
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Pediatr. , 13 February 2023
Sec. Pediatric Infectious Diseases
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1128126
This article is part of the Research Topic An Update on Pediatric Skeletal System Infections View all 6 articles
Editorial on the Research Topic
An Update on Pediatric Skeletal System Infections
Osteoarticular infections (OAIs) in pediatric patients are medical emergencies that require early diagnosis and adequate treatment to avoid severe septic complications, prolonged morbidity, and long-term functional disability (1). Although traumatic inoculation of bacteria into the joint space and bones or dissemination from a contiguous soft tissue focus may occur, in most cases, pathogens reach the skeletal tissues through the hematogenous route (2). Because the incidence of bacteremia is elevated in immunologically naïve young children, the incidence of septic arthritis and osteomyelitis is higher in early childhood (1).
Early diagnosis of skeletal infections, identification of their etiologic agent, and prompt administration of adequate antimicrobial therapy are cornerstones of managing the disease, avoiding clinical deterioration, and preventing permanent orthopedic sequelae (3). Traditionally, culture isolation of the etiologic agent, its identification, and determination of its antibiotic susceptibility was performed by conventional methods. However, causative organisms may be present in the specimen at low concentrations, difficult to isolate, and/or children may have been empirically administered antibiotics before cultures were obtained. Consequently, many pediatric joint and bone infections remained bacteriologically unconfirmed (4). Because the microbiology laboratory results are obtained with a 2–3-day delay and the danger of administering an inappropriate or poorly efficient antibiotic, the initial therapy of bone and joint infections usually consists of wide-spectrum antimicrobial drugs, including those that provide appropriate coverage of Staphylococcus aureus (3). To ensure therapeutic success, antibiotics are administered through the intravenous route in the early phase and switched to oral therapy after body temperature has normalized for 24 h, local findings and motion have improved, and C-reactive protein (CRP) levels have decreased (1).
Technological progress has revolutionized the epidemiology, diagnosis, and management of pediatric OAI in recent decades, as summarized in Table 1.
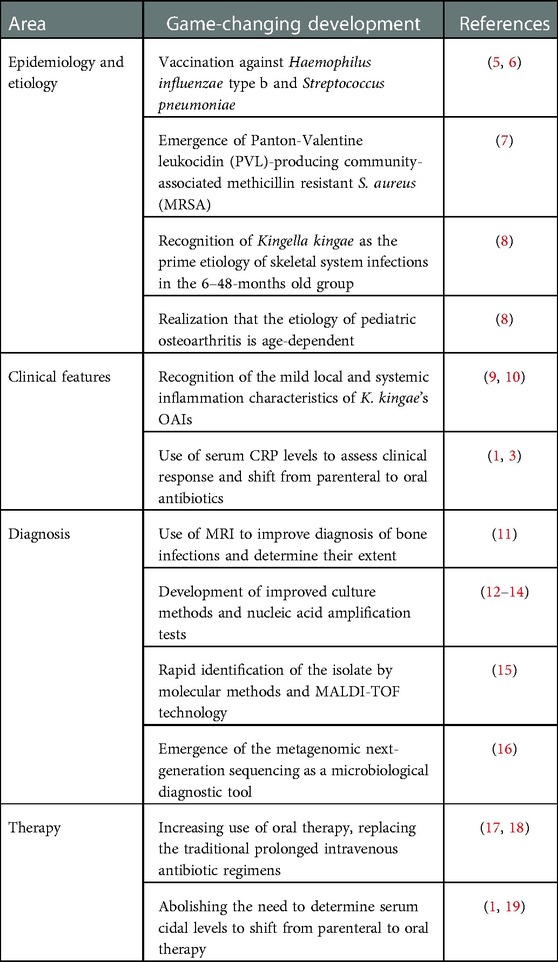
Table 1. Main developments that have changed the management of pediatric skeletal system infections in recent years.
The present Special Issue provides an update on managing these skeletal system infections in children and the areas of controversy and current research to improve the diagnosis, shorten the duration of intravenous therapy, and reduce hospitalization length.
The article by Porsch et al. summarizes our current knowledge of the virulence factors of Kingella kingae. Advances in the culture methods and especially the increasing use of molecular detection methods have resulted in recognition of the bacterium as the prime etiology of hematogenous septic arthritis, osteomyelitis, spondylodiscitis, and tenosynovitis in children aged 6–48 months (8). Intensive research conducted over the last two decades has identified a wide range of bacterial surface factors that play crucial roles in the transition from the colonized oropharyngeal surfaces to the invasion of the skeletal system tissues (20).
