
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr., 04 May 2023
Sec. General Pediatrics and Pediatric Emergency Care
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1125112
Background: Guidance for preparing powdered infant formula (PIF) helps to ensure it meets the nutritional needs of infants and is safe to consume. Among safety concerns is Cronobacter sakazakii contamination which can lead to serious infections and death. PIF preparation guidance varies; there is a lack of consensus on whether there is a need to boil water to inactivate potential Cronobacter and for how long to let the water cool before reconstitution. We sought to quantify the burden of burn injuries among infants related to water heating for PIF preparation. Estimating this burden may help inform preparation recommendations.
Methods: Burn injuries among infants <18 months of age were identified from 2017 to 2019 National Electronic Injury Surveillance System data collected from sampled hospital emergency departments. Injuries were classified as related to PIF water heating, potentially related to PIF water heating but with undetermined causation, related to other infant feeding aspects, or unrelated to infant formula or breast milk feeding. Unweighted case counts for each injury classification were determined.
Results: Across sampled emergency departments, 7 PIF water heating injuries were seen among the 44,395 injuries reported for infants <18 months. No reported PIF water heating injuries were fatal, but 3 required hospitalization. Another 238 injuries potentially related to PIF water heating but with undetermined causation were also seen.
Conclusion: Preparation guidance should consider both the potential risk for Cronobacter infection and the potential risk for burns.
Although breast milk is the optimal source of infant nutrition (1), most infants in the United States receive infant formula at some point during their first year of life. Just 1 in 4 US infants born in 2019 were exclusively breastfed through 6 months, as recommended (1, 2). Guidance for preparing powdered infant formula (PIF) helps to ensure the formula meets the nutritional needs of infants and is safe to consume. Safety concerns include the use of a safe water source to reconstitute PIF and potential contamination of formula from the gram-negative bacteria Cronobacter sakazakii, formerly known as Enterobacter sakazakii, which can result from both environmental exposure (i.e., unclean bottles and surfaces) and intrinsically contaminated PIF (3–5). Since 2014, US manufacturers have been required to test PIF for Cronobacter before further distribution (6). Following this testing mandate, from 2015 to 2018, 2–4 cases of infant Cronobacter infection were reported each year (7). More recently, in 2022, following the investigation of 2 infant Cronobacter cases, a voluntary recall of powdered infant formula led to supply chain issues and a nationwide infant formula shortage, which brought more public attention to the safety of PIF and preparation methods (8, 9).
In 2006, the World Health Organization (WHO) released guidelines on the safe preparation, storage, and handing of PIF following a series of Cronobacter infection outbreaks in neonatal intensive care units (10–12). These guidelines for reconstituting PIF instruct parents and home-based caregivers to clean and disinfect surfaces and hands, bring water to a rolling boil, pour water that has been cooled to no less than 70°C/158°F (cool for no more than 30 min) into a clean and sterilized bottle, add PIF to the bottle and mix, and then further cool the reconstituted PIF to a safe feeding temperature before serving (12). PIF preparation guidance has varied in the United States, and there has been a lack of consensus on whether there is a need to boil water and for how long to let the water cool before reconstituting PIF (13). Water may be boiled and cooled before mixing PIF, which is primarily to ensure a safe water source (3). Alternatively, water may be boiled and mixed with PIF while still hot (at least 70°C/158°F), which is intended to inactivate any potential Cronobacter (11, 12).
A 2019 commentary highlighted the variation in PIF preparation guidance and the lack of agreement surrounding water heating and water temperatures when reconstituting PIF (13). Considering more than 300 children are estimated to be treated in emergency departments (EDs) each day for burn-related injuries (14), it is important to consider the potential risk of burns from boiling water for PIF preparation along with the potential risk of Cronobacter infection (13).
In this cross-sectional study, we sought to better quantify the burden of burn injuries among infants related to water heating for PIF preparation (hereafter referred to as PIF water heating). Estimating the burden of burn injuries related to PIF water heating may help inform discussions related to the risks and benefits of water heating for PIF preparation and wider PIF preparation recommendations.
