
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 12 April 2023
Sec. Pediatric Critical Care
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1129156
This article is part of the Research Topic Insights in Pediatric Critical Care 2022 View all 8 articles
Purpose: We aimed to explore the relationship between serum phosphate concentration and 90-day mortality in critically ill children receiving continuous renal replacement therapy (CRRT).
Methods: Data from the medical records of children aged <13 years who received CRRT at the Pediatric Intensive Care Unit of Hunan Children's Hospital, China from January 2015 to June 2020 were retrospectively collected. Children were grouped into four categories according to the baseline phosphate concentration before CRRT and mean serum phosphate concentration during CRRT: <0.81 mmol/L (hypophosphatemia), 0.81–1.19 mmol/L, 1.2–2.4 mmol/L (normal phosphate concentration), and >2.4 mmol/L (hyperphosphatemia), with the normal phosphate group serving as the comparator group. The correlation of the serum phosphate concentration before and during CRRT with the 90-day mortality after CRRT initiation was analyzed using logistic regression.
Results: A total of 177 children were included in our study. The mean serum phosphate concentration before CRRT was 1.46 mmol/L (quartiles: 1.04, 2.20). The 90-day mortality rate was increased in children with a serum phosphate concentration >2.4 mmol/L before CRRT (adjusted odds ratio [aOR] 3.74, 95% confidence interval [CI] 1.42–9.86, P = 0.008). The mean serum phosphate concentration during CRRT was 1.2 mmol/L (quartiles: 0.91, 1.49). The 90-day mortality rate was increased in children with a mean serum phosphate concentration >2.4 mmol/L during CRRT (aOR 7.34, 95% CI 1.59–33.88, P = 0.011).
Conclusion: Hyperphosphatemia before and during CRRT predicts a higher 90-day mortality rate.
Although serum phosphate accounts for only approximately 1% of phosphorus in the body, it plays an important role in energy metabolism, bone metabolism, cellular signal transduction, and oxygen transport (1–3). Currently, the generally accepted normal range of serum phosphate in adults is 0.8–1.5 mmol/L (2.5–4.5 mg/dl) (4). Phosphate homeostasis is a complex process, and its abnormalities can cause dysfunction of multiple organ systems, including the cardiovascular, respiratory, immune, neuromuscular, and hematologic systems (5).
Phosphate concentration is typically abnormal in critically ill patients. There have been an increasing number of studies on the relationship between phosphate abnormalities and clinical outcomes (6–9). A recent large-scale meta-analysis showed that hyperphosphatemia is associated with all-cause mortality in critically ill patients (10). Additionally, some studies have shown that hypophosphatemia is an independent risk factor for morbidity and mortality (11, 12), whereas other reports have shown no significant association with either (6, 13). Therefore, it remains unclear whether an abnormal serum phosphate concentration is directly associated with increased mortality or is merely a marker of disease severity, in the context of intensive care unit (ICU) patients.
Phosphate, a small inorganic solute, is removed through diffusion or convection during continuous renal replacement therapy (CRRT). Patients receiving CRRT have an increased risk of developing hypophosphatemia, and this has been associated with poor prognosis (4, 14). Patients may require phosphate supplementation after experiencing hypophosphatemia; however, inappropriate supplementation may trigger hyperphosphatemia. Furthermore, large magnitude changes in the serum phosphate concentration have been associated with an increased in-hospital mortality rate (9). One of the goals of CRRT is correcting electrolyte disturbances; nevertheless, in some patients, phosphate abnormalities persist during CRRT. Patients requiring CRRT are often the most severely ill patients in the ICU—the mortality rate of these patients in the pediatric ICU (PICU) is more than eight times that of the total PICU population (15). Currently, only a limited number of studies have probed the effect of serum phosphate concentration on patient mortality in CRRT cases. In particular, there is no information available regarding the relationship between serum phosphate concentrations (before and during CRRT) and mortality in children. Therefore, this study aimed to assess the relationship between serum phosphate concentration before and after CRRT initiation with the 90-day mortality rate in critically ill children.
Data from the medical records of children who underwent CRRT in the Department of Critical Care Medicine at Hunan Children's Hospital from January 2015 to June 2020 were retrospectively collected. The normal serum phosphate concentration of children fluctuates slightly with age, whereas the reference value for children aged >13 years fluctuates greatly. Therefore, to facilitate group statistics, children >13 years of age were excluded from this study (4, 16). The inclusion criteria were as follows: (1) serum phosphate test results available within 24 h prior to CRRT initiation; (2) at least one serum phosphate test result during CRRT; and (3) a CRRT duration ≥24 h. The exclusion criteria were as follows: (1) mortality within 48 h of CRRT initiation; (2) oncologic disease; and (3) chronic kidney disease (17). If a child received multiple courses of CRRT while in the PICU, only the first course was included in our analysis.
