
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 26 June 2023
Sec. Neonatology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1127885
This article is part of the Research TopicCare During Pregnancy and Early Childhood for Growth and Development in Low- and Middle- Income CountriesView all 20 articles
 Sitanshi Sharma1
Sitanshi Sharma1 Ranadip Chowdhury1*
Ranadip Chowdhury1* Sunita Taneja1
Sunita Taneja1 Sarmila Mazumder1
Sarmila Mazumder1 Kiran Bhatia1
Kiran Bhatia1 Runa Ghosh1
Runa Ghosh1 Sowmya C. Karantha1
Sowmya C. Karantha1 Neeta Dhabhai1
Neeta Dhabhai1 Harish Chellani2
Harish Chellani2 Rajiv Bahl3
Rajiv Bahl3 Nita Bhandari1
Nita Bhandari1
Background: Short and long term benefits of early Initiation of breastfeeding (EIBF) and exclusive breastfeeding (EBF) in the first six months of life are well established and recommended globally. However, reliable estimates of breastfeeding practices and impact of breastfeeding counselling interventions according to gestational age and weight at birth are not available in low and middle income countries.
Objective: To assess the impact of breastfeeding counselling on EIBF and EBF during the first 6 months of life according to gestational age and weight at birth.
Methods: We analysed the data collected from the Women and Infants Integrated Interventions for Growth Study (WINGS), an individually randomized factorial design trial. Mothers were counselled on EIBF during third trimester of pregnancy. They were supported throughout the first 6 months to continue EBF by early problem identification, frequent home visits and assistance in expressing breastmilk when direct breastfeeding was not possible. Breastfeeding practices were ascertained through 24 h recalls at infant ages 1, 3 and 5 months for both the intervention and control groups by an independent outcome ascertainment team. The World Health Organization (WHO) definitions were used for classification of infant breastfeeding practices. Generalized linear models of the Poisson family with a log-link function were used to estimate the effect of interventions on breastfeeding practices. The relative measures of effect on breastfeeding practices were estimated in term appropriate for gestational age (T-AGA), term small for gestational age (T-SGA), preterm AGA (PT-AGA), preterm SGA (PT-SGA) infants.
Results: Amongst all infants irrespective of gestational age and weight at birth, EIBF was (51.7%) higher amongst the intervention group (IRR 1.38, 95% CI 1.28–1.48) compared with the control group. The proportion of exclusively breastfed infants at ages 1 month (IRR 1.37, 95% CI 1.28–1.48), 3 months (IRR 2.13, 95% CI 1.30–1.44) and 5 months (IRR 2.78, 95% CI 2.58–3.00) were higher in intervention group than control group. We identified significant interaction (p value for interaction <0.05) between intervention and infant size and gestation at birth on exclusive breastfeeding at 3 and 5 months of age. Subgroup analysis showed that the impact of the intervention was greater on exclusive breastfeeding in PT- SGA infants at 3 months (IRR 3.30, 95% CI 2.20–4.96) and 5 months of age (IRR 5.26, 95% CI 2.98–9.28).
Conclusion: This is one of the first studies wherein impact of breastfeeding counselling interventions in the first 6 months of life was assessed according to infant size and gestation at birth wherein gestational age was reliably estimated. The impact of this intervention was higher in preterm and SGA babies compared to other infants. This finding is important as preterm and SGA infants have a higher burden of mortality and morbidity during early infancy. Intensive breastfeeding counselling to these vulnerable infants is likely to improve overall breastfeeding rates and reduce the adverse outcomes.
Clinical Trial Registration: [http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=19339%26EncHid=%26userName=societyforappliedstudies], identifier [#CTRI/2017/06/008908].
Exclusive breastfeeding (EBF) during the first 6 months of life is universally recommended as it reduces the risk of neonatal and childhood morbidity and mortality. EBF supports optimal growth, neurodevelopment and better school performance (1). Furthermore, evidence from literature supports maternal benefits for breastfeeding like reduced risk of type 2 diabetes mellitus, prolonging lactation amenorrhoea and protection from various cancers like ovarian and breast cancers (2).
Despite the evidence, EBF rates continue to be sub-optimal in the low-middle income countries (LMIC’s) (3). Promotion of EBF for first 6 months of life is a part of all national and international guidelines. Breastfeeding practices of vulnerable babies like preterm and small for gestational age (SGA) babies are not reflected in demographic surveys (4).
