
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr. , 14 February 2023
Sec. Pediatric Neurology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1123348
This article is part of the Research Topic Stress Neurobiology in COVID 19: diagnosis, neuroimaging and therapeutic tools View all 9 articles
COVID-19 in the pediatric population is mostly asymptomatic. However, 1 out of 5 children presents non-specific neurologic symptoms such as headache, weakness, or myalgia. Furthermore, rarer forms of neurological diseases are increasingly being described in association to a SARS-CoV-2 infection. Encephalitis, stroke, cranial nerves impairment, Guillain-Barré syndrome or acute transverse myelitis have been reported and account for around 1% of pediatric COVID-19 cases. Some of these pathologies may occur during or after the SARS-CoV-2 infection. The pathophysiological mechanisms range from direct invasion of the central nervous system (CNS) by SARS-CoV-2 itself to postinfectious immune-mediated CNS inflammation. In most cases, patients presenting neurological pathologies related to SARS-CoV-2 infection are at greater risk of life-threatening complications and should be closely monitored. Further studies are needed to acknowledge the potential long-term neurodevelopmental consequences of the infection.
According to the world health organization (WHO), pediatric cases of COVID-19 account for up to 8.5% of cases in 2020. However, this proportion turns out to be much higher in some countries such as the United States where children represent 18.5% of cases (1, 2). Given the absence or poor symptoms of COVID-19 in this population, this prevalence is probably still underestimated (3–5).
When symptomatic, the most common symptoms are fever, cough and rhinorrhea (6) and some gastrointestinal symptoms such as diarrhea, vomiting or abdominal pain, can be present as well (6). The main reported complication related to SARS-CoV-2 infection in children is the multisystem inflammatory syndrome (MIS-C). The most recent definition of MIS-C is an illness in a person aged <21 years associated to (i) a subjective or documented fever, (ii) a clinical severity requiring hospitalization or resulting in death, (iii) the evidence of a systemic inflammation and (iv) new onset manifestations in at least two of the following categories (Shock, cardiac, mucocutaneous, gastrointestinal or hematologic involvement) in the absence of a more likely alternative diagnosis (7). These clinical criteria must be associated to laboratory confirmation of a SARS-CoV-2 infection to confirm the diagnosis of MIS-C (Table 1).
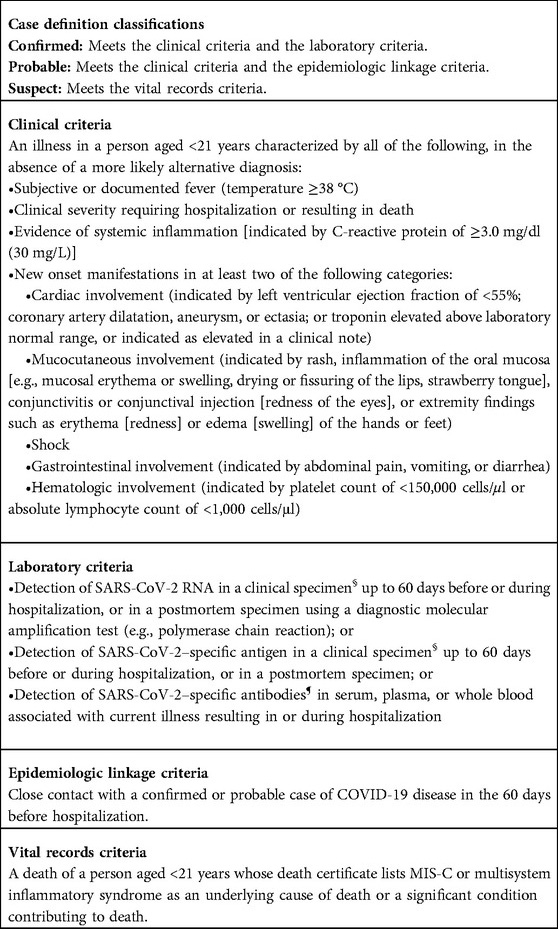
Table 1. Council of state and territorial epidemiologists/CDC surveillance case definition for multisystem inflammatory syndrome (MIS-C) in children associated with SARS-CoV-2 infection – June 2022 (7).
Neurological complications have also been described, in both adults and children (8–10). They can be differentiated into (i) non-specific neurological symptoms, like headache, weakness, myalgia or (ii) specific central or peripheral nervous system pathologies, like encephalitis, cranial nerves impairment, stroke, or Guillain-Barré syndrome (10–13). Non-specific neurological symptoms have been reported in up to 22% of pediatric patients. Specific neurologic pathologies account for 1% of cases (10, 12, 14–16) and are generally associated to more severe forms of COVID-19 (14, 17).
The aim of this manuscript is to present the different pathophysiological pathways underlying the neurological consequences of SARS-CoV-2, describe their clinical presentations and discuss therapeutics and prevention.
The mechanisms underlying the pathophysiological pathways of neurological symptoms of SARS-CoV-2 infection are still under study. However, a number of hypotheses have already been put forward. Some may explain (i) the occurrence of neurologic pathologies during acute SARS-CoV-2 infection, while others (ii) the neurologic pathologies showing after recovery.
The neurological symptoms occurring during the SARS-CoV-2 infection seem to be related to two different mechanisms: the direct invasion of the central nervous system (CNS) by the virus and an abnormal host inflammatory response.