This study was conducted with approval from the Ethics Committee of Hunan Children's Hospital (Approval Number: HCHLL-2022-150). This was a retrospective observational study; data were acquired from the electronic case records database; this research did not involve aspects related to patient privacy; and there was no commercial interest involved. Thus, the ethics committee waived the requirement for informed patient consent.
The equipment used for CRRT at our center was the multiFiltrate system (Fresenius Medical Care, Bad Homburg, Germany) or PRISMAflex System (Gambro, Lund, Sweden), including the ancillary tubing and filters. For vascular access, a double-lumen central venous catheter was inserted in the femoral or internal jugular vein. The treatment mode was continuous veno-venous hemodialysis or hemodiafiltration, heparin or sodium citrate was the anticoagulation agent, and phosphate-free replacement and dialysis fluids were used. Electrolytes were routinely monitored, every 6–8 h, and vital signs, hemodynamics, and coagulation were closely monitored throughout the CRRT treatment process. CRRT treatment parameters were titrated, as needed.
Clinical and biochemical parameters that were recorded included sex, age (months), weight, Pediatric Critical Illness Score (18), whether or not mechanical ventilation was used, whether or not vasopressors were used, serum creatinine, albumin, sodium, potassium, and calcium concentrations, blood pH, and the blood platelet count.
The main predictive factors in this study were as follows: (1) serum phosphate concentration before CRRT initiation and (2) mean serum phosphate concentration during CRRT. The serum phosphate concentration before CRRT initiation was defined as the test result closest and within 24 h prior to CRRT initiation. The primary outcome was the 90-day mortality rate after CRRT initiation.
The range of normal serum phosphate concentration in children under 13 years of age fluctuated slightly with age, and the range from the lower limit of reference value 1.2 mmol/L (3.7 mg/dl) to the upper limit 2.4 mmol/L (7.4 mg/dl) was considered the range of normal serum phosphate concentration in these children (16, 19). Currently, there is no unified reference value for hypophosphatemia in children (8). In adult studies, hypophosphatemia has been defined as a serum phosphate concentration < 0.81 mmol/L (2.5 mg/dl) (4). Therefore, in this study, we have also used serum phosphate concentration < 0.81 mmol/L to define hypophosphatemia. Furthermore, there is also no clear standard for hyperphosphatemia. Nevertheless, we defined hyperphosphatemia as a serum phosphate concentration > 2.4 mmol/L.
When the serum phosphate concentration was <0.81 mmol/L, 0.5–1 ml/kg/session sodium glycerophosphate was empirically administered via slow intravenous infusion, with a maximum of 10 ml/session, and the decision to reintroduce intravenous phosphate was made according to the serum phosphate concentration.
Participants were divided into four groups according to their serum phosphate concentration: <0.81 mmol/L, 0.81–1.19 mmol/L, 1.2–2.4 mmol/L, and >2.4 mmol/L, with the 1.2–2.4 mmol/L group—the normal range for serum phosphate in children—set as the comparator group.
Data were analyzed using IBM SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY). Data with a normal distribution are expressed as mean ± standard deviation (), and analysis of variance was used for comparisons between groups. Count data are expressed as number of cases (percentage), and comparisons were performed using the test. Measurement data with a non-normal distribution are expressed as median (quartiles) [M(P25, P75)], and the Kruskal–Wallis H test was used for comparisons between groups.
Logistic regression was used to analyze the odds ratios (OR) of the relationship between the serum phosphate concentration and the 90-day mortality rate. Confounding factors were controlled by adjusting for clinically relevant variables, including sex, age (in months), weight, Pediatric Critical Illness Score, whether or not mechanical ventilation was used, whether or not vasopressors were used, serum creatinine, albumin, sodium, potassium, and calcium concentrations, blood pH, and blood platelet count. Covariables were selected by force entry. Four-node restricted cubic splines were constructed to study the nonlinear relationship of the serum phosphate concentration before (baseline) and during CRRT with the 90-day mortality rate.
Differences were considered statistically significant at P < 0.05. Regression analyses and model constructions were performed using R-studio, version 4.2.1 (Integrated Development for R. RStudio, Inc., Boston, Massachusetts, USA); glm was used for the logistic regression, and rms was used for the restricted cubic spline curves.