The review by Searns et al. covers the highly debated issue of delaying the administration of antimicrobial therapy to children with suspected joint and bone infections. Postponing the initiation of antibiotics enables to obtain of surgical specimens for the identification of the pathogen and determining its antibiotic susceptibility. However, this delay may result in the patient's clinical deterioration. The increasing prevalence of the highly virulent and multiresistant PVL-positive MRSA in many regions requires a clear-cut answer (7). Septic children are at high risk for life-threatening complications and mortality and require immediate initiation of intravenous antibiotics. For well-appearing children, additional evidence is needed to define what period is acceptable to withhold antimicrobial therapy while awaiting surgical, diagnostic procedures.
Alcobendas et al. legitimately question the traditional recommendations of OAI treatment and suggest treating the patient individually, advocating short courses or no intravenous therapy (21). They highlight growing evidence of good outcomes in patients with primary OAI treated with a minimally invasive approach consisting of stricter surgical indications and short courses or no intravenous therapy. They consider that most children with K. kingae OAI do not usually require invasive surgical procedures to achieve clinical improvement. In contrast, invasive surgery remains indicated when causal pathogens are pyogenic and express specific virulence genes. The same concept is thereafter applied to the antibiotic treatment; if some children with severe OAI are more likely to respond better in children who initially received intravenous antibiotics, an exclusively oral administration could be a safe option in patients with OAI caused by K. kingae.
De Marco et al. articulate a full reflection about a better understanding of the etiology of OAIs, which could lead to major changes in their therapeutic management. They underline that it currently needs to be a consensus about the treatment of OAIs, especially about who can be safely treated solely using medicines and who requires and will benefit from a surgical approach. The indications that justify, in their opinion, the performance of a surgical procedure are reviewed. In the authors’ opinion, there are three basic indications for surgical procedures during pediatric OAIs: obtain a bacteriological diagnosis, ensure control of the infectious source, and preserve the maximal function of the affected bone segment. They advise therapeutic management according to the causal germs, and surgery remains essential when pyogenic bacteria are incriminated.
Finally, Moez et al. propose a new strategy for investigating spondylodiscitis in children younger than 4 years. The new diagnostic approach is based on the premise that detecting K. kingae RTX toxin genes in the oropharynx by swab real-time PCR assay provides strong evidence that this microorganism is responsible for the patient's OAI (22). This study demonstrated that K. kingae's RTX toxin operon is detected on the oropharynx of approximately 90% of toddlers with confirmed spondylodiscitis. These results provided robust arguments that K. kingae should be considered as the primary etiology of spinal infections in children aged 6–48 months. The oropharyngeal swab PCR assay could become an early decision-making tool for indirectly identifying the etiology of spondylodiscitis where needle aspiration or open biopsy cannot be regarded as desirable diagnostic procedures due to their poor performance and surgical/anesthetic risks.
We want to thank all the authors and reviewers for their valuable time and contributions that made this publication possible.
PY has described the recent developments in the epidemiology, clinical presentation, diagnosis, and therapy of pediatric osteoarthritis. DC has summarized the contributions of the individual papers. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1128126/full#supplementary-material.
1. Pääkkönen M, Peltola H. Simplifying the treatment of acute bacterial bone and joint infections in children. Expert Rev Anti Infect Ther. (2011) 9:1125–31. doi: 10.1586/eri.11.140
2. Trujillo M, Nelson JD. Suppurative and reactive arthritis in children. Semin Pediatr Infect Dis. (1997) 8:242–9. doi: 10.1016/S1045-1870(97)80018-0
3. Pääkkönen M, Peltola H. Bone and joint infections. Pediatr Clin North Am. (2013) 60:425–36. doi: 10.1016/j.pcl.2012.12.006
4. Spyridakis E, Jeffrey S, Gerber JS, Schriver E, Grundmeier RW, Porsch EA, et al. Clinical features and outcomes of children with culture-negative septic arthritis. J Pediatic Infect Dis Soc. (2019) 8:228–34. doi: 10.1093/jpids/piy034
