
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 23 May 2023
Sec. Pediatric Pulmonology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1123237
Background: We sought to classify patients with congenital tracheal stenosis (CTS) according to tracheobronchial morphology and determine anatomic features associated with tracheobronchial anomalies (TBAs) and concurrent cardiovascular defects (CVDs).
Methods: We enrolled 254 patients who underwent tracheoplasty between November 1, 2009 and December 30, 2018. The anatomic features of the tracheobronchial tree and cardiovascular system were abstracted from bronchoscopy, echocardiography, computerized