In total, 177 children were included in this study; details are shown in Figure 1. The male-to-female ratio was 112/65 (males, 63.3%). The median age was 27 months (quartiles: 9, 67), and the median weight was 12.4 kg (quartiles: 8, 20). The most common indication for CRRT was sepsis (35.6%). The median Pediatric Critical Illness Score was 78 (quartiles: 74, 80); 45.8% of participants (n = 81) required mechanical ventilation and 13.6% (n = 24) required vasopressors (Table 1).
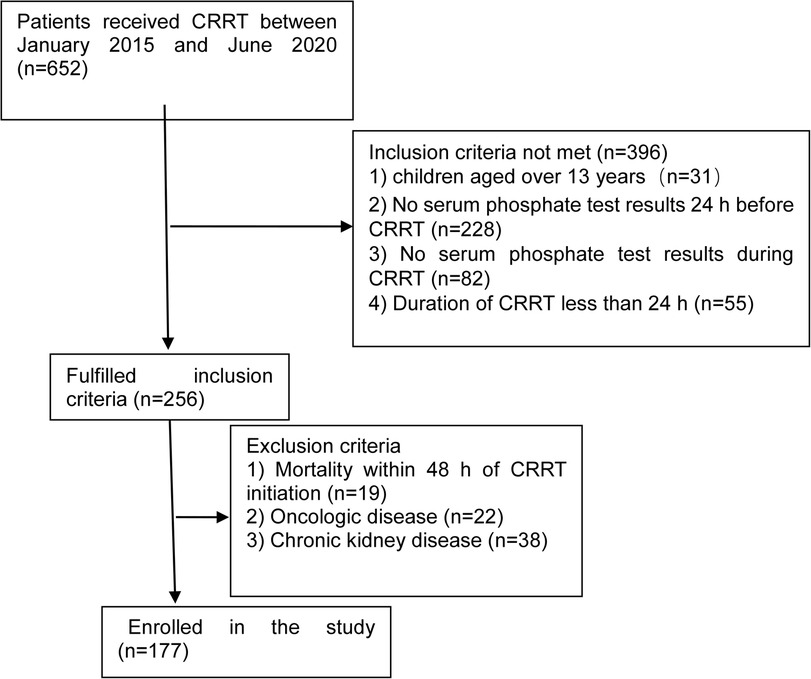
Figure 1. Flow chart showing participant categorization and interventions for this study. CRRT, continuous renal replacement therapy.
The mean serum phosphate concentration before CRRT initiation was 1.46 mmol/L (quartiles: 1.04, 2.20). The 90-day mortality rates of children with serum phosphate concentrations <0.81, 0.81–1.19, 1.2–2.4, and >2.4 mmol/L before CRRT were 50%, 35.7%, 26.6%, and 55.3%, respectively. Multivariate logistic regression analysis showed a higher 90-day mortality rate in children with a serum phosphate concentration >2.4 mmol/L before CRRT than in children with a serum phosphate concentration of 1.2–2.4 mmol/L [adjusted OR 3.74, confidence interval (CI) 1.42–9.86, P = 0.008]. Serum phosphate concentrations <0.81 and 0.81–1.19 mmol/L were not associated with significant increases in the 90-day mortality rate (Table 2). Figure 2 shows restricted cubic splines for the adjusted OR of the serum phosphate concentration before CRRT and the corresponding 90-day mortality rates.
