. (ex Absidia/Mycocladus) in ICU. Possible cross-transmission in an intensive care unit between 2 cases. J Mycol Med. (2013) 23:265–9. doi: 10.1016/j.mycmed.2013.09.002
22. Millon L, Larosa F, Lepiller Q, Legrand F, Rocchi S, Daguindau E, et al. Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin Infect Dis. (2013) 56:e95–e101. doi: 10.1093/cid/cit094
23. Kutlu M, Ergin C, Bir F, Hilmioğlu-Polat S, Gümral R, Necan C, et al. Pulmonary mucormycosis due to Lichtheimia ramosa in a patient with HIV infection. Mycopathologia. (2014) 178:111–5. doi: 10.1007/s11046-014-9761-5
24. Irtan S, Lamerain M, Lesage F, Verkarre V, Bougnoux ME, Lanternier F, et al. Mucormycosis as a rare cause of severe gastrointestinal bleeding after multivisceral transplantation. Transpl Infect Dis. (2013) 15:E235–8. doi: 10.1111/tid.12147
25. Fréalle E, Rocchi S, Bacus M, Bachelet H, Pasquesoone L, Tavernier B, et al. Real-time polymerase chain reaction detection of Lichtheimia species in bandages associated with cutaneous mucormycosis in burn patients. J Hosp Infect. (2018) 99:68–74. doi: 10.1016/j.jhin.2018.02.004
26. Kaur R, Bala K, Ahuja RB, Srivastav P, Bansal U. Primary cutaneous mucormycosis in a patient with burn wounds due to Lichtheimia ramosa. Mycopathologia. (2014) 178:291–5. doi: 10.1007/s11046-014-9805-x
27. Chowdhary A, Kathuria S, Singh PK, Sharma B, Dolatabadi S, Hagen F, et al. Molecular characterization and in vitro antifungal susceptibility of 80 clinical isolates of mucormycetes in Delhi, India. Mycoses. (2014) 57(Suppl 3):97–107. doi: 10.1111/myc.12234
28. Zaki SM, Elkholy IM, Elkady NA, Abdel-Ghany K. Mucormycosis in Cairo, Egypt: review of 10 reported cases. Med Mycol. (2014) 52:73–80. doi: 10.3109/13693786.2013.809629
29. Bala K, Chander J, Handa U, Punia RS, Attri AK. A prospective study of mucormycosis in north India: experience from a tertiary care hospital. Med Mycol. (2015) 53:248–57. doi: 10.1093/mmy/myu086
30. Suzuki D, Kobayashi R, Hori D, Kishimoto K, Sano H, Yasuda K, et al. Stem cell transplantation for acute myeloid leukemia with pulmonary and cerebral mucormycosis. Pediatr Int. (2016) 58:569–72. doi: 10.1111/ped.12866
31. Mouronte-Roibás C, Leiro-Fernández V, Botana-Rial M, Ramos-Hernández C, Lago-Preciado G, Fiaño-Valverde C, et al. Lichtheimia ramosa: a fatal case of mucormycosis. Can Respir J. (2016) 2016:2178218. doi: 10.1155/2016/2178218
32. Winstead M, Ozolek J, Nowalk A, Williams J, Vander Lugt M, Lin P. Disseminated Lichtheimia ramosa infection after hematopoietic stem cell transplantation in a child with chronic granulomatous disease. Pediatr Infect Dis J. (2017) 36:1222–4. doi: 10.1097/INF.0000000000001589
33. Guinea J, Escribano P, Vena A, Munoz P, Martinez-Jimenez MDC, Padilla B, et al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: epidemiology and microbiological characterization of the isolates. PLoS One. (2017) 12:e0179136. doi: 10.1371/journal.pone.0179136
34. Kaneko Y, Oinuma KI, Terachi T, Arimura Y, Niki M, Yamada K, et al. Successful treatment of intestinal mycosis caused by a simultaneous infection with Lichtheimia ramosa and Aspergillus calidoustus. Intern Med. (2018) 57:2421–4. doi: 10.2169/internalmedicine.0254-17
35. Geng C, Lv X, Li J, Jiang Q, Yang R, Zhan P. Chronic subcutaneous infection due to Lichtheimia ramosa. J Eur Acad Dermatol Venereol. (2019) 33:e26–9. doi: 10.1111/jdv.15137
36. Spithoven EM, Bruns AHW, Petri BJ, Haas PJ, Nguyen TQ, Hagen F, et al. Renal transplant patient survives a donor-derived abdominal invasive mucormycosis (Lichtheimia ramos a). Med Mycol Case Rep. (2020) 30:39–42. doi: 10.1016/j.mmcr.2020.10.002
37. La Via WV, Dalai S, de Vries CR, Macintyre A, Ahmed AA. 390. Non-invasive detection of co-infections in hospitalized patients with COVID-19 using the Karius test, a plasma-based next-generation sequencing test for microbial cell-free DNA, p S264. In (ed), Oxford University Press.