Babies born preterm (born before completing 37 weeks of gestation) and small for gestational age (born with birth weight <10th centile for gestational age at birth) are vulnerable to serious infections and feeding difficulties (5). This contributes to increased risk of growth failure, death in early and later life, and neurodevelopmental deficits (6, 7). Breastmilk provides protection for preterm and SGA infants by reducing severity of necrotizing enterocolitis, sepsis, and retinopathy of pre- maturity. Breastfeeding also improves neuropsychological performance, strengthens mother-child bond, reduces the length of hospital stay with lesser incidence of readmissions (8). In spite of the evidence on benefits of breastfeeding for preterm and small for gestational age (SGA) babies, the knowledge on their breastfeeding practices is scanty in LMICs when compared with term infants (9, 10). The data available on preterm births is robust in high income countries with higher birth registration coverage for preterm births in contrast to LMIC’s, where birth registration coverage is low and data on preterm birth is scarce (11). Though, birthweight continues to be the primary measure for birth outcomes, it does not help in differentiating between growth restricted or preterm infants. Gestational age is a better indicator but difficult to ascertain accurately, in low resource settings (12).
We conducted a secondary analysis from a population-based cohort in urban and peri-urban low-to-mid socio-economic neighbourhoods of South Delhi to estimate the impact of breastfeeding counselling on early initiation of breastfeeding (EIBF) and EBF during the first 6 months of life for infants according to both gestational age and weight at birth. We had reliable and early estimates of gestational age by dating ultrasounds and reliable measure of birth weights to be able to categorise infants in the groups: term appropriate for gestational age (T-AGA), term small for gestational age (T-SGA), preterm AGA (PT-AGA), preterm SGA (PT-SGA) infants.
Data collected from a recently concluded randomized controlled study, Women and Infants Integrated Interventions for Growth Study (WINGS) was used (13). Total live births which occurred during the course of the study were included in this analysis. The infants who were born to mothers who received interventions during pregnancy continued to receive the interventions throughout early childhood, and those infants who were born to mothers who were in control group during pregnancy were part of the control group during early childhood. The women underwent randomisation twice during the study due to the factorial design of the trial. The first randomisation was done when eligible women were identified through a door-to-door survey and enrolled after getting a written consent. The second randomisation was done at the time when pregnancy was confirmed. Details on the main study design and randomisation have been previously published (14). The gestational age was assessed between 9 and 13 weeks of gestation using Intergrowth-21st standards by calculating fetal crown-rump length (CRL), if CRL was >95 mm, femur length and head circumference was be used to assess gestational age (14–16). Birth weight was taken at the place of birth or at home at age day 7 (±6) days after birth by pair of trained and standardised study team workers using calibrated digital weighing scale (model 354; Seca, California, USA) to the nearest 10 gram.
Women in the intervention group received breastfeeding support which was initiated as soon as the woman reached the third trimester of pregnancy. Pregnant women were counselled on benefits of EIBF and EBF at each contact with the study team. Post birth, the study team supported mothers in EIBF within first hour of birth or as early as possible if birth occurred at the collaborating hospital. Home visits were made by study workers who were referred to as Prernas (inspiration) for the mothers in intervention group for all infants on days 3, 7, 10, 14, 28 after birth and thereafter monthly from 2 to 6 months of life to enquire about infant wellbeing, establish breastfeeding and promoting EBF for first 6 months (13, 14, 17). During these visits, mothers were counselled and supported for establishing and sustaining exclusive breastfeeding during the first 6 months of infant’s life. Extra support through additional home visits was given by workers trained in breastfeeding support called lactation counsellors. The lactation counsellors supported those mothers who reported breastfeeding problems to Prernas. They addressed breastfeeding problems like inverted nipple and breast engorgement or helped mothers with preterm babies. In addition, breast pumps for expressing breastmilk were provided to the mothers of small babies (mostly preterm or SGA) who were unable to suckle effectively and for some mothers with breast problems. Repeat visit were made whenever required or requested by mothers. Monthly anthropometric measures for routine growth monitoring was taken by an independent team, to observe infant growth. When inadequate weight gain (<15th centile of weight/velocity per month from birth to 6 months) was reported, the infants were referred to physicians for evaluation (18). Nutritional supplementation included vitamin D (400 IU), to all infants and Iron supplementation was given from two weeks for the very low birth weight (VLBW) and from six weeks to low birth weight (LBW) infants until six months of age according to WHO guidelines (14, 19). Snacks, milk (600 kcal, 20 g protein), micronutrient supplements, iron folic acid, calcium, and vitamin D were given to mothers for six months to meet additional requirements during lactation. In addition, counselling on positive thinking, screening and management of depressive symptoms, and WaSH interventions were provided to all mothers in intervention group (14, 20–22).