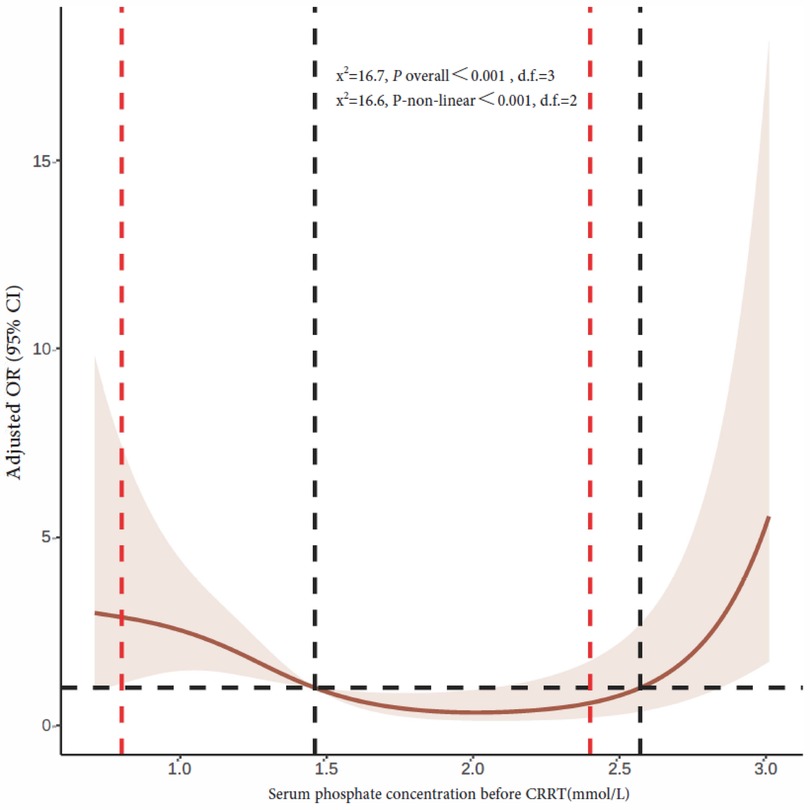
Figure 2. Restricted cubic splines of the correlation between serum phosphate concentration and 90-day mortality rate prior to CRRT initiation. The dotted red line on the left indicates hypophosphatemia cutoff, while the dotted red line on the right indicates hyperphosphatemia cutoff. The horizontal black dashed line represents OR = 1, and the vertical black dashed line is the serum phosphate concentration at the point where the curve intersects with OR value 1 that are 1.46 and 2.57 mmol/L, respectively. CRRT, continuous renal replacement therapy; OR, odds ratio; CI, confidence interval.
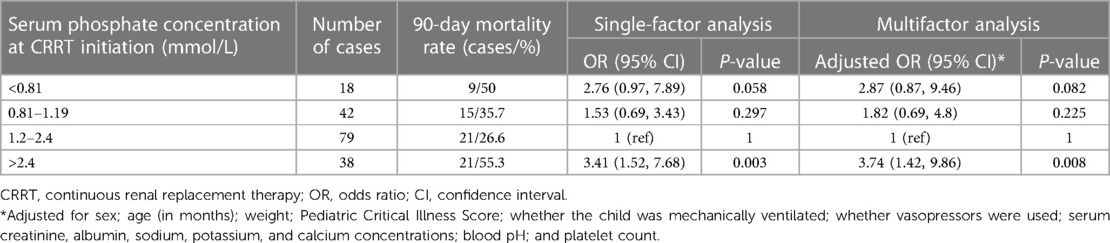
Table 2. Relationship between serum phosphate concentration and 90-day mortality rate before CRRT initiation.
The mean serum phosphate concentration during CRRT was 1.2 mmol/L (quartiles: 0.91, 1.49). The 90-day mortality rates of children with a mean serum phosphate concentration <0.81, 0.81–1.19, 1.2–2.4, and >2.4 mmol/L during CRRT were 34.5%, 32.2%, 33.3%, and 63.6%, respectively. Multivariate logistic regression analysis showed a higher 90-day mortality rate in children with a mean serum phosphate concentration >2.4 mmol/L during CRRT than in children with a serum phosphate concentration of 1.2–2.4 mmol/L (adjusted OR 7.34, 95% CI 1.59–33.88, P = 0.011). Hypophosphatemia during CRRT was not significantly correlated with mortality (Table 3). Figure 3 shows restricted cubic splines for the adjusted OR of the mean serum phosphate concentration during CRRT and the corresponding 90-day mortality rates.