The National Electronic Injury Surveillance System (NEISS) collects data on consumer product-related injuries from a probability sample of approximately 100 hospital EDs representing more than 5,000 hospitals with EDs across the United States and US territories. Trained NEISS coordinators in each hospital abstract data from medical records of patients presenting to the ED with a consumer product-related injury, assign each case at least 1 product code from a standardized coding manual corresponding to the related consumer product, and provide a brief 1–2 line narrative describing the incident scenario (15–17).
To identify cases for review, the authors first restricted 2017–2019 NEISS data to infants <18 months of age (n = 44,395) (Figure 1). Next, the authors queried the dataset for potential burn injuries (n = 1,924) using diagnosis codes 48 (burns, scald), 51 (burns, thermal), and 47 (burns, not specified). Injuries associated with diagnosis code 71 (other/not stated) were also included if their associated narratives referred to “burn” or “scald.” Beginning in 2019, injuries could have a second associated diagnosis code; for consistency, the authors examined only the primary diagnosis code across all years.
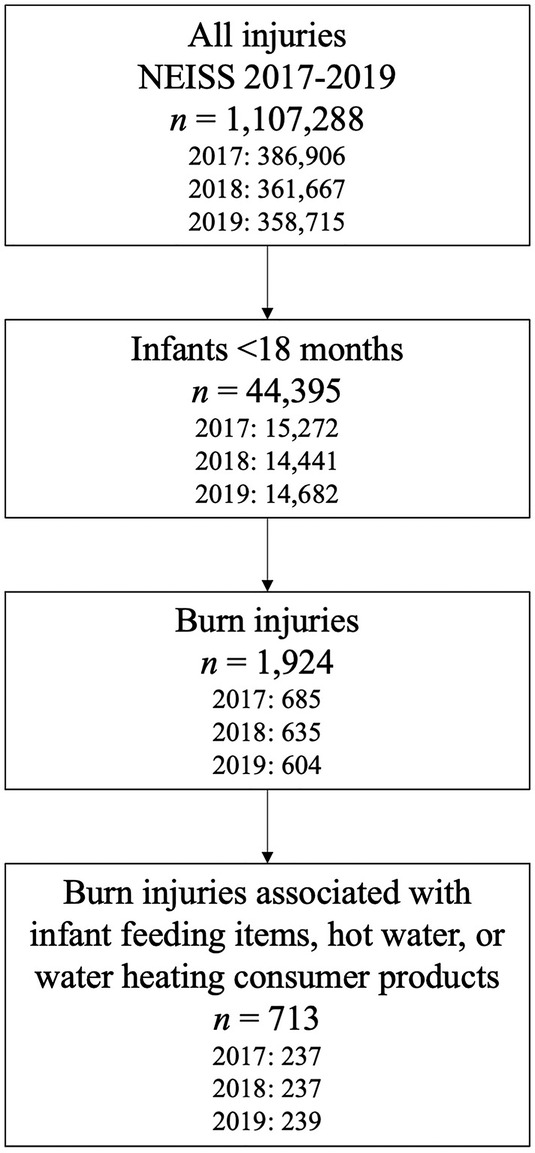
Figure 1. Identification of burn injuries associated with infant feeding items, hot water, and water heating consumer products for further review for relation to water heating during powdered infant formula preparation.