In the control group, during pregnancy, women were encouraged to register for antenatal care at a government or private facility, have at least four antenatal care check- ups, consume iron folic acid, calcium, vitamin D daily throughout pregnancy, access supplementary foods through the Integrated Child Development Services (ICDS) scheme and deliver in health facilities. After child birth, mothers were advised to go for a postnatal health check-up, and to consume iron folic acid, calcium, vitamin D, and supplementary foods daily through the ICDS scheme. They were also encouraged to allow home visits by the health workers from national health system like ASHA workers in the first 42 days of life. For early childhood, mothers were advised to breastfeed their babies exclusively for the first six months, and continue breastfeeding for at least two years and to collect supplementary food from ICDS (13).
Outcomes were assessed at birth and at infant ages 1, 3 and 5 months. These included rates of EIBF, EBF and breastfeeding practices. An independent outcome assessment team collected data on breastfeeding practices at birth (within 7 days of birth) and at 1,3, 5 months (150–170 days) of age through 24-hour recall from the mothers.
Birth weight was defined as weight taken by the study team at day 7 (±6 days) after birth.
T-AGA was defined as gestational age ≥37 weeks at birth and birth weight ≥10th centile to ≤90th centile; T-SGA: gestational age ≥37 weeks at birth and birth weight <10th centile; PT-AGA: gestational age <37 weeks at birth and birth weight ≥10th centile to ≤90th centile; PT- SGA: gestational age <37 weeks at birth and birth weight <10th centile, all in accordance to the Intergrowth-21st standards (15). Sub-group analysis included those infants whose gestational age and birth weight were available.
All breastfeeding practices were defined using WHO definitions (23, 24). Early Initiation of breastfeeding was defined as initiation of breastfeeding the infant within 1 h of birth. Exclusive breastfeeding was defined as feeding the infant only breast milk (including expressed breast milk) and no other food or drink, not even water, for first 6 months of life, other than ORS, vitamins, minerals and medicines.
Predominant breastfeeding was defined as feeding the infant breast milk (including expressed breastmilk). However, the infant may also have received other liquids like water and water-based drinks, fruit juice, ritual fluids or any other liquids.
Partial breastfeeding was defined as giving the infant some breastfeeds along with either packaged or powdered milk or cereal based feeds, or any food other than breastmilk.
No breastfeeding was defined as, any infant who did not receive either direct or expressed breastmilk (23, 24).
Data collection for the outcomes ended on 30th June 2021. For this analysis, we compared the mothers and infants received interventions during pregnancy and early childhood with the group who did not receive the interventions during that period. We assessed similarity of proportions of baseline characteristics across groups to check for successful randomisations. Intention to treat analysis was used. We used generalised linear models of the Poisson family with a log link function to estimate the effect (Incidence rate ration IRR, 95% CI) of intervention on EIBF and EBF at1, 3 and 5 months of age. The final models were adjusted for place of birth, family possessing a below poverty line card, women’s height, and women’s body mass index which were potential confounders. We also adjusted the analysis for clustering due to twins. The relative measures of effect on EBF in T-AGA, T- SGA, PT-AGA and PT-SGA were estimated and presented as forest plots. Data analysis was conducted with Stata version 16.0. (Stata Corp., College Station, TX, USA.
In the analyses all live births were included at the baseline (Table 1). For describing the breastfeeding practices at different time points, a cross sectional approach was followed. Out of the total live births at baseline, the number of infants who had the information on breastfeeding practices at age 1, 3 and 5 months were selected for the analysis. Reasons for exclusions included family moved away, refused for interview, child died or were not available at the time of interview (Figure 1).
Sociodemographic characteristics and infant details were represented as means (SD) or proportions as appropriate. The total number of live births were 2,382 in intervention group and 2,369 in control group. Baseline characteristics were comparable except for women’s height, proportion of women underweight, families possessing a below poverty line card, and place of birth (Table 1).