38. Liu Y, Zhang J, Han B, Du L, Shi Z, Wang C, et al. Case report: diagnostic value of metagenomics next generation sequencing in intracranial infection caused by Mucor. Front Med (Lausanne). (2021) 8:682758. doi: 10.3389/fmed.2021.682758
39. Colman S, Giusiano G, Colman C, Sosa M, Rojas F. Hepatic failure and malnutrition as predisposing factors of cutaneous mucormycosis in a pediatric patient. Med Mycol Case Rep. (2022) 35:26–9. doi: 10.1016/j.mmcr.2021.12.005
40. Buil JB, van Zanten ARH, Bentvelsen RG, Rijpstra TA, Goorhuis B, van der Voort S, et al. Case series of four secondary mucormycosis infections in COVID-19 patients, The Netherlands, December 2020 to May 2021. Euro Surveill. (2021) 26(23):pii = 2100510. doi: 10.2807/1560-7917.ES.2021.26.23.2100510
41. Arana C, Cuevas Ramirez RE, Xipell M, Casals J, Moreno A, Herrera S, et al. Mucormycosis associated with COVID-19 in two kidney transplant patients. Transpl Infect Dis. (2021) 23:e13652. doi: 10.1111/tid.13652
42. Hassan MIA, Cseresnyes Z, Al-Zaben N, Dahse HM, Vilela de Oliveira RJ, Walther G, et al. The geographical region of origin determines the phagocytic vulnerability of Lichtheimia strains. Environ Microbiol. (2019) 21:4563–81. doi: 10.1111/1462-2920.14752
43. Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. (2020) 185:599–606. doi: 10.1007/s11046-020-00462-9
44. Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J Ophthalmol. (2021) 69:1563–8. doi: 10.4103/ijo.IJO_310_21
45. Guinea J, Escribano P, Vena A, Muñoz P, Martínez-Jiménez MDC, Padilla B, et al. Correction: Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: epidemiology and microbiological characterization of the isolates. PLoS One. (2020) 15:e0229347. doi: 10.1371/journal.pone.0229347
46. Pan J, Tsui C, Li M, Xiao K, de Hoog GS, Verweij PE, et al. First case of rhinocerebral mucormycosis caused by lichtheimia ornata, with a review of lichtheimia infections. Mycopathologia. (2020) 185:555–67. doi: 10.1007/s11046-020-00451-y
47. Colon NC, Chung DH. Neuroblastoma. Adv Pediatr. (2011) 58:297–311. doi: 10.1016/j.yapd.2011.03.011
48. Garcia-Hermoso D, Hoinard D, Gantier JC, Grenouillet F, Dromer F, Dannaoui E. Molecular and phenotypic evaluation of Lichtheimia corymbifera (formerly Absidia corymbifera) complex isolates associated with human mucormycosis: rehabilitation of L. ramosa. J Clin Microbiol. (2009) 47:3862–70. doi: 10.1128/JCM.02094-08
49. Miller S, Chiu C, Rodino KG, Miller MB. Point-counterpoint: should we be performing metagenomic next-generation sequencing for infectious disease diagnosis in the clinical laboratory? J Clin Microbiol. (2020) 58:e01739–19. doi: 10.1128/jcm.01739-19
50. Schneidawind D, Nann D, Vogel W, Faul C, Fend F, Horger M, et al. Allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia and pulmonary mucormycosis. Transpl Infect Dis. (2012) 14:E166–72. doi: 10.1111/tid.12019
51. Serio B, Rosamilio R, Giudice V, Zeppa P, Esposito S, Fontana R, et al. Successful management of pulmonary mucormycosis with liposomal amphotericin B and surgery treatment: a case report. Infez Med. (2012) 20(Suppl 2):43–7.23042005
Keywords: Lichtheimia ramosa, mucormycosis, pediatric, neuroblastoma, mNGS (metagenomic next-generation sequencing)
Citation: Shen H, Cai X, Liu J, Yan G, Ye Y, Dong R, Wu J, Li L, Shen Q, Ma Y, Ou Q, Shen M, Chen W and Lu G (2023) Case report: The clinical utility of metagenomic next-generation sequencing in mucormycosis diagnosis caused by fatal Lichtheimia ramosa infection in pediatric neuroblastoma. Front. Pediatr. 11:1130775. doi: 10.3389/fped.2023.1130775
Received: 18 January 2023; Accepted: 30 May 2023;
Published: 19 June 2023.
Edited by:
Ramos Amador Jose T, Complutense University of Madrid, SpainReviewed by:
Fabianne Carlesse, University of São Paulo, BrazilKazem Ahmadikia, Tehran University of Medical Sciences, Iran
© 2023 Shen, Cai, Liu, Yan, Ye, Dong, Wu, Li, Shen, Ma, Ou, Shen, Chen and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoping Lu MTM3ODg5MDQxNTBAMTYzLmNvbQ== Weiming Chen UG9sYXJpc2NoZW4yMDEwQDE2My5jb20=
†These authors have contributed equally to this work
 Huili Shen
Huili Shen Xiaodi Cai1,†
Xiaodi Cai1,† Jing Liu
Jing Liu Gangfeng Yan
Gangfeng Yan Rui Dong
Rui Dong Quanli Shen
Quanli Shen Yutong Ma
Yutong Ma Qiuxiang Ou
Qiuxiang Ou Meili Shen
Meili Shen Weiming Chen
Weiming Chen Guoping Lu
Guoping Lu