The authors then further restricted injuries to those associated with infant feeding items, hot water, and water heating consumer products (n = 713) by querying product codes 1509 (baby bottles or nipples), 1934 (hot water), 1510 (bottle warmers), 264 (microwave ovens), 278 (electric ranges or ovens), 279 (gas ranges or ovens), 280 (other ranges or ovens), 281 (ranges or ovens, not specified), 217 (electric coffee makers or teapots), 405 (unpowered coffee makers or teapots), 452 (coffee makers or teapots, not specified), 269 (electric kettles or hot pots), and 224 (hot plates) (Figure 1). Each injury could list up to 3 associated product codes; all 3 product code fields were queried. Because NEISS does not include injuries related to food products, queries specific to infant formula products were not possible (18). To determine each injury's association with PIF water heating, 2 authors (KC, EA) first reviewed a sample of injury narratives and collaboratively developed 14 injury classification codes based on likely causes of each injury. Classification codes included 1) PIF preparation; 2) hot water, use not specified; 3) hot surface used to heat water, use not specified; 4) bottle warming; and 5) infant feeding item sanitation. Injuries coded as “PIF preparation” (code 1) included narratives that described formula or bottle preparation with hot water; however, PIF reconstitution did not have to be explicitly mentioned for inclusion. Injuries coded as “hot water, use not specified” (code 2) or “hot surface used to heat water, use not specified” (code 3) involved hot water or water heating appliances or devices such as stoves and hotplates without descriptions of stated uses for the heated water. Injuries coded as “bottle warming” (code 4) had narratives similar but distinct from injuries coded as “PIF preparation” (code 1). Rather than descriptions of bottle preparation, narratives coded as “bottle warming” (code 4) included frequently used methods for bottle warming such as using bottle warming devices, heating prepared bottles in microwaves, or placing prepared bottles in hot water to warm the bottles' contents. Similarly, narratives of injuries coded as “infant feeding item sanitation” (code 5) included frequently used methods for bottle cleaning such as boiling bottle parts. Remaining classification codes (codes 6–14) included injuries determined to be unrelated to PIF water heating, such as those related to hot water used for bathing, or miscellaneous injuries unrelated to infant feeding (see Supplementary Material Table S1 for a complete description of all codes).
The two authors (KC, EA) then independently reviewed and coded each case, reaching a concordance of 91.2%. KC and EA then discussed discrepancies in coding (n = 63) and either came to a consensus (n = 59) or consulted a third author (CP) to determine the injury's classification (n = 4). KC, EA, and CP then further delineated injuries as those related to PIF water heating (code 1), those potentially related to PIF water heating but with undetermined causation (codes 2 and 3), those related to other infant feeding aspects (codes 4 and 5), and those unrelated to infant formula or breast milk feeding (codes 6–14) (Supplementary Material Table S1).
The authors determined unweighted case counts for each burn injury classification. Small case counts prevented the authors from further applying complex survey procedures to calculate stable national estimates such as weighted case counts and stable estimates of occurrence such as incidence rates. The authors also examined disposition of each PIF water heating case. This secondary data analysis of public use, de-identified data is not research involving human subjects and did not require Centers for Disease Control and Prevention (CDC) Institutional Review Board review.
Of the 713 burn injuries among infants <18 months of age associated with infant feeding items, hot water, or water heating consumer products in this 2017–2019 review, 442 injuries were determined to be unrelated to infant milk feeding. Most of these unrelated injuries were associated with bathing or food and beverage preparation other than infant formula or breast milk.
A total of 7 injuries related to PIF water heating were identified. Another 238 injuries potentially related to PIF water heating but with undetermined causation were also identified. Of these potential PIF water heating injuries, 188 were associated with contact with hot water, use not specified and 50 were associated with contact with hot surfaces used to heat water, use not specified, such as a stove or hot plate. A further 26 injuries related to other infant feeding aspects were also identified, including 24 associated with warming bottles and 2 associated with infant feeding item sanitation (Table 1).
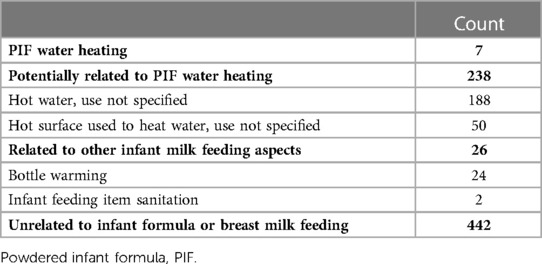
Table 1. Burn injuries associated with infant feeding items, hot water, and water heating consumer products and relation to infant feeding—NEISS, 2017-2019.
The 7 injuries directly related to PIF water heating ranged from 1 to 4 cases a year across 2017–2019 and occurred among infants aged 2–14 months. Among these cases, 4 were examined or treated and released or not admitted but transferred for treatment to another department of the same facility, 1 was treated and transferred to another hospital, and 2 were treated and admitted for hospitalization (Table 2). No injuries collected by NEISS related to PIF water heating resulted in fatalities.