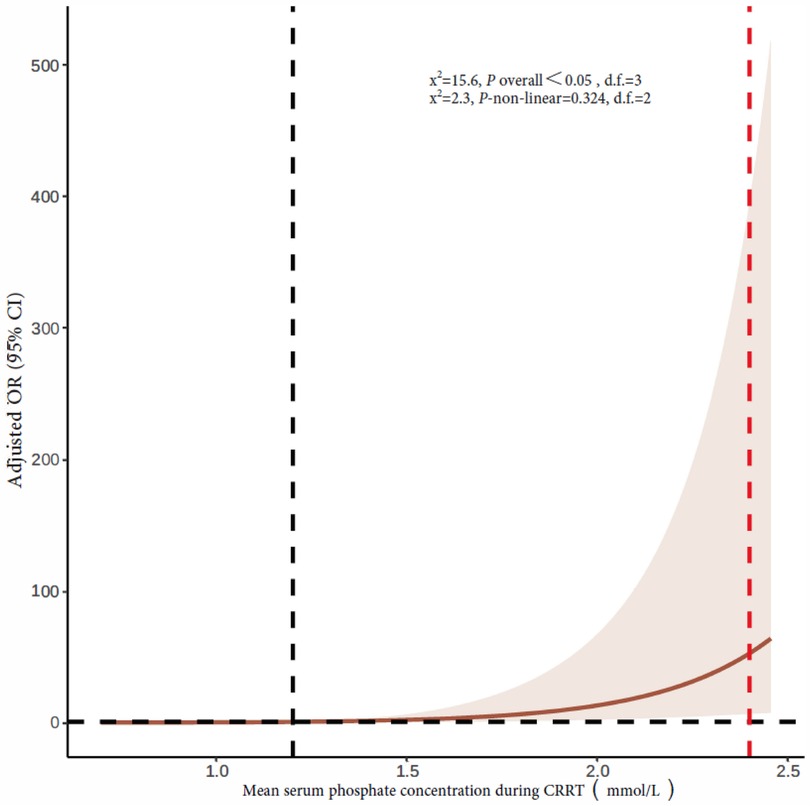
Figure 3. Restricted cubic splines of the association between mean serum phosphate concentration during CRRT and 90-day mortality rate. The vertical dotted red line indicates hyperphosphatemia cutoff, and the dotted black line indicates an OR of 1. CRRT, continuous renal replacement therapy; OR, odds ratio; CI, confidence interval.
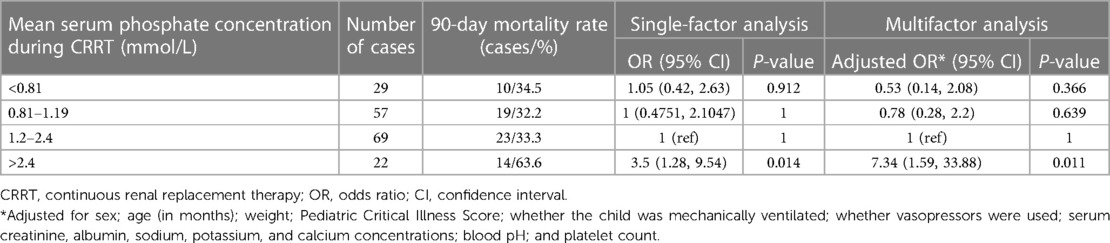
Table 3. Relationship between mean serum phosphate concentration during CRRT and 90-day mortality rate.
Phosphate, the most abundant intracellular anion in the body, is an important component of multiple physiological processes affecting many different organ systems. Some studies have correlated phosphate metabolism disturbances with prognosis in critically ill patients (6, 7, 10, 11, 20). However, few studies have focused on the relationship between serum phosphate concentration and clinical outcomes in patients receiving CRRT in the ICU (11, 21–24). In particular, the relationship between serum phosphate concentration and mortality in critically ill children undergoing CRRT in the PICU has not been reported. To our knowledge, this is the first cohort study to address this relationship.
A recent study by Thongprayoon et al. (21), conducted among adult ICU patients, showed that hyperphosphatemia was more common than hypo- and normophosphatemia before undergoing CRRT, patients with hyperphosphatemia could maintain normal serum phosphate levels during CRRT, and hyper- and hypophosphatemia before and during CRRT predicted a higher 90-day mortality rate. In our study, the baseline serum phosphate concentration before CRRT was usually within the normal range. Importantly, the included patients were from different types of ICUs, and patients in the hyperphosphatemia group had higher serum creatinine concentrations and lower glomerular filtration rates. However, in our study, patients were predominantly from the PICU, and there was no significant difference in the creatinine concentration before CRRT initiation between groups with different serum phosphate concentration ranges. The different sources of patients may explain the varied distribution of serum phosphate concentration in the Thongprayoon et al. study. In our study, patients with hyperphosphatemia before CRRT had a higher 90-day mortality rate than those with hypo- and normophosphatemia. Wang et al. (22) conducted a study among 796 patients with sepsis receiving CRRT and confirmed that when the serum phosphate concentration before CRRT was 5.6–8.7 mg/dl (1.8–2.8 mmol/L), patients had a significantly increased risk of 28-day mortality. Similar results were obtained in an observational study conducted among 1,144 critically ill patients with acute kidney injury who underwent CRRT (23); this study revealed that hyperphosphatemia during CRRT initiation was significantly associated with increased 28-day [hazard ratio (HR) 1.05, 95% CI 1.02–1.08, P = 0.001] and 90-day (HR 1.05, 95% CI 1.02–1.08, P = 0.001) mortality rates. In an earlier study, Thongprayoon et al. (21) also reported that hypophosphatemia before CRRT was associated with higher mortality rates. However, we have not observed an association of hypophosphatemia before CRRT with the 90-day mortality rate in our present study, and this is consistent with the results of two similar studies (23, 24); nevertheless, one of these studies has suggested that hypophosphatemia may prolong the length of stay in ICU for patients receiving CRRT (25). The relationship between hypophosphatemia before CRRT and prognosis varies between studies, and this variability may stem from differences in the study population, sample size, timing of CRRT intervention, and disease severity.