The direct invasion of the CNS is possible by hematogenous or neuronal dissemination. In both cases, the spike protein of the virus binds both angiotensin-converting enzyme 2 (ACE2) receptors or transmembrane serine protease 2 (TMPRSS2) to infect the cells (18–20). In the hematogenous pathway, the virus first infects the respiratory tract, and then, from the bloodstream it crosses the blood-brain barrier (BBB) to enter the CNS. Once the BBB is disrupted, leukocytes infected by the SARS-CoV-2 can pass through, leading to their dissemination in the CNS and subsequent inflammation. Regarding the viral dissemination by retrograde axonal transport, the main hypothesis is that SARS-CoV-2 infects the olfactory receptor neurons (the only part of the CNS that is not protected by the dura), crosses the neuroepithelium reaching the olfactory bulb and then invades the brainstem, cortex and basal ganglia via the nerves (8, 10, 13, 19–21). The direct invasion of the CNS, leading to encephalitis, is however probably infrequent. Indeed, PCR of SARS-CoV2 performed in CSF when patients presented neurologic complications yielded positive in a very small proportion of cases (6% in a systematic review reporting 304 CSF studies) (22, 23). This may be due to the low sensitivity of PCR as it is currently performed, or to the fact that the SARS-CoV-2 has a weak direct neurotrophic effect.
Strokes and vasculitis have also been described during an acute SARS-CoV-2 infection (10, 19, 24, 25). They could be the consequence of an aberrant host inflammatory response to the virus. Indeed, it has been observed that coronaviruses, particularly SARS-CoV-2, trigger molecular mechanisms that interfere with the host adaptative immune response (ineffective IFN response, reduction of lymphocyte count etc) (26). Moreover, the association with a pro-coagulable state and endothelial dysfunction (vascular disruption, activation of the clotting cascade by exposure of thrombogenic activator) increases the risk of thrombotic complications.
In this case, too, two hypotheses are to be considered. The first one is based on a retarded multisystemic inflammatory response to the viremia. This uncontrolled inflammatory state leads to the multi-organ damages that have been described in MIS-C. The release of inflammatory agents leads to a cytokine storm causing the disruption of the integrity of the BBB allowing various molecules (tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, IL-6, IL-12, and interferon-gamma (INFγ)), as well as infected and immunes cells to penetrate into the CNS (10–13, 25, 27). Neurologic complications during MIS-C seems to be both non-specific and specific neurologic pathologies (13, 28–30).
The second hypothesis relies on postinfectious immune-mediated mechanisms: SARS-CoV-2 might induce an autoimmune response by activating antibodies against brain components or via “molecular mimicry”: the spike protein of the SARS-CoV-2 could cross-reactive with CNS components explaining some of the neurologic complications (10, 12, 19, 27, 31–33). Indeed, the SARS-CoV-2 spike protein binds not only to the ACE-2 receptor but also to sialic acid-containing glycoproteins and gangliosides on cell surfaces. There could be a cross-reactivity between components of the spike protein binding normally to gangliosides and some of surface peripheral nerve glycolipids residues. The molecular mimicry could explain reported post-infectious pathologies like Guillain Barré-syndrome (GBS) or acute disseminated encephalomyelitis (ADEM) (19, 34).
Besides, as in many viral infections, neurological manifestations can be caused or aggravated by hypoxemia, septic shock, metabolic or electrolyte disorders that COVID-19 infection can induce (10, 12, 27, 35).
It has been well documented and described that children present less severe forms of SARS-CoV-2 than adults. The mortality rate for children has been estimated under 0.1% with about 2% of children admitted to intensive care units (36). Different factors may contribute to this difference. First of all, children usually have no comorbidities, so, they do not suffer from the baseline pro-inflammatory state (increased by hypertension, smoking, hypercholesterolemia, obesity etc.) more commonly observed in aging adults (26). Secondly, the host immune response in children is more efficient than in adults (26) (increased number of adaptive immunity cells CD4+/CD8+, better functional capacity of B-cells) and the trained innate immunity (37) (frequent exposure to antigens as vaccine or infections) increases its baseline reactivity to pathogens. These particularities may explain the smaller number of severe COVID-19 cases in children, but they do not completely prevent immune dysregulation mediated by the coronaviruses and its consequence.
Encephalitis is an inflammation of the brain causing neurologic dysfunction. Etiologies are multiples, as reported above: direct invasion, post-infectious pathologies such as acute disseminated encephalomyelitis (ADEM), or non-infectious condition such as N-methyl-D-aspartate receptor (NMDAR) encephalitis. Non-autoimmune encephalitis could concern between 7.5% and 49% of hospitalized adult COVID-19 patients. No incidence has been established in children due to its low incidence (38), however, many case reports have been described (Table 2). The main symptoms of meningoencephalitis due to COVID-19 infection are seizures (29.5%), confusion (23.2%), headache (20.5%), alteration of mental status (11.6%) (39). Psychotic symptoms, cerebellar ataxia and focal deficit have also been described but they seem rarer (8, 19, 39). In most cases, when the study of the CSF has been carried out, the RT-PCR in search of SARS-CoV-2 was negative, or a non-specific lymphocytic pleocytosis could be found (11, 40). Cases of N-methyl-Aspartate receptor (NMDAR) encephalitis as well as MOG antibody-associated encephalitis have been described (39, 41–45).
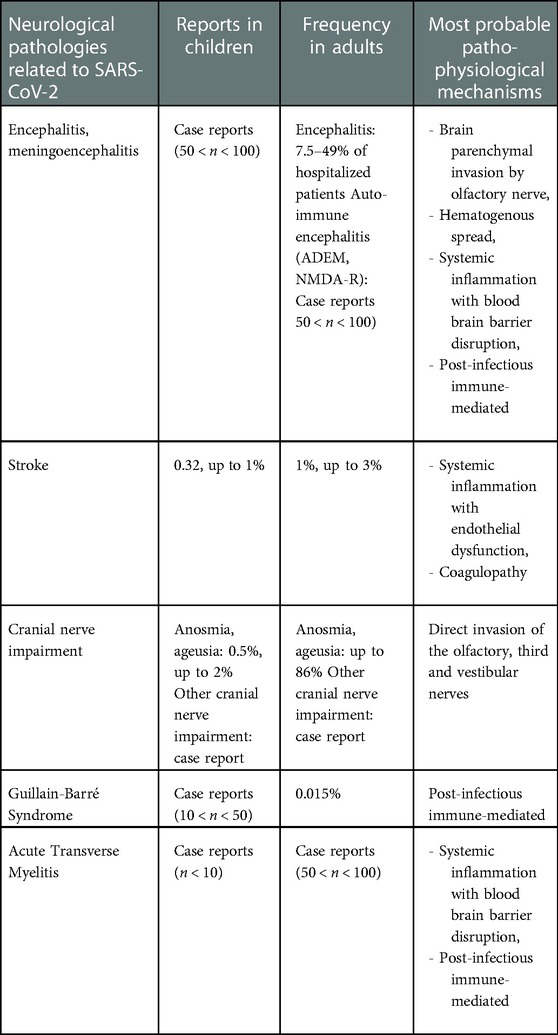
Table 2. Summary of frequencies of neurological pathologies related to SARS-CoV-2 in children and in adults, and most probable pathophysiological mechanisms.