Although the true burden of burns related to water heating for PIF preparation is difficult to determine, these counts offer some insights. Over 3 years of data collection, 7 PIF water heating injuries were seen in EDs in NEISS hospitals across the United States. Although 3 reported injuries required hospitalization, none were fatal; however, fatalities may be underreported as NEISS is not a good source for fatal injuries. Another 238 injuries potentially related to PIF water heating but with undetermined causation were also seen in EDs, although many of these were likely not related to PIF preparation. These data demonstrate that some infants in the United States are burned during PIF preparation, and that, though uncommon, some of these injuries are serious enough for ED visits and sometimes further hospitalization.
Notably, this analysis may have underestimated the true burden of PIF water heating-related injuries. These counts likely underestimate PIF water heating-related injuries as only cases appearing in EDs are accounted for in NEISS data, thus less severe burns not requiring emergency medical attention were not captured. Further, the 1–4 PIF water heating injuries estimated to occur each year are drawn from unweighted case counts among a sample of approximately 100 hospitals out of more than 5,000 hospitals with EDs across the United States. Small case counts prevented further extrapolation of these 1–4 annual cases into stable nationally representative weighted estimates for PIF water heating injuries. However, combining the 7 cases related to PIF water heating and those 238 cases potentially related to PIF water heating but with undetermined causation yields stable weighted estimates for possible PIF water heating burn injuries across the 3-year study period. Using this methodology, an estimated 1,706 infants presented to EDs for burn injuries possibly related to PIF water heating in the United States from 2017 to 2019. The true burden of infant burns related to PIF water heating requiring emergency care and presenting to the ED between 2017 and 2019 lies between 7 and 1,706 cases, likely far towards the lower end as the authors' definition of possible cases was liberal. Additionally, parents, caregivers, and other household children could also have experienced burns related to PIF preparation that were not captured in this study.
A 2012 research study among parents in the United Kingdom suggested that although many parents were confident in their ability to prepare PIF safely, most were not aware PIF was not sterile before opened and found it difficult to judge water temperature when reconstituting PIF (19). Parents and caregivers may lack awareness of underlying reasons for water boiling and correct, safe methods for PIF preparation. Those they turn to for expertise may have disparate recommendations.
Consensus is lacking on when water boiling during PIF preparation is necessary, for whom water boiling is needed, and if water is boiled, at which temperature to reconstitute PIF. CDC does not recommend heating water universally when preparing PIF as recommended by WHO, but cautions that if infants are at increased risk of infection (those who are younger than 2 months, were born prematurely, or have weakened immune systems), parents and caregivers may consider taking extra precautions to protect against Cronobacter by boiling water and then letting it cool for about 5 minutes (water should be at least 70°C/158°F) before mixing with PIF and further cooling to body temperature before consumption (3, 4). Following the PIF recall related to the 2022 Cronobacter investigation, consensus around PIF preparation guidelines has increased among federal agencies and professional organizations, such as the American Academy of Pediatrics (20, 21). However, PIF preparation instructions from Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) programs may vary from state to state (22–25). Similarly, PIF manufacturers also provide preparation instructions on their product containers and product webpages. Although their instructions warn that PIF is not a sterile product, manufacturers do not provide instructions on preparing PIF with hot water (at least 70°C/158°F) to destroy potential bacterial contamination such as Cronobacter. Rather, the manufacturers' instructions generally advise parents and caregivers to ask their baby's doctor if they should use water that has been boiled and then cooled when preparing PIF, with the intention to protect infants when the safety of the water source is uncertain (26–28).
These inconsistencies in guidelines might be resulting in confusion among parents, caregivers, and pediatricians concerning when water heating is needed. Water heating might be occurring when it is not needed, thus increasing risk of related burn injuries. Further research on parental and caregiver knowledge of PIF preparation recommendations and the extent to which recommendations are followed might be useful in identifying opportunities to prevent both Cronobacter infection and burn injuries.