The present study found that the 90-day mortality rate increased when the mean serum phosphate concentration during CRRT was >2.4 mmol/L (adjusted OR 7.34, 95% CI 1.59–33.88, P = 0.011). This finding was consistent with those of several recent studies conducted among adults which have reported that hyperphosphatemia during CRRT predicts a higher risk of mortality (21–23). Wang et al. (22) also found that when the phosphate concentration was >3.8 mg/dl (1.2 mmol/L) 24 h after CRRT initiation, each 1 mg/dl increase raised the risk of 28-day mortality by 23% (adjusted OR 1.23, 95% CI 1.15–1.33). Patients with an elevated phosphate concentration during CRRT had significantly shorter survival times. Compared with patients with low phosphate concentrations, those with elevated phosphate concentrations had a 1.51-fold (95% CI 1.24–1.86, P < 0.001) and 1.50-fold (95% CI 1.24–1.82, P < 0.001) increased risk of 28-day and 90-day mortality, respectively (23).
Elevated phosphate concentrations increase fibroblast growth factor 23 (FGF23) levels, and this directly induces the in vitro hypertrophic growth of cardiomyocytes. High FGF23 concentrations are associated with left ventricular hypertrophy and cardiovascular toxicity, increasing the risk of adverse cardiovascular outcomes and ultimately leading to poor prognosis (26–28). Hyperphosphatemia causes vascular calcification via the downregulation of transcription factor EB in vascular smooth muscle cells. Meanwhile, microparticles from hyperphosphatemia-stimulated endothelial cells promote vascular calcification through astrocyte-elevated gene-1, and vascular calcification leads to a higher mortality rate (29, 30).
This study has some limitations. First, it is a single-center retrospective observational study, and there may be a potential for bias as well as confounding factors that could have affected our interpretation of the results. Second, the data were captured from an electronic medical records database and the system neither archived information on the causes of phosphate disturbances at baseline and during CRRT, nor denoted the causes of mortality. Therefore, the causal relationship between serum phosphate concentration and mortality remains unclear, and it is not possible to ascertain whether patient mortality was due to hyperphosphatemia or other underlying causes. Third, the efficacy of CRRT in phosphate removal varies with the effluent dose (24). We did not assess the actual effluent dose achieved during CRRT nor the effect of phosphate changes on mortality. Fourth, patients without baseline phosphate test results, those with undetectable phosphate levels during CRRT, and children older than 13 years were excluded—this may have potentially biased the results. Fifth, in our study, serum creatinine levels were included in the multifactorial analysis, while urine volume data were not collected or included in the analysis. Serum creatinine levels and urine volume have been reported to correlate with the severity of acute kidney injury, while serum phosphate levels have been shown to be dependent on renal function (31). Children in our study were grouped according to different phosphate levels, and there was no significant difference in baseline creatinine levels between the groups (P > 0.05). However, urine volume was not included in the multivariate analysis to take into account the effect of pRIFLE score on results, and this is indeed a limitation. In addition, according to standard practice, the ratio of the population to variables should be 10:1. However, in the present study, we did not select covariables through single-factor analysis, and all clinically relevant variables were included: this is a potential limitation of this study.
Phosphate is a potential biomarker of disease severity in critically ill patients receiving CRRT and is associated with a high mortality rate. Early identification and treatment of hyperphosphatemia is therefore important in patients receiving CRRT in the ICU. In future, we plan to conduct a randomized controlled trial that incorporates the phosphate concentration into the risk stratification and explores whether reducing the phosphate concentration, such as by CRRT, may reduce mortality risk in patients with hyperphosphatemia.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee Hunan Children's Hospital. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
XZ: research design and manuscript writing; DZ: data organization and analysis; ZY: research guidance and data analysis; DP: data organization and analysis; JH: research design and guidance; XZ: research design, guidance, and paper revision. All authors contributed to the article and approved the submitted version.
This work was funded by the Scientific Research Plan Project of Hunan Provincial Health Commission (Project Number: C202306018490) and the Science and Technology Innovation Plan Project of Hunan Province (Project Numbers: 2020SK50509 and 2021SK50518).