Mothers of 51.7% of the infants in intervention group initiated breastfeeding within the first hour of birth, in contrast to only 35.6% mothers in the control group. Breastfeeding practices of infants at 1 and 5 months of age are graphically represented in Figures 2A,B respectively. At 1 month of age, the rates of EBF was higher (75%), in the intervention group as compared to the control group (54%). Predominant breastfeeding (11.1%) and partial breastfeeding (32.2%) were higher in control group which received no breastfeeding counselling and support through the study, when compared with intervention group (Figure 2A).
At 5 months of age, EBF rate in the intervention group was sustained at 73.8% (Figure 2B). The rates of predominant (23.7%) and partial (42%) breastfeeding further increased in control group from the first month. EIBF which was 51% (IRR 1.38, 95% CI 1.28–1.48) in the intervention group compared to control group (35.6%) (Table 2). The proportion of exclusively breastfed infants at ages 1 month (IRR 1.37, 95% CI 1.28–1.48), 3 months (IRR 2.13, 95%CI 1.30–1.44) and 5 months (IRR 2.78, 95%CI 2.58–3.00) were higher in intervention as compared to the control group.
The relative measures of effect on breastfeeding practice, estimated in term appropriate for gestation age (T-AGA), term small for gestation age (T-SGA), preterm AGA (PT-AGA), preterm SGA (PT-SGA) infants are shown in Figure 3. We identified significant interaction (p value for interaction <0.05) between intervention and birth weight and gestational age at birth on exclusive breastfeeding at 3 and 5 months of age. Subgroup analysis showed that the impact of the intervention was greater on exclusive breastfeeding in PT- SGA infants at 3 months (IRR 3.30, 95% CI 2.20–4.96) and 5 months of age (IRR 5.26, 95% CI 2.98–9.28).
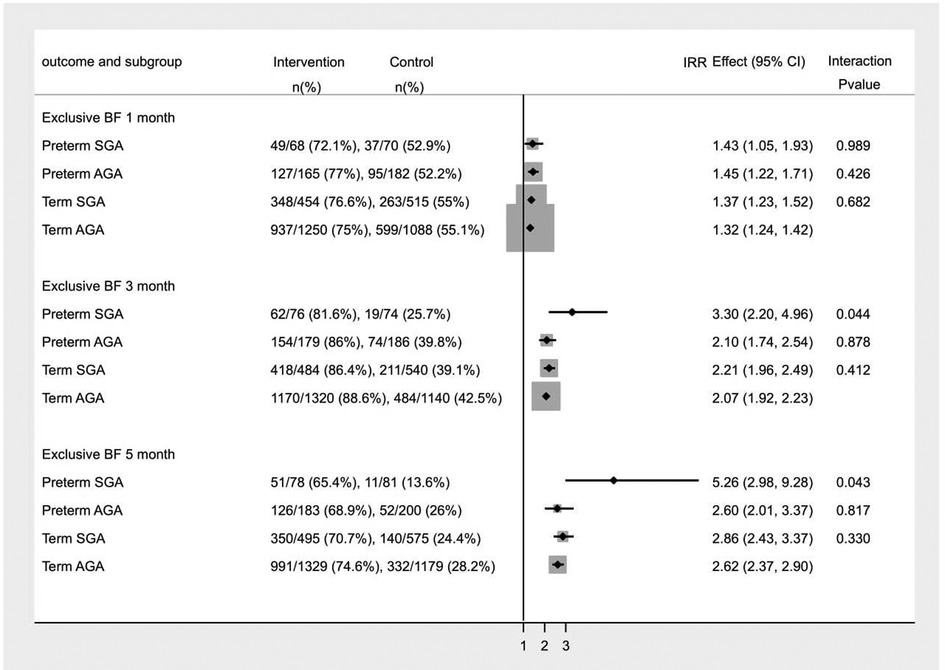
Figure 3. Subgroup analysis for Incidence of Exclusive breastfeeding in infants at 1, 3 and 5 months of age.
These analyses showed that provision of counselling and support to mothers for breastfeeding, improved practices on EIBF by 38% and lead to 2 times increase in practices of EBF in the first six months of life. The impact of this intervention was higher in preterm and SGA babies compared to other infants.