Efforts to clarify existing recommendations and to offer additional guidance on how to safely put recommendations into practice might help to reduce burn injuries. Guidance does not always clearly differentiate between water heating to ensure a safe, clean water source, such as during emergencies (29), and water heating to inactivate Cronobacter, such as for infants at increased risk of infection (4). Highlighting both the rationale for precautions and recommended steps to take during PIF preparation in both scenarios might help increase understanding and reduce ineffective or unnecessary water heating. Factors such as the correct water temperature for reconstitution of PIF to inactivate Cronobacter and safe water temperature for feeding and how long it takes boiled water to cool to those temperatures could also be further explained and clarified. Additionally, pediatricians and others who provide guidance on infant feeding might offer further education on how to safely implement PIF preparation recommendations to avoid burn injuries.
Parent and caregiver education on general hot water safety might also help reduce PIF water heating-related burns among infants and other burns related to daily household tasks such as bathing and food and beverage preparation. Additional research on effective prevention of scalds in children may also be warranted (30). Further, increased education on bottle warming might also be needed. Warming bottles is not necessary, but if a parent or caregiver wishes to warm a bottle, they should avoid using a microwave (which can create hot spots that can cause scalds) and instead hold the bottle under running warm water (3). Bottle warming devices heat infant formula and breast milk to precise temperatures but may be cost-prohibitive.
Although this study utilized recent national data and the methodology used to identify cases was inclusive of many potentially related causes, findings from this study are subject to several limitations. First, exhaustive information was not available for each case as narratives were limited to only a few sentences. The authors may have misclassified an injury's association with PIF water heating. For example, injuries that were described as resulting from bottle preparation with hot water and were thus classified as being related to PIF preparation by the authors may have been truly caused by bottle warming but were described using preparation rather than warming phrasing. Additionally, as previously mentioned, case counts may be underestimated due to severity bias as only burns serious enough to prompt an ED visit were examined. However, to the authors' knowledge, national data for burns examined and treated in outpatient settings that include narrative incident scenarios allowing for identification of underlying cause are not available. Finally, because NEISS does not capture non-consumer product-related injuries, some burns related to PIF water heating may not have been captured in the data because infant formula is not classified as a consumer product by the Consumer Product Safety Commission. Since many other products used in PIF preparation (i.e., hot water, stoves, microwaves, and bottles) were queried, the impact on estimates would likely be minimal; however, cases that mentioned only infant formula with no other consumer product would not be captured in NEISS data.
Over the past 2 decades, the threat of Cronobacter infections resulting from contaminated infant formula has led to the convening of global expert meetings, the development of formula preparation guidelines, and the mandating of manufacturer testing requirements (6, 10, 11, 12, 31). More recently, both the potential contamination of PIF (8, 9) and the potential unintended consequences of increased risk of infant burns as a result of stringent PIF water heating recommendations have received attention (13). Increased parental and caregiver education on hot water safety by pediatricians and others who guide infant feeding may help to reduce these burn injuries. These estimates of the burden of burn injuries related to PIF water heating can be used to help inform discussions related to the risks and benefits of water heating for PIF preparation and wider PIF preparation recommendations. Guidance on PIF preparation should consider the potential for risk of burns to infants as a result of PIF water heating.
Note: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cpsc.gov/cgibin/NEISSQuery/home.aspx.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
KVC conceptualized and designed the study, conducted analyses, and drafted the initial manuscript. EHA conducted analyses and critically reviewed and revised the manuscript. SAA provided expert subject matter guidance and critically reviewed and revised the manuscript. CGP conceptualized and designed the study, oversaw analyses, and critically reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
This project was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the Centers for Disease Control and Prevention.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1125112/full#supplementary-material.
PIF, powdered infant formula; WHO, World Health Organization; NEISS, National Electronic Injury Surveillance System; ED, emergency department; CDC, Centers for Disease Control and Prevention.