We would like to thank all the hemodialysis specialist nurses of the ICU at Hunan Children's Hospital, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
aOR, adjusted odds ratio; CI, confidence interval; CRRT, continuous renal replacement therapy; ICU, intensive care unit; OR, odds ratio; PICU, pediatric ICU
1. Hernando N, Wagner CA. Mechanisms and regulation of intestinal phosphate absorption. Compr Physiol. (2018) 8:1065–90. doi: 10.1002/cphy.c170024
2. Broman M, Wilsson AM, Hansson F, Klarin B. Analysis of hypo- and hyperphosphatemia in an intensive care unit cohort. Anesth Analg. (2017) 124:1897–905. doi: 10.1213/ANE.0000000000002077
3. Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultz MJ. Treatment of hypophosphatemia in the intensive care unit: a review. Crit Care. (2010) 14:1–8. doi: 10.1186/cc9215
4. Thompson BM, Adams PM, Nerusu S, Morris PE, Mayer KP, Neyra JA. Association of phosphate containing solutions with incident hypophosphatemia in critically ill patients requiring continuous renal replacement therapy. Blood Purif. (2022) 51:122–9. doi: 10.1159/000514418
5. Shore RM. Disorders of phosphate homeostasis in children, part 1: primer on mineral ion homeostasis and the roles of phosphate in skeletal biology. Pediatr Radiol. (2022) 52:2278–89. doi: 10.1007/s00247-022-05374-y
6. Chen Y, Luo M, Xu H, Zhao W, He Q. Association between serum phosphate and mortality in critically ill patients: a large retrospective cohort study. BMJ Open. (2021) 11:e44473. doi: 10.1136/bmjopen-2020-044473
7. Sin JC, King L, Ballard E, Llewellyn S, Laupland KB, Tabah A. Hypophosphatemia and outcomes in ICU: a systematic review and meta-analysis. J Intensive Care Med. (2021) 36:1025–35. doi: 10.1177/0885066620940274
8. Reintam BA, Gunst J, Ichai C, Casaer MP, Benstoem C, Besch G, et al. Hypophosphatemia in critically ill adults and children—a systematic review. Clin Nutr. (2021) 40:1744–54. doi: 10.1016/j.clnu.2020.09.045
9. Thongprayoon C, Cheungpasitporn W, Hansrivijit P, Thirunavukkarasu S, Chewcharat A, Medaura J, et al. Impact of serum phosphate changes on in-hospital mortality. BMC Nephrol. (2020) 21:1–7. doi: 10.1186/s12882-020-02090-3
10. Zheng WH, Yao Y, Zhou H, Xu Y, Huang HB. Hyperphosphatemia and outcomes in critically ill patients: a systematic review and meta-analysis. Front Med. (2022) 9:870637. doi: 10.3389/fmed.2022.870637
11. Wang L, Xiao C, Chen L, Zhang X, Kou Q. Impact of hypophosphatemia on outcome of patients in intensive care unit: a retrospective cohort study. BMC Anesthesiol. (2019) 19:1–7. doi: 10.1186/s12871-018-0673-7
12. Shazly AN E, Soliman DR, Assar EH, Behiry EG, Ahmed IA. Phosphate disturbance in critically ill children: incidence, associated risk factors and clinical outcomes. Ann Med Surg. (2017) 21:118–23. doi: 10.1016/j.amsu.2017.07.079
13. Federspiel CK, Itenov TS, Thormar K, Liu KD, Bestle MH. Hypophosphatemia and duration of respiratory failure and mortality in critically ill patients. Acta Anaesthesiol Scand. (2018) 62:1098–104. doi: 10.1111/aas.13136
14. Song YH, Seo EH, Yoo YS, Jo YI. Phosphate supplementation for hypophosphatemia during continuous renal replacement therapy in adults. Ren Fail. (2019) 41:72–9. doi: 10.1080/0886022X.2018.1561374
15. Miklaszewska M, Korohoda P, Zachwieja K, Sobczak A, Kobylarz K, Stefanidis CJ, et al. Factors affecting mortality in children requiring continuous renal replacement therapy in pediatric intensive care unit. Adv Clin Exp Med. (2019) 28:615–23. doi: 10.17219/acem/81051
16. Bacchetta J, Bernardor J, Garnier C, Naud C, Ranchin B. Hyperphosphatemia and chronic kidney disease: a major daily concern both in adults and in children. Calcif Tissue Int. (2021) 108:116–27. doi: 10.1007/s00223-020-00665-8
17. Stevens PE, Levin A. Kidney disease: improving global outcomes chronic kidney disease guideline development work group members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. (2013) 158:825–30. doi: 10.7326/0003-4819-158-11-201306040-00007
18. Chinese Medical Association Pediatrics Association Emergency Medicine Education Group. Memo of the 4th national pediatric emergency medicine symposium. Chinese J Pediatr. (1995) 33:370–3.