Similar findings have been observed from the evidence on breastfeeding counselling in LMIC’s. Interventions delivered concurrently in health facility and home settings lead to improvement in rates of EIBF (OR: 4.96; 95% CI: 2.88, 8.54) compared with interventions delivered individually at home or at health facility. For effect on EBF, pooled estimates showed that the odds of EBF at 1–5 months increased 3-fold (OR: 3.08; 95% CI: 2.57, 3.68) with only breastfeeding promotion interventions. This impact was highest when interventions were delivered in combination settings which was both in health facility and home (OR: 6.80; 95% CI: 3.75, 12.33) (25). Best outcomes are thus achieved when several interventions are delivered simultaneously and through multiple channels (26).
However, there is no population based evidence documenting effects of counselling on breastfeeding practices amongst infants based on gestational age and weight at birth from LMIC’s. It is widely known and accepted that small babies are at higher risk of non-exclusive breastfeeding due to factors like NICU admission, feeding intolerance and susceptibility to infections which are influenced by the gestational age at birth. NICU practices also play an important role in establishing the EBF in these babies, however most evidence is limited to high-income settings (27–29). In our study, while the infant was in hospital after birth, the hospital guidelines were followed for feeding as per treating physicians judgment. The study team only provided extra support to babies in intervention group for early initiation of breastfeeding and establishing breastfeeding. All our interventions were based on counselling about initiating breastfeeding and maintaining exclusive breastfeeding. All infants across both groups received standard of care at the health facility immediately post birth and intervention babies had no additional benefits at the hospital.
Through our study we have shown that through breastfeeding counselling centred around the small, at risk babies, the rates of EIBF and EBF can be improved which in turn will reduce the burden of morbidity and mortality in the first 6 months of life. This is the first study from India which has demonstrated the effects of breastfeeding counselling of mothers based on their infant’s gestational age and weight at birth. In addition, it reinforces that by providing breastfeeding counselling in both health facility and home settings and by offering hands-on support to mothers to tackle breastfeeding problems there were significant improvements in the breastfeeding rates of preterm and SGA babies, and improved adherence to exclusive breastfeeding in the first 6 months of life.
The strengths of the study include standardised outcome assessments, early pregnancy ultrasound based gestational age assessment, and generalisability to low and middle income urban populations.It is the first population-based studies from India where breastfeeding practices were ascertained in a randomized control trial based on gestational age at birth and birth weight of the infant.
The lockdowns due to the covid-19 pandemic affected intervention delivery which was one challenge we faced which may have affected outcome assessments for some infants. Restrictions in mobility made it difficult to conduct home visits for observing breastfeeding and to provide support and hands-on help to mothers. However, we overcame this through virtual contacts by lactation counsellors with the mothers.
The key priority is support to small babies in breastfeeding right form birth and continued up to 6 months. This can only be achieved by early identification of these babies through Ultrasonography (USG) to ascertain accurate GA. The importance of EBF has been widely disseminated through various National programs, but the measures to be taken to improve the breastfeeding practices for vulnerable infants separately from the term and AGA infants needs to be defined. Special consideration should be given to understanding the barriers in implementation of breastfeeding interventions for these preterm and SGA babies. The availability of USG facility at different levels of the health systems to ascertain at risk infants (born preterm or SGA), though an expensive but one-time investment will improve lives of small babies by addressing the problems in breastfeeding in vulnerable infants right from birth.
This is the first study where impact of breastfeeding counselling interventions in the first 6 months of life was assessed according to infant’s gestational age and weight at birth. The impact of this intervention was higher in preterm and SGA babies compared to other infants. In summary, the analyses show that breastfeeding counselling at both the health facility and at home can be translated to higher rates of EIBF and EBF, amongst the preterm and SGA babies thereby reducing adverse health outcomes during infancy and childhood. This is an important finding as preterm and SGA babies have a higher burden of mortality and morbidity during the first 6 months of life.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Review Committee of the Society for Applied Studies, New Delhi, India. Vardhman Medical College and Safdarjung Hospital, New Delhi, India. World Health Organization, Geneva. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
SS and RC were involved in conceptualisation and literature search and drafting first draft of the manuscript. SS, RC, ST and KB were responsible for conducting data analysis. SS, RC, NB, ST, SM, RG, SC and ND were involved in developing interventions and implementation. RB, NB and HC provided technical guidance. All authors contributed to the article and approved the submitted version.