Brain MRI studies are reported for only a few children, and are usually normal. Some authors report T2-hyperintense lesions associated with restricted diffusion in the splenium of the corpus callosum (SCC) (14, 15, 19, 46). These lesions seem to be more frequent when encephalitic symptoms occurs during MIS-C, and they are similar to the ones described in other inflammatory syndromes such as Kawasaki disease with reversible encephalopathy (47, 48). Comparing to the adult population with encephalitis, Ellul and al report 6 out of 8 cases with a normal MRI, while two other studies report between 44% and 100% of abnormal MRI, with FLAIR signals in the frontotemporal cortex (8, 31, 49). Electroencephalogram (EEG), when performed, shows nonspecific abnormalities such as diffuse slowing. Focal abnormalities are rarely reported, particularly in cases of encephalitis associated with seizures. In the great majority of cases, the EEG normalized within 2 weeks (8, 11–13, 31, 50).
The prognosis of encephalitis during a SARS-CoV-2 infection is quite hard to define. Only a few cases have been described, and they are quite heterogeneous: with or without a positive PCR in the CSF, presence or absence of NMDAR antibodies, normal or abnormal neuroimaging, treated or not by steroids and so on. Some studies report a complete recovery (19, 41, 50, 51) whereas others report an unfavorable outcome (14).
Cases of ADEM post-COVID do not clinically differ from ADEM post other viruses (41, 48, 52–55). So, the encephalitic presentation described above is enriched by focal symptoms: motor deficit, loss of reflexes or hyperreflexia, impairment of cranial nerves. Moreover, acute hemorrhagic necrotizing encephalitis have also been reported (39, 56, 57). All patients (adults and children) had a fatal outcome, except for a recent report of an 11-year-old boy treated with tocilizumab (57).
An ICU monitoring seems mandatory in COVID-19 encephalitis because of a great risk of life-threatening complications (12–14).
SARS-CoV-2 infection - as other viruses - with its degree of coagulopathy, inflammation and endothelial damage can cause secondary cerebral arteriopathy and lead to stroke (41). According to studies, 0.32 to 1% of children admitted for COVID-19 presented stroke, compared to 1 to 3% of adults (8, 58–62) (Table 2).
The majority of the described pediatric cases presented risk factors for strokes (14, 35, 41, 58) such as cardiac disease, arteriopathy/arteritis, or prothrombotic disorders. However, LaRovere et al. (14) described 4 healthy children out of 12 stroke cases, for which, COVID-19 seemed to be the only causative factor. A recent review of 23 pediatric cases found similar results (62). Interestingly, even asymptomatic children could suffer from strokes, suggesting that the aberrant host immune response would contribute to increase the risk of coagulopathy, thrombocytopathy and endotheliopathy conducting to strokes (63) even in non-symptomatic cases (64, 65). Indeed, more than 80% had a biological inflammatory syndrome and all presented either arteritis or focal arteriopathy on brain MRI. Elevated D-Dimer appeared as an independent biomarker for SARS-CoV-2-related ischemic stroke in adults and as a risk factor of thrombotic-events in children (62, 64). D-Dimer, could be a parameter to monitor whilst SARS-CoV-2 infection as a stroke predictive factor.
Regarding the prognosis for these patients, it mainly depended on the size and localization of the stroke, as well as the presence of risk factors.
The loss of smell and taste is commonly associated with SARS-CoV-2 infection in the adult population (8, 31). The pathophysiology is not yet fully understood but is likely associated to direct olfactory invasion (12, 66). Its prevalence has been estimated at its highest to 86%, but varies widely according to studies. This could be partly explained by the subjective nature of this symptom (8). In children compared to adult, this complication seems to be less frequent, but it is very difficult to diagnose, if not impossible under a certain age (13, 21, 61–64). Thus, the incidence in children has been estimated to be around 0.5% to 2% and is probably under-reported (10, 14, 16) (Table 2).
Explorations carried out when children presented a loss of taste or smell are practically non-existent, and the few cases reporting brain MRI results do not seem to show any anomaly of the olfactory region (48, 67). Some studies in adults have shown an increased olfactory bulb size with a pathological signal at MRI (68–70).
The prognosis is usually good, and symptoms disappear within 1 to 2 weeks. In some cases, they can persist after other symptoms have resolved. Treatments for dysgeusia are rarely reported. Several treatments have been tested for anosmia: saline irrigations, nasal or oral corticosteroids (67, 71). The only one that seems to have shown its effectiveness in adults, is olfactory rehabilitation (72).
Vestibular neuritis as well as facial nerve palsy have been reported in children during a COVID-19 infection (73–75). SARS-CoV-2 could explain the increase of facial nerve palsy cases during the pandemic in both adults and children (76–78). Other cranial nerve palsies, with enhancement of the nerve at brain MRI, have been described (48). Particularly, cases of third cranial nerve palsy (79–81) revealed by unilateral diplopia and ptosis have been described with good outcomes after corticosteroid treatment.