5. Olarte L, Romerom J, Barson W, Bradley J, Lin PL, Givner L, et al. Osteoarticular infections caused by Streptococcus pneumoniae in children in the post-pneumococcal conjugate vaccine era. Pediatr Infect Dis J. (2017) 36:1201–4. doi: 10.1097/INF.0000000000001697
6. Howard AW, Viskontas D, Sabbagh C. Reduction in osteomyelitis and septic arthritis related to Haemophilus influenzae type b vaccination. J Pediatr Orthop. (1999) 19:705–9.10573336
7. Vardakas KZ, Kontopidis I, Gkegkes ID, Rafailidis PI, Falagas ME. Incidence, characteristics, and outcome of patients with bone and joint infections due to community-associated methicillin-resistant Staphylococcus aureus: a systematic review. Eur J Clin Microbiol Infect Dis. (2013) 32:711–21. doi: 10.1007/s10096-012-1807-3
8. Juchler C, Spyropoulou V, Wagner N, Merlini L, Dhouib A, Manzano S, et al. The contemporary bacteriologic epidemiology of osteoarticular infections in children in Switzerland. J Pediatr. (2018) 194:190–96.e1. doi: 10.1016/j.jpeds.2017.11.025
9. Ceroni D, Cherkaoui A, Combescure C, François P, Kaelin A, Schrenzel J. Differentiating osteoarticular infections caused by Kingella kingae from those due to typical pathogens in young children. Pediatr Infect Dis J. (2011) 30:906–9. doi: 10.1097/INF.0b013e31821c3aee
10. Basmaci R, Lorrot M, Bidet P, Doit C, Vitoux C, Penneçot G, et al. Comparison of clinical and biologic features of Kingella kingae and Staphylococcus aureus arthritis at initial evaluation. Pediatr Infect Dis J. (2011) 30:902–4. doi: 10.1097/INF.0b013e31821fe0f7
11. Woods CR, Bradley JS, Chatterjee A, Copley LA, Robinson J, Kronman MP, et al. Clinical practice guideline by the pediatric infectious diseases society and the infectious diseases society of America: 2021 guideline on diagnosis and management of acute hematogenous osteomyelitis in pediatrics. J Pediatr Infect Dis Soc. (2021) 10:801–44. doi: 10.1093/jpids/piab027
12. Hughes JG, Vetter EA, Patel R, Schleck CD, Harmsen S, Turgeant LT, et al. Culture with BACTEC peds plus/F bottle compared with conventional methods for detection of bacteria in synovial fluid. J Clin Microbiol. (2001) 39:4468–71. doi: 10.1128/JCM.39.12.4468-4471.2001
13. Chometon S, Benito Y, Chaker M, Boisset S, Ploton C, Bérard J, et al. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J. (2007) 26:377–81. doi: 10.1097/01.inf.0000259954.88139.f4
14. Rosey AL, Abachin E, Quesnes G, Cadilhac C, Pejin Z, Glorion C, et al. Development of a broad range 16S rDNA real-time PCR for the diagnosis of septic arthritis in children. J Microbiol Meth. (2007) 68:88–93. doi: 10.1016/j.mimet.2006.06.010
15. Singhal N, Kumar M, Kanaujia PK, Virdi JS. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol. (2015) 6:791. doi: 10.3389/fmicb.2015.00791
16. Ahmed AA, Arun A, Lindner MS, Sarmiento A, Del Valle Penella A, Laufer PM, et al. Rapid, non-invasive plasma-based microbial cell-free DNA next generation sequencing (the karius test) outperforms the standard of care for the detection of Kingella kingae pediatric spinal infections. Proceedings of the pediatric academic societies (PAS) meeting; Denver, CO, USA (2022).
17. Ossurarson F, Thors V, Heraldsson A. Simplified antibiotic treatment for paediatric osteoarticular infections achieved good outcomes. Acta Paediatr. (2022) 111:2188–94. doi: 10.1111/apa.16510
18. Peltola H, Paakkonen M, Kallio P, Kallio MJ, Osteomyelitis-Septic Arthritis Study Group. Prospective, randomized trial of 10 days versus 30 days of antimicrobial treatment, including a short-term course of parenteral therapy, for childhood septic arthritis. Clin Infect Dis. (2009) 48:1201–10. doi: 10.1086/597582
19. Pääkkönen M, Peltola H. Management of a child with suspected acute septic arthritis. Arch Dis Child. (2012) 97:287–92. doi: 10.1136/archdischild-2011-300462
20. Porsch EA. Kingella kingae virulence factors and insights into pathogenicity. Microorganisms. (2022) 10:997. doi: 10.3390/microorganisms10050997
21. Alcobendas Rueda RM, Núñez E, Martín L, Hernández MB, Saavedra-Lozano J, Udaondo C, et al. Oral versus intravenous antibiotics for pediatric osteoarticular infection: when and to whom? Pediatr Infect Dis J. (2022) 41:e351–7. doi: 10.1097/INF.0000000000003619
Keywords: osteoarthritis, children, epidemiology, etiology, diagnosis, therapy
Citation: Yagupsky P and Ceroni D (2023) Editorial: An update on pediatric skeletal system infections. Front. Pediatr. 11:1128126. doi: 10.3389/fped.2023.1128126
Received: 20 December 2022; Accepted: 17 January 2023;
Published: 13 February 2023.
Edited by:
Maurizio Aricò, Director of the Dept. of Pediatrics, PescaraReviewed by:
Daniela Dibello, Azienda Ospedaliero Universitaria Consorziale Policlinico di Bari, Italy© 2023 Yagupsky and Ceroni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pablo Yagupsky cHlhZ3Vwc2t5QGdtYWlsLmNvbQ==
Specialty Section: This article was submitted to Pediatric Infectious Diseases, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.