The primary study is funded by Biotechnology Industry Research Assistance Council (BIRAC) (Grant No. BIRAC/GCI/0085/03/14-ACT) of the Department of Biotechnology, Government of India and by the Bill & Melinda Gates Foundation (Grant No. #OPP1191052), USA. The funding agency did not play any role in the design of study and are neither involved in nor have any influence over the collection, analyses or interpretation of data.
We acknowledge the contribution and support of the enrolled women and their families. We are thankful to the community leaders for their cooperation and support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet (London, England). (2016) 387(10017):475–90. doi: 10.1016/S0140-6736(15)01024-7
2. Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatrica (Oslo, Norway: 1992). (2015) 104(467):96–113. doi: 10.1111/apa.13102
3. Collaborators GRF. Global burden of 87 risk factors in 204 countries and territories, 1990−2019: a systematic analysis for the global burden of disease study 2019. Lancet (London, England). (2020) 396(10258):1223–49. doi: 10.1016/S0140-6736(20)30752-2
5. Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet (London, England). (2014) 384(9938):189–205. doi: 10.1016/S0140-6736(14)60496-7
6. WHO. The WHO application of ICD-10 to deaths during the perinatal period: ICD-PM, Geneva: World Health Organization (2016) pg 20–21. ISBN 978 92 4 154975 2.
7. Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet (London, England). (2014) 384(9946):857–68. doi: 10.1016/S0140-6736(14)60932-6
8. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. (2014) (4):Cd002971. doi: 10.1002/14651858.CD002971.pub3
9. Akindolire A, Talbert A, Sinha I, Embleton N, Allen S. Evidence that informs feeding practices in very low birthweight and very preterm infants in sub-saharan Africa: an overview of systematic reviews. BMJ Paediatr Open. (2020) 4(1):e000724. doi: 10.1136/bmjpo-2020-000724
10. Seródio Michelin N, Nunes HRC, Carvalhaes M, Parada C. The influence of gestational age at term on breastfeeding: a cohort study. Rev Esc Enferm USP. (2021) 55:e20200381. doi: 10.1590/1980-220x-reeusp-2020-0381
11. Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7(1):e37–e46. doi: 10.1016/S2214-109X(18)30451-0
12. McGuire W, Henderson G, Fowlie PW. Feeding the preterm infant. BMJ (Clin Res Ed). (2004) 329(7476):1227–30. doi: 10.1136/bmj.329.7476.1227
13. Taneja S, Chowdhury R, Dhabhai N, Upadhyay RP, Mazumder S, Sharma S, et al. Impact of a package of health, nutrition, psychosocial support, and WaSH interventions delivered during preconception, pregnancy, and early childhood periods on birth outcomes and on linear growth at 24 months of age: factorial, individually randomised controlled trial. BMJ (Clin Res Ed). (2022) 379:e072046. doi: 10.1136/bmj-2022-072046
14. Taneja S, Chowdhury R, Dhabhai N, Mazumder S, Upadhyay RP, Sharma S, et al. Impact of an integrated nutrition, health, water sanitation and hygiene, psychosocial care and support intervention package delivered during the pre- and peri-conception period and/or during pregnancy and early childhood on linear growth of infants in the first two years of life, birth outcomes and nutritional status of mothers: study protocol of a factorial, individually randomized controlled trial in India. Trials. (2020) 21(1):127. doi: 10.1186/s13063-020-4059-z
15. Consortium IFaNG. The International Fetal and Newborn Growth Standards for the 21st Century (INTERGROWTH-21st) Study Protocol. (2009).
16. Oxford University. Correct measurement of fetal crown rump length and standardization of ultrasonographers. INTERGROWTH-21st CRL standardization. (2010): 15.
17. Ministry of Health and Family Welfare & Ministry of Women and Child Development. Home Based Care for Young Child (HBYC) Operational Guidelines, New Delhi, India: National Health Mission (2018).