Guillain-Barré syndrome is a post-infectious immune-mediated polyneuropathy. The estimated incidence of GBS is 15 cases per 100,000 SARS-CoV-2 infections (82). As previously described for GBS not related to COVID-19 infection, male gender appears to be a risk factor even in the pediatric population (82–84) (Table 2). The exact pathophysiology has not been elucidated yet (19, 35). The syndrome may appear between a few days and several weeks after a SARS-CoV-2 infection (11). According to the reported cases, the clinical presentation is similar to non-COVID 19 related cases: rapid ascending bilateral decrease of the motor force, diminished or abolished tendon reflex and distal paresthesia. Dysautonomia and pain may accompany the course of the disease. Electrophysiological studies have shown acute inflammatory demyelinating polyneuropathy. MRI of the spine showed abnormal enhancement of the nerve roots. CSF analysis confirmed the cytological dissociation of albumin (42, 55, 85–89).
The outcome after treatment with intravenous immunoglobulin varies between a total recovery and the persistence of a severe handicap or even death. A systematic review carried out in an adult population reported more than 70% of patients with a good prognosis (90). It seems that COVID-19 does not influence the prognosis of the disease (82).
The pediatric literature reports rare but typical cases of transverse myelitis in patients with no medical history (Table 2) and in whom SARS-CoV-2 infection seemed to be the only triggering event (91–94). This complication occurs during or longer after the SARS-CoV-2 infection and has been more often described in adults (95). The clinical presentation is characterized by the evolution towards flaccid quadriparesis with areflexia. Involvement may be cervical, thoracic or extended longitudinally. MRI commonly finds a hyperintense T2 signal localized in the affected cord (48). Depending on the severity of the disease, the patients received boluses of methylprednisolone either alone or with plasma exchanges or immunoglobulin. Treatment aimed to prevent permanent disabilities in these case reports (94).
SARS-CoV-2 infection in children can be responsible for rare and various neurological consequences. In view of the different pathophysiologic mechanisms and the adult literature, there is little evidence supporting the interest of antiviral therapy in central or peripheral nervous system pathologies related to SARS-CoV-2 previously described (38). Indeed, neurological manifestations are mainly immune-mediated, so they should be better managed by anti-inflammatory (corticosteroids) or immunomodulatory treatments such as immunoglobulin, colchicine or tocilizumab (anti-IL6 antibodies) (96).
As for today, there is no reports, in the literature (97), of neurological consequences of the various SARS-CoV-2 vaccines in the pediatric population. Regarding immunogenicity, studies (98, 99) report very small numbers of MIS-C after receiving one dose or more of COVID-19 vaccine. Moreover, in the adult population, the incidence of GBS (100) or central veinous thrombosis (101) is lower after COVID-19 vaccination, supporting the safety and interest of the vaccine to prevent neurological consequences as well as severe forms of COVID-19 and the transmission of SARS-CoV-2.
Since the beginning of the SARS-CoV-2 pandemic, the progressive increase of pediatric cases has raised attention on rare forms of COVID-19 that were not highlighted previously. Reports of neurological consequences of SARS-CoV-2 range from non-specific neurological symptoms to specific central or peripheral nervous system diseases like encephalitis, cranial nerves impairment, stroke or Guillain-Barré syndrome. The host immune response of children being more efficient and not interfered by a baseline pro-inflammatory state, these neurological consequences of SARS-CoV-2 seem to be less frequent in children than in adultes. In most cases, patients presenting neurological pathologies related to SARS-CoV-2 infection have poorer outcomes, and are at risk of life-threatening complications.
The long-term neurodevelopmental consequences after a neurological complication of COVID-19 are unknown and need further investigations.
MC investigated the literature, wrote the original draft and edited the manuscript. CC investigated the literature and edited the manuscript. LT conceptualized the methodology of the review, and reviewed the manuscript. LL conceptualized the methodology of the review, reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. World Health Organization. Coronavirus disease (COVID-19): Schools. 2020. Disponible sur: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-schools
2. American Academy of Pediatrics. Children and COVID-19 State Data Report 1.20.22. United States; 2022 févr. Disponible sur: https://downloads.aap.org/AAP/PDF/AAP%20and%20CHA%20-%20Children%20and%20COVID-19%20State%20Data%20Report%201.20.22_FINAL.pdf
3. Nikolopoulou GB, Maltezou HC. COVID-19 in children: where do we stand? Arch Med Res. (2022) 53(1):1–8. doi: 10.1016/j.arcmed.2021.07.002
4. Parri N, Lenge M, Buonsenso D. Children with COVID-19 in pediatric emergency departments in Italy. N Engl J Med. (2020) 383(2):187–90. doi: 10.1056/NEJMc2007617
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. (2020) 323(13):1239–42. doi: 10.1001/jama.2020.2648
6. Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. (2020) 4(9):653–61. doi: 10.1016/S2352-4642(20)30177-2
7. Melgar M, Lee EH, Miller AD, Lim S, Brown CM, Yousaf AR, et al. Council of state and territorial epidemiologists/CDC surveillance case definition for multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection - United States. MMWR Recomm Rep. (2022) 71(4):1–14. doi: 10.15585/mmwr.rr7104a1
8. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. (2020) 19(9):767–83. doi: 10.1016/S1474-4422(20)30221-0
9. Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. (2020) 143(10):3104–20. doi: 10.1093/brain/awaa240
10. Schober ME, Pavia AT, Bohnsack JF. Neurologic manifestations of COVID-19 in children: emerging pathophysiologic insights. Pediatr Crit Care Med. (2021) 22(7):655–61. doi: 10.1097/PCC.0000000000002774
11. Govil-Dalela T, Sivaswamy L. Neurological effects of COVID-19 in children. Pediatr Clin North Am. (2021) 68(5):1081–91. doi: 10.1016/j.pcl.2021.05.010
12. Panda PK, Sharawat IK, Panda P, Natarajan V, Bhakat R, Dawman L. Neurological complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis. J Trop Pediatr. (2021) 67(3):fmaa070. doi: 10.1093/tropej/fmaa070
13. Siracusa L, Cascio A, Giordano S, Medaglia AA, Restivo GA, Pirrone I, et al. Neurological complications in pediatric patients with SARS-CoV-2 infection: a systematic review of the literature. Ital J Pediatr. (2021) 47(1):123. doi: 10.1186/s13052-021-01066-9
14. LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. (2021) 78(5):536–47. doi: 10.1001/jamaneurol.2021.0504
15. Abdel-Mannan O, Eyre M, Löbel U, Bamford A, Eltze C, Hameed B, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. (2020) 77(11):1440–5. doi: 10.1001/jamaneurol.2020.2687
16. Pousa PA, Mendonça TSC, Oliveira EA, Simões-E-Silva AC. Extrapulmonary manifestations of COVID-19 in children: a comprehensive review and pathophysiological considerations. J Pediatr (Rio J). (2021) 97(2):116–39. doi: 10.1016/j.jped.2020.08.007
17. Antoon JW, Hall M, Howard LM, Herndon A, Freundlich KL, Grijalva CG, et al. COVID-19 and acute neurologic complications in children. Pediatrics. (2022) 150(5):e2022058167. doi: 10.1542/peds.2022-058167
18. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. (2020) 367(6485):1444–8. doi: 10.1126/science.abb2762
19. Lin JE, Asfour A, Sewell TB, Hooe B, Pryce P, Earley C, et al. Neurological issues in children with COVID-19. Neurosci Lett. (2021) 743:135567. doi: 10.1016/j.neulet.2020.135567
20. Nordvig AS, Fong KT, Willey JZ, Thakur KT, Boehme AK, Vargas WS, et al. Potential neurologic manifestations of COVID-19. Neurol Clin Pract. (2021) 11(2):e135–46. doi: 10.1212/CPJ.0000000000000897
21. Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol. (2020) 16(11):636–44. doi: 10.1038/s41582-020-0398-3
22. Lewis A, Frontera J, Placantonakis DG, Lighter J, Galetta S, Balcer L, et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J Neurol Sci. (2021) 421:117316. doi: 10.1016/j.jns.2021.117316
23. Cosentino G, Todisco M, Hota N, Della Porta G, Morbini P, Tassorelli C, et al. Neuropathological findings from COVID-19 patients with neurological symptoms argue against a direct brain invasion of SARS-CoV-2: a critical systematic review. Eur J Neurol. (2021) 28(11):3856–65. doi: 10.1111/ene.15045
24. Gulko E, Overby P, Ali S, Mehta H, Al-Mufti F, Gomes W. Vessel wall enhancement and focal cerebral arteriopathy in a pediatric patient with acute infarct and COVID-19 infection. Am J Neuroradiol. (2020) 41(12):2348–50. doi: 10.3174/ajnr.A6778
25. Valderas C, Méndez G, Echeverría A, Suarez N, Julio K, Sandoval F. COVID-19 and neurologic manifestations: a synthesis from the child neurologist's Corner. World J Pediatr. (2022) 18(6):373–82. doi: 10.1007/s12519-022-00550-4
26. Costagliola G, Spada E, Consolini R. Age-related differences in the immune response could contribute to determine the spectrum of severity of COVID-19. Immun Inflamm Dis. (2021) 9(2):331–9. doi: 10.1002/iid3.404
27. Principi N, Esposito S. Are we sure that the neurological impact of COVID 19 in childhood has not been underestimated? Ital J Pediatr. (2021) 47(1):191. doi: 10.1186/s13052-021-01144-y
28. Santos MO, Gonçalves LC, Silva PAN, Moreira ALE, Ito CRM, Peixoto FAO, et al. Multisystem inflammatory syndrome (MIS-C): a systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. J Pediatr (Rio J). (2022) 98(4):338–49. doi: 10.1016/j.jped.2021.08.006
29. Hennon TR, Penque MD, Abdul-Aziz R, Alibrahim OS, McGreevy MB, Prout AJ, et al. COVID-19 associated multisystem inflammatory syndrome in children (MIS-C) guidelines; a Western New York approach. Prog Pediatr Cardiol. (2020) 29:101232. doi: 10.1016/j.ppedcard.2020.101232
30. Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. (2020) 7(7):69. doi: 10.3390/children7070069
31. Aghagoli G, Gallo Marin B, Katchur NJ, Chaves-Sell F, Asaad WF, Murphy SA. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocrit Care. (2021) 34(3):1062–71. doi: 10.1007/s12028-020-01049-4
32. Kreye J, Reincke SM, Prüss H. Do cross-reactive antibodies cause neuropathology in COVID-19? Nat Rev Immunol. (2020) 20(11):645–6. doi: 10.1038/s41577-020-00458-y
33. Saini L, Krishna D, Tiwari S, Goyal JP, Kumar P, Khera D, et al. Post-COVID-19 immune-mediated neurological complications in children: an ambispective study. Pediatr Neurol. (2022) 136:20–7. doi: 10.1016/j.pediatrneurol.2022.06.010
34. Dalakas MC. Guillain-Barré syndrome: the first documented COVID-19–triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflammation. (2020) 7(5). Disponible sur: https://nn.neurology.org/content/7/5/e781. doi: 10.1212/NXI.0000000000000781
35. Valderas C, Méndez G, Echeverría A, Suarez N, Julio J, Sandoval F. COVID-19 and neurologic manifestations: a synthesis from the child neurologist's Corner. World J Pediatr : WJP. (2022) 18(6). doi: 10.1007/s12519-022-00550-4 Disponible sur: https://pubmed.ncbi.nlm.nih.gov/35476245/.
36. Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. (2020) 179(7):1029–46. doi: 10.1007/s00431-020-03684-7
37. Mantovani A, Netea MG. Trained innate immunity, epigenetics, and COVID-19. Phimister EG, éditeur. N Engl J Med. (2020) 383(11):1078–80. doi: 10.1056/NEJMcibr2011679
38. Graham EL, Koralnik IJ, Liotta EM. Therapeutic approaches to the neurologic manifestations of COVID-19. Neurotherapeutics. (2022) 19(5):1435–66. doi: 10.1007/s13311-022-01267-y
39. Islam MA, Cavestro C, Alam SS, Kundu S, Kamal MA, Reza F. Encephalitis in patients with COVID-19: a systematic evidence-based analysis. Cells. (2022) 11(16):2575. doi: 10.3390/cells11162575
40. Chen TH. Neurological involvement associated with COVID-19 infection in children. J Neurol Sci. (2020) 418:117096. doi: 10.1016/j.jns.2020.117096
41. O’Loughlin L, Alvarez Toledo N, Budrie L, Waechter R, Rayner J. A systematic review of severe neurological manifestations in pediatric patients with coexisting SARS-CoV-2 infection. Neurol Int. (2021) 13(3):410–27. doi: 10.3390/neurolint13030041
42. Sánchez-Morales AE, Urrutia-Osorio M, Camacho-Mendoza E, Rosales-Pedraza G, Dávila-Maldonado L, González-Duarte A, et al. Neurological manifestations temporally associated with SARS-CoV-2 infection in pediatric patients in Mexico. Childs Nerv Syst. (2021) 37(7):2305–12. doi: 10.1007/s00381-021-05104-z
43. Burr T, Barton C, Doll E, Lakhotia A, Sweeney M. N-Methyl-d-Aspartate receptor encephalitis associated with COVID-19 infection in a toddler. Pediatr Neurol. (2021) 114:75–6. doi: 10.1016/j.pediatrneurol.2020.10.002
44. Sarigecili E, Arslan I, Ucar HK, Celik U. Pediatric anti-NMDA receptor encephalitis associated with COVID-19. Childs Nerv Syst. (2021) 37(12):3919–22. doi: 10.1007/s00381-021-05155-2
45. Ahsan N, Jafarpour S, Santoro JD. Myelin oligodendrocyte glycoprotein antibody encephalitis following severe acute respiratory syndrome coronavirus 2 in a pediatric patient. Clin Exp Pediatr. (2021) 64(6):310–2. doi: 10.3345/cep.2020.01963
46. Abel D, Shen MY, Abid Z, Hennigan C, Boneparth A, Miller EH, et al. Encephalopathy and bilateral thalamic lesions in a child with MIS-C associated with COVID-19. Neurology. (2020) 95(16):745–8. doi: 10.1212/WNL.0000000000010652
47. Takanashi Ji, Shirai K, Sugawara Y, Okamoto Y, Obonai T, Terada H. Kawasaki disease complicated by mild encephalopathy with a reversible splenial lesion (MERS). J Neurol Sci. (2012) 315(1):167–9. doi: 10.1016/j.jns.2011.11.022
48. Lindan CE, Mankad K, Ram D, Kociolek LK, Silvera VM, Boddaert N, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. (2021) 5(3):167–77. doi: 10.1016/S2352-4642(20)30362-X
49. Kandemirli SG, Dogan L, Sarikaya ZT, Kara S, Akinci C, Kaya D, et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. (2020) 297(1):E232–5. doi: 10.1148/radiol.2020201697
50. Olivotto S, Basso E, Lavatelli R, Previtali R, Parenti L, Fiori L, et al. Acute encephalitis in pediatric multisystem inflammatory syndrome associated with COVID-19. Eur J Paediatr Neurol. (2021) 34:84–90. doi: 10.1016/j.ejpn.2021.07.010
51. Nathan N, Prevost B, Corvol H. Atypical presentation of COVID-19 in young infants. Lancet. (2020) 395(10235):1481. doi: 10.1016/S0140-6736(20)30980-6
52. Akçay N, Bektaş G, Menentoğlu ME, Oğur M, Sofuoğlu Aİ, Palabiyik FB, et al. COVID-19-associated acute disseminated encephalomyelitis-like disease in 2 children. Pediatr Infect Dis J. (2021) 40(11):e445–50. doi: 10.1097/INF.0000000000003295
53. de Miranda Henriques-Souza AM, de Melo ACMG, de Aguiar Coelho Silva Madeiro B, Freitas LF, Sampaio Rocha-Filho PA. Gonçalves FG. Acute disseminated encephalomyelitis in a COVID-19 pediatric patient. Neuroradiology. (2021) 63(1):5–141. doi: 10.1007/s00234-020-02571-0
54. McLendon LA, Rao CK, Da Hora CC, Islamovic F, Galan FN. Post–COVID-19 acute disseminated encephalomyelitis in a 17-month-old. Pediatrics. (2021) 147(6):e2020049678. doi: 10.1542/peds.2020-049678
55. Ray STJ, Abdel-Mannan O, Sa M, Fuller C, Wood GK, Pysden K, et al. Neurological manifestations of SARS-CoV-2 infection in hospitalised children and adolescents in the UK: a prospective national cohort study. Lancet Child Adolesc Health. (2021) 5(9):631–41. doi: 10.1016/S2352-4642(21)00193-0
56. Mierzewska-Schmidt M, Baranowski A, Szymanska K, Ciaston M, Kuchar E, Ploski R, et al. The case of fatal acute hemorrhagic necrotizing encephalitis in a two-month-old boy with COVID-19. Int J Infect Dis. (2022) 116:151–3. doi: 10.1016/j.ijid.2021.12.334
57. Ho JHY, Lee CYM, Chiong YK, Aoyama R, Fan LJ, Tan AHS, et al. SARS-CoV-2-Related acute necrotizing encephalopathy of childhood with good response to tocilizumab in an adolescent. Pediatr Neurol. (2023) 139:65–9. doi: 10.1016/j.pediatrneurol.2022.11.010
58. Beslow LA, Linds AB, Fox CK, Kossorotoff M, Zuñiga Zambrano YC, Hernández-Chávez M, et al. Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann Neurol. (2021) 89(4):657–65. doi: 10.1002/ana.25991