19. KDOQI Work Group. KDOQI Clinical practice guideline for nutrition in children with CKD: 2008 update. Executive summary. Am J Kidney Dis. (2009) 53:S11–104. doi: 10.1053/j.ajkd.2008.11.017
20. Shore RM. Disorders of phosphate homeostasis in children, part 2: hypophosphatemic and hyperphosphatemic disorders. Pediatr Radiol. (2022) 52:2290–305. doi: 10.1007/s00247-022-05373-z
21. Thongprayoon C, Radhakrishnan Y, Cheungpasitporn W, Petnak T, Qureshi F, Mao MA, et al. Association of serum phosphate derangement with mortality in patients on continuous renal replacement therapy. Can J Kidney Health Dis. (2022) 9:1025069897. doi: 10.1177/20543581221114697
22. Wang H, Bai ZH, Lv JH, Sun JL, Shi Y, Zhang ZL, et al. The relationship and threshold of serum phosphate with regard to the 28-day mortality risk in sepsis patients undergoing continuous renal replacement therapy. J Int Med Res. (2020) 48:0300060519831896. doi: 10.1177/0300060519831896
23. Jung SY, Kwon J, Park S, Jhee JH, Yun HR, Kim HN, et al. Phosphate is a potential biomarker of disease severity and predicts adverse outcomes in acute kidney injury patients undergoing continuous renal replacement therapy. PLoS One. (2018) 13:e191290. doi: 10.1371/journal.pone.0191290. eCollection 2018
24. Kim SY, Kim YN, Shin HS, Jung Y, Rim H. The influence of hypophosphatemia on outcomes of low- and high-intensity continuous renal replacement therapy in critically ill patients with acute kidney injury. Kidney Res Clin Pract. (2017) 36:240–9. doi: 10.23876/j.krcp.2017.36.3.240
25. Hendrix RJ, Hastings MC, Samarin M, Hudson JQ. Predictors of hypophosphatemia and outcomes during continuous renal replacement therapy. Blood Purif. (2020) 49:700–7. doi: 10.1159/000507421
26. Mehta RC, Cho ME, Cai X, Lee J, Chen J, He J, et al. Iron status, fibroblast growth factor 23 and cardiovascular and kidney outcomes in chronic kidney disease. Kidney Int. (2021) 100:1292–302. doi: 10.1016/j.kint.2021.07.013
27. Grund A, Sinha MD, Haffner D, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease—a pediatric perspective. Front Pediatr. (2021) 9:702719. doi: 10.3389/fped.2021.702719
28. Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol. (2014) 10:268–78. doi: 10.1038/nrneph.2014.49
29. Ishiwata R, Morimoto Y. Hyperphosphatemia-induced degradation of transcription factor EB exacerbates vascular calcification. Biochim Biophys Acta Mol Basis Dis. (2022) 1868:166323. doi: 10.1016/j.bbadis.2021.166323
30. Xiang Y, Duan Y, Peng Z, Huang H, Ding W, Chen E, et al. Microparticles from hyperphosphatemia-stimulated endothelial cells promote vascular calcification through astrocyte-elevated gene-1. Calcif Tissue Int. (2022) 111:73–86. doi: 10.1007/s00223-022-00960-6
Keywords: continuous renal replacement therapy, children, phosphate, mortality, pediatric intensive care unit
Citation: Zhou X, He J, Zhu D, Yao Z, Peng D and Zhang X (2023) Relationship between serum phosphate and mortality in critically ill children receiving continuous renal replacement therapy. Front. Pediatr. 11:1129156. doi: 10.3389/fped.2023.1129156
Received: 21 December 2022; Accepted: 29 March 2023;
Published: 12 April 2023.
Edited by:
Nicole Ann Shilkofski, Johns Hopkins University, United StatesReviewed by:
Michael Seifert, University of Alabama at Birmingham, United States© 2023 Zhou, He, Zhu, Yao, Peng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinping Zhang emhhbmd4aW5waW5ncGljdUAxNjMuY29t
Specialty Section: This article was submitted to Pediatric Critical Care, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.