18. World Health Organization. WHO child growth standards: growth velocity based on weight, length and head circumference: methods and development. Geneva, Switzerland: World Health Organization (2009). https://apps.who.int/iris/handle/10665/44026
19. World Health Organization. Guidelines on Optimal feeding of low birth weight infants in low-and middle-income countries. Geneva, Switzerland: WHO (2011). https://apps.who.int/iris/handle/10665/85670
21. WHO. Thinking healthy: a manual for psychosocial management of perinatal depression, WHO generic field-trial version 1.0, 2015. Geneva: World Health Organization (2015). Report No.: 9789754004113 (Turkish) Contract No.: WHO/MSD/MER/15.1.
22. Kochhar PH, Rajadhyaksha SS, Suvarna VR. Translation and validation of brief patient health questionnaire against DSM IV as a tool to diagnose major depressive disorder in Indian patients. J Postgrad Med. (2007) 53(2):102–7. doi: 10.4103/0022-3859.32209
23. WHO. Implementing the global strategy for infant and young child feeding. Report of a technical meeting. (2003).
24. WHO. Indicators for assessing infant and young child feeding practices: part 1 definitions. (2007).
25. Sinha B, Chowdhury R, Upadhyay RP, Taneja S, Martines J, Bahl R, et al. Integrated interventions delivered in health systems, home, and community have the highest impact on breastfeeding outcomes in low- and middle-income countries. J Nutr. (2017) 147(11):2179s–87s. doi: 10.3945/jn.116.242321
26. Rollins NC, Bhandari N, Hajeebhoy N, Horton S, Lutter CK, Martines JC, et al. Why invest, and what it will take to improve breastfeeding practices? Lancet (London, England). (2016) 387(10017):491–504. doi: 10.1016/S0140-6736(15)01044-2
27. Méio MDBB, Villela LD, Gomes Júnior SCDS, Tovar CM, Moreira MEL. Breastfeeding of preterm newborn infants following hospital discharge: follow-up during the first year of life. Cien Saude Colet. (2018) 23:2403–12. doi: 10.1590/1413-81232018237.15742016
28. Dong D, Ru X, Huang X, Sang T, Li S, Wang Y, et al. A prospective cohort study on lactation status and breastfeeding challenges in mothers giving birth to preterm infants. Int Breastfeed J. (2022) 17(1):6. doi: 10.1186/s13006-021-00447-4
29. Tran HT, Murray JCS, Sobel HL, Mannava P, Huynh LT, Nguyen PTT, et al. Early essential newborn care is associated with improved newborn outcomes following caesarean section births in a tertiary hospital in Da nang, Vietnam: a pre/post-intervention study. BMJ Open Qual. (2021) 10(3):4–5. doi: 10.1136/bmjoq-2020-001089
Keywords: preterm, SGA, gestational age (GA), birth weight, breastfeeding, exclusive breast feeding (EBF)
Citation: Sharma S, Chowdhury R, Taneja S, Mazumder S, Bhatia K, Ghosh R, Karantha SC, Dhabhai N, Chellani H, Bahl R and Bhandari N (2023) Breastfeeding practices based on the gestational age and weight at birth in the first six months of life in a population-based cohort of infants from North India. Front. Pediatr. 11:1127885. doi: 10.3389/fped.2023.1127885
Received: 20 December 2022; Accepted: 14 June 2023;
Published: 26 June 2023.
Edited by:
Dagrun Engeset, University of Agder, Norway© 2023 Sharma, Chowdhury, Taneja, Mazumder, Bhatia, Ghosh, Karantha, Dhabhai, Chellani, Bahl and Bhandari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranadip Chowdhury cmFuYWRpcC5jaG93ZGh1cnlAc2FzLm9yZy5pbg==
Abbreviations EBF, Exclusive Breastfeeding; LMIC, Low middle income countries; SGA, Small for gestational age; EIBF, Early initiation of breastfeeding; T-AGA, Term appropriate for gestational age; T-SGA, Term small for gestational age; PT-AGA, Preterm appropriate for gestational age; PT-SGA, Preterm small for gestational age; WINGS, Women and Infants Integrated Growth Study; USG, Ultrasonography; CRL, Crown rump length; LBW, Low Birth Weight; VLBW, Very Low Birth Weight; WHO, World Health Organization; PHQ-2, Patient Health Questionnaire-2; ORS, Oral Rehydration Therapy; ICDS, Integrated Child Development Services; IRR, Incidence Rate Ratio; OR, Odds ratio; CI, Confidence Interval; SD, Standard Deviation; NICU, Neonatal Intensive Care Unit.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.