59. Vogrig A, Gigli GL, Bnà C, Morassi M. Stroke in patients with COVID-19: clinical and neuroimaging characteristics. Neurosci Lett. (2021) 743:135564. doi: 10.1016/j.neulet.2020.135564
60. Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. (2020) 51(9):e219–22. doi: 10.1161/STROKEAHA.120.030995
61. Ranabothu S, Onteddu S, Nalleballe K, Dandu V, Veerapaneni K, Veerapandiyan A. Spectrum of COVID-19 in children. Acta Paediatr. (2020) 109(9):1899–900. doi: 10.1111/apa.15412
62. Beslow LA, Agner SC, Santoro JD, Ram D, Wilson JL, Harrar D, et al. International prevalence and mechanisms of SARS-CoV-2 in childhood arterial ischemic stroke during the COVID-19 pandemic. Stroke. (2022) 53(8):2497. doi: 10.1161/STROKEAHA.121.038250
63. Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. (2021) 18(3):194–209. doi: 10.1038/s41569-020-00469-1
64. Whitworth H, Sartain SE, Kumar R, Armstrong K, Ballester L, Betensky M, et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. (2021) 138(2):190–8. doi: 10.1182/blood.2020010218
65. Diorio C, McNerney KO, Lambert M, Paessler M, Anderson EM, Henrickson SE, et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. (2020) 4(23):6051–63. doi: 10.1182/bloodadvances.2020003471
66. Sandoval F, Julio K, Méndez G, Valderas C, Echeverría AC, Perinetti MJ, et al. Neurologic features associated with SARS-CoV-2 infection in children: a case series report. J Child Neurol. (2021) 36(10):853–66. doi: 10.1177/0883073821989164
67. Hatipoglu N, Yazici ZM, Palabiyik F, Gulustan F, Sayin I. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia in pediatric cases. Int J Pediatr Otorhinolaryngol. (2020) 139:110469. doi: 10.1016/j.ijporl.2020.110469
68. Chetrit A, Lechien JR, Ammar A, Chekkoury-Idrissi Y, Distinguin L, Circiu M, et al. Magnetic resonance imaging of COVID-19 anosmic patients reveals abnormalities of the olfactory bulb: preliminary prospective study. J Infect. (2020) 81(5):816–46. doi: 10.1016/j.jinf.2020.07.028
69. Li CW, Syue LS, Tsai YS, Li MC, Lo CL, Tsai CS, et al. Anosmia and olfactory tract neuropathy in a case of COVID-19. J Microbiol Immunol Infect. (2021) 54(1):93–6. doi: 10.1016/j.jmii.2020.05.017
70. Aragão MFVV, Leal MC, Filho OQC, Fonseca TM, Valença MM. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. Am J Neuroradiol. (2020) 41(9):1703–6. doi: 10.3174/ajnr.A6675
71. Sayin I, Yazici ZM. Taste and smell impairment in SARS-CoV-2 recovers early and spontaneously: experimental data strongly linked to clinical data. ACS Chem Neurosci. (2020) 11(14):2031–3. doi: 10.1021/acschemneuro.0c00296
72. de Santé HA. Les troubles du goût et de l’odorat au cours des symptômes prolongés de la Covid-19. France; 2021 févr. Disponible sur: https://www.has-sante.fr/upload/docs/application/pdf/2021-11/fiche_troubles_du_gout_et_de_l_odorat.pdf
73. Tannous D, Klepper K. Pediatric COVID-19 vestibular neuritis. Perm J. (2022) 26(2):162–5. doi: 10.7812/TPP/21.221
74. Giannantonio S, Scorpecci A, Montemurri B, Marsella P. Case of COVID-19-induced vestibular neuritis in a child. BMJ Case Rep. (2021) 14(6):e242978. doi: 10.1136/bcr-2021-242978
75. Mat Q, Noël A, Loiselet L, Tainmont S, Chiesa-Estomba CM, Lechien JR, et al. Vestibular neuritis as clinical presentation of COVID-19. Ear Nose Throat J. (2021):145561321995021. doi: 10.1177/0145561321995021
76. Decio A, Mazza A, Quadri V, Ronconi MS, Brusadelli C, Ruggeri M, et al. Neurological manifestations of COVID-19 in children: a case of facial nerve palsy. Pediatr Neurol. (2021) 116:59. doi: 10.1016/j.pediatrneurol.2020.12.006
77. Codeluppi L, Venturelli F, Rossi J, Fasano A, Toschi G, Pacillo F, et al. Facial palsy during the COVID-19 pandemic. Brain Behav. (2021) 11(1):e01939. doi: 10.1002/brb3.1939
78. Brisca G, Garbarino F, Carta S, Palmieri A, Vandone M, Severino M, et al. Increased childhood peripheral facial palsy in the emergency department during COVID-19 pandemic. Pediatr Emerg Care. (2020) 36(10):e595–6. doi: 10.1097/PEC.0000000000002231
79. Elenga N, Martin E, Gerard M, Osei L, Rasouly N. Unilateral diplopia and ptosis in a child with COVID-19 revealing third cranial nerve palsy. J Infect Public Health. (2021) 14(9):1198–200. doi: 10.1016/j.jiph.2021.08.007
80. de Oliveira MR, Lucena ARVP, Higino TMM, Ventura CV. Oculomotor nerve palsy in an asymptomatic child with COVID-19. J Am Assoc Pediatr Ophthalmol Strabismus. (2021) 25(3):169–70. doi: 10.1016/j.jaapos.2021.02.001
81. Lonardi V, Meneghesso D, Debertolis G, Pin JN, Nosadini M, Sartori S. Isolated third cranial nerve palsy and COVID-19 infection in a child. Pediatr Neurol. (2021) 120:11. doi: 10.1016/j.pediatrneurol.2021.03.011
82. Palaiodimou L, Stefanou MI, Katsanos AH, Fragkou PC, Papadopoulou M, Moschovos C, et al. Prevalence, clinical characteristics and outcomes of Guillain-Barré syndrome spectrum associated with COVID-19: a systematic review and meta-analysis. Eur J Neurol. (2021) 28(10):3517–29. doi: 10.1111/ene.14860
83. Levison LS, Thomsen RW, Markvardsen LK, Christensen DH, Sindrup SH, Andersen H. Pediatric guillain-barré syndrome in a 30-year nationwide cohort. Pediatr Neurol. (2020) 107:57–63. doi: 10.1016/j.pediatrneurol.2020.01.017
84. Pourbakhtyaran E, Heidari M, Akbari MG, Mohammadi M, Badv RS, Zamani G, et al. Childhood Guillain–Barre syndrome in the SARS-CoV-2 era: is there any causative relation? Clin Case Rep. (2022) 10(12):e6772. doi: 10.1002/ccr3.6772
85. Curtis M, Bhumbra S, Felker MV, Jordan BL, Kim J, Weber M, et al. Guillain-Barré syndrome in a child with COVID-19 infection. Pediatrics. (2021) 147(4):e2020015115. doi: 10.1542/peds.2020-015115
86. Khalifa M, Zakaria F, Ragab Y, Saad A, Bamaga A, Emad Y, et al. Guillain-Barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J Pediatric Infect Dis Soc. (2020) 9(4):510–3. doi: 10.1093/jpids/piaa086
87. Frank CHM, Almeida TVR, Marques EA, de Sousa Monteiro Q, Feitoza PVS, Borba MGS, et al. Guillain–Barré syndrome associated with SARS-CoV-2 infection in a pediatric patient. J Trop Pediatr. (2021) 67(3):fmaa044. doi: 10.1093/tropej/fmaa044
88. Araújo NM, Ferreira LC, Dantas DP, Silva DS, dos Santos CA, Cipolotti R, et al. First report of SARS-CoV-2 detection in cerebrospinal fluid in a child with Guillain-Barré syndrome. Pediatr Infect Dis J. (2021) 40(7):e274. doi: 10.1097/INF.0000000000003146
89. Singer TG, Evankovich KD, Fisher K, Demmler-Harrison GJ, Risen SR. Coronavirus infections in the nervous system of children: a scoping review making the case for long-term neurodevelopmental surveillance. Pediatr Neurol. (2021) 117:47–63. doi: 10.1016/j.pediatrneurol.2021.01.007
90. Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. (2021) 268(4):1133–70. doi: 10.1007/s00415-020-10124-x
91. Güler MA, Keski˙n F, Tan H. Acute myelitis secondary to COVID-19 in an adolescent: causality or coincidence ? New Trends Med Sci. (2020) 1(2):132–6.
92. Nejad Biglari H, Sinaei R, Pezeshki S, Khajeh Hasani F. Acute transverse myelitis of childhood due to novel coronavirus disease 2019: the first pediatric case report and review of literature. Iran J Child Neurol. (2021) 15(1):107–12. doi: 10.22037/ijcn.v15i1.31579
93. Kaur H, Mason JA, Bajracharya M, McGee J, Gunderson MD, Hart BL, et al. Transverse myelitis in a child with COVID-19. Pediatr Neurol. (2020) 112:5–6. doi: 10.1016/j.pediatrneurol.2020.07.017
94. Brisca G, Sotgiu S, Pirlo D, Tubino B, Siri L, Chianucci B, et al. Longitudinally Extensive Transverse Myelitis (LETM) and myopericarditis in a 7-month-old child with SARs-CoV-2 infection. Neuropediatrics. (2022) 53(01):061–4. doi: 10.1055/s-0041-1732364
95. Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute Transverse Myelitis (ATM):clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222). Front Immunol. (2021) 12:653786. doi: 10.3389/fimmu.2021.653786
96. Costagliola G, Spada E, Consolini R. Severe COVID-19 in pediatric age: an update on the role of the anti-rheumatic agents. Pediatr Rheumatol. (2021) 19(1):68. doi: 10.1186/s12969-021-00559-5
97. Stein M, Ashkenazi-Hoffnung L, Greenberg D, Dalal I, Livni G, Chapnick G, et al. The burden of COVID-19 in children and its prevention by vaccination: a joint statement of the Israeli pediatric association and the Israeli society for pediatric infectious diseases. Vaccines (Basel). (2022) 10(1):81. doi: 10.3390/vaccines10010081
98. Ouldali N, Bagheri H, Salvo F, Antona D, Pariente A, Leblanc C, et al. Hyper inflammatory syndrome following COVID-19 mRNA vaccine in children: a national post-authorization pharmacovigilance study. Lancet Reg Health Eur. (2022) 17:100393. doi: 10.1016/j.lanepe.2022.100393
99. Yousaf AR, Cortese MM, Taylor AW, Broder KR, Oster ME, Wong JM, et al. Reported cases of multisystem inflammatory syndrome in children aged 12–20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: a surveillance investigation. Lancet Child Adolesc Health. (2022) 6(5):303–12. doi: 10.1016/S2352-4642(22)00028-1
100. Shao SC, Wang CH, Chang KC, Hung MJ, Chen HY, Liao SC. Guillain-Barré syndrome associated with COVID-19 vaccination. Emerg Infect Dis. (2021) 27(12):3175–8. doi: 10.3201/eid2712.211634
Keywords: SARS-CoV-2, encephalitis, children, COVID-19, anosmia
Citation: Casabianca M, Caula C, Titomanlio L and Lenglart L (2023) Neurological consequences of SARS-CoV-2 infections in the pediatric population. Front. Pediatr. 11:1123348. doi: 10.3389/fped.2023.1123348
Received: 13 December 2022; Accepted: 16 January 2023;
Published: 14 February 2023.
Edited by:
Alberto Spalice, Sapienza University of Rome, ItalyReviewed by:
Giacomo Brisca, Giannina Gaslini Institute (IRCCS), Italy© 2023 Casabianca, Caula, Titomanlio and Lenglart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Léa Lenglart bGVhLmxlbmdsYXJ0QGFwaHAuZnI=
†ORCID Manon Casabianca orcid.org/0000-0002-4025-7279 Luigi Titomanlio orcid.org/0000-0003-4909-803X Léa Lenglart orcid.org/0000-0003-2325-8998
Specialty Section: This article was submitted to Pediatric Neurology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.