1. US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th ed. December 2020. Accessed August 5, 2021. https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials
2. Breastfeeding among US children born 2012-2019, CDC National Immunization Survey. Centers for Disease Control and Prevention. Updated August 2022. Accessed April 6, 2023. https://www.cdc.gov/breastfeeding/data/nis_data/results.html
3. Infant formula preparation and storage. Centers for Disease Control and Prevention. Updated July 13, 2022. Accessed April 6, 2023. https://www.cdc.gov/nutrition/infantandtoddlernutrition/formula-feeding/infant-formula-preparation-and-storage.html
4. Cronobacter infection and infants. Centers for Disease Control and Prevention. Updated February 14, 2023. Accessed April 6, 2023. https://www.cdc.gov/cronobacter/infection-and-infants.html
5. Cronobacter frequently asked questions. Centers for Disease Control and Prevention. Updated December 29, 2022. Accessed April 6, 2023. https://www.cdc.gov/cronobacter/technical.html
7. Strysko J, Cope JR, Martin H, Tarr C, Hise K, Collier S, et al. Food safety and invasive Cronobacter infections during early infancy, 1961-2018. Emerg Infect Dis. (2020) 26(5):857–65. doi: 10.3201/eid2605.190858
8. FDA Investigation of Cronobacter Infections: Powdered Infant Formula (February 2022). Food and Drug Administration. Updated August 1, 2022. Accessed April 6, 2023. https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigation-cronobacter-infections-powdered-infant-formula-february-2022#622f1cc391bf6
9. Cronobacter and Powdered Infant Formula Investigation. Centers for Disease Control and Prevention. Updated May 24, 2022. Accessed April 6, 2023. https://www.cdc.gov/cronobacter/outbreaks/infant-formula.html
10. Food and Agriculture Organization of the United Nations and World Health Organization. Enterobacter sakazakii and other microorganisms in powdered infant formula: meeting report. Microbiological Risk Assessment Series No. 6. 2004. Accessed August 5, 2021. http://www.fao.org/documents/card/en/c/3ffdb8bc-8c2c-5d73-87a4-6e9698b164ae/
11. Food and Agriculture Organization of the United Nations and World Health Organization. Enterobacter sakazakii and Salmonella in powdered infant formula: meeting report. Microbiological Risk Assessment Series No. 10. 2006. Accessed August 5, 2021. http://www.fao.org/documents/card/en/c/50a37040-c71d-5f10-8a25-e37370a38ebd/
12. World Health Organization. Safe preparation, storage and handling of powdered infant formula: guidelines. 2007. Accessed August 5, 2021. https://www.who.int/publications/i/item/9789241595414
13. Wilkinson TA, Scott EK, Carroll AE. Mixed message on formula mixing. Pediatrics. (2019) 143(6):e20182525. doi: 10.1542/peds.2018-2525
14. Burn prevention. Centers for Disease Control and Prevention. Updated February 6, 2019. Accessed August 5, 2021. https://www.cdc.gov/safechild/burns/index.html
15. National Electronic Injury Surveillance System (NEISS). US Consumer Product Safety Commission. Accessed August 5, 2021. https://www.cpsc.gov/Research–Statistics/NEISS-Injury-Data
16. US Consumer Product Safety Commission. NEISS the National Electronic Injury Surveillance System a tool for researchers. March 2000. Accessed August 5, 2021. https://www.cpsc.gov/s3fs-public/pdfs/blk_media_2000d015.pdf
17. US Consumer Product Safety Commission. The NEISS sample (design and implementation) 1997 to present. June 2001. Accessed August 5, 2021. https://www.cpsc.gov/s3fs-public/pdfs/blk_media_2001d011-6b6.pdf
18. US Consumer Product Safety Commission. NEISS coding manual January 2019. January 2019. Accessed August 5, 2021. https://www.cpsc.gov/s3fs-public/2019-NEISS-Codng-Manual.pdf?H9rT3ZRKAHmrYCJUl5ta3nVPjSKeN3QK
19. Redmond E, Griffith C. Food Standards Agency Research Project B13008 an investigation into the attitudes and behaviors of consumers and caregivers in the preparation, handling, and storage of powdered infant formula inside and outside the home. Food Standards Agency. Updated January 8, 2014. Accessed August 5, 2021. https://www.food.gov.uk/research/microbial-risk-assessment-b13/an-investigation-into-the-attitudes-and-behaviours-of-consumers-and-caregivers-in-the-preparation-handling-storage-and-feeding
20. Food and Drug Administration. How to Prevent Cronobacter Illness: Prepare and Store Powdered Infant Formula Safely. Accessed April 6, 2023. https://www.fda.gov/media/158904/download
21. How to Safely Prepare Baby Formula with Water. American Academy of Pediatrics. Updated May 17, 2022. Accessed April 6, 2023. https://healthychildren.org/English/ages-stages/baby/formula-feeding/Pages/How-to-Safely-Prepare-Formula-with-Water.aspx
22. How to Mix Infant Formula. Texas WIC. Accessed April 6, 2023. https://www.texaswic.org/health-nutrition/baby/how-mix-infant-formula
23. California WIC. When you feed me formula. Updated May 2022. Accessed April 6, 2023. https://myfamily.wic.ca.gov/Content/Documents/NutritionHealth/Infants/WIC-When-You-Feed-Me-2022-Mobile-EN.pdf
24. Maryland WIC. Help Me Be Healthy Birth to 6 Months. Accessed April 6, 2023. https://health.maryland.gov/phpa/wic/Documents/wic_nutrition/N-54_HMBH_0%20to%206%20mos_Eng_proof_pages_0619.pdf
25. Florida WIC. Food for Baby's First Year. Accessed April 6, 2023. https://www.floridahealth.gov/programs-and-services/wic/nutrition-materials/_documents/food-for-babys-first-year-eng.pdf
26. How to make a baby bottle. Similac. Accessed April 6, 2023. https://www.similac.com/baby-feeding/formula-guide/how-to-make-a-bottle.html
27. Enfamil® Infant Formula Powder Enfamil. Accessed April 6, 2023. https://www.enfamil.com/products/enfamil-infant-formula/powder-can-29-4-oz-can/
28. Gerber® Good Start® GentlePro Powder Infant Formula. Gerber. Accessed April 6, 2023. https://www.gerber.com/gerber-baby-good-start-gentle-powder-formula
29. How to Prepare and Store Powdered Infant Formula During an Emergency. Centers for Disease Control and Prevention. Updated March 3, 2023. Accessed April 6, 2023. https://www.cdc.gov/nutrition/emergencies-infant-feeding/powdered-infant-formula.html
30. Zou K, Wynn PM, Miller P, Hindmarch P, Majsak-Newman G, Young B, et al. Preventing childhood scalds within the home: overview of systematic reviews and a systematic review of primary studies. Burns. 2015;41(5):907–24. doi: 10.1016/j.burns.2014.11.002
31. Food and Drug Administration. Outline of FDA's Strategy to Help Prevent Cronobacter sakazakii Illnesses Associated with Consumption of Powdered Infant Formula. Updated November 15, 2022. Accessed April 6, 2023. https://www.fda.gov/food/new-era-smarter-food-safety/outline-fdas-strategy-help-prevent-cronobacter-sakazakii-illnesses-associated-consumption-powdered
Keywords: infant formula, infant nutrition, burns, injuries, nutrition guidelines
Citation: Chiang KV, Anstey EH, Abrams SA and Perrine CG (2023) Infant burn injuries related to water heating for powdered infant formula preparation. Front. Pediatr. 11:1125112. doi: 10.3389/fped.2023.1125112
Received: 15 December 2022; Accepted: 17 April 2023;
Published: 4 May 2023.
Edited by:
Jérémie F. Cohen, Necker-Enfants malades Hospital, FranceReviewed by:
Nathan M. Money, The University of Utah, United States© 2023 Chiang, Anstey, Abrams and Perrine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katelyn V. Chiang a2F0ZWx5bi5jaGlhbmdAZW1vcnkuZWR1
†Present Address: Katelyn V. Chiang, Oak Ridge Institute for Science and Education, Oak Ridge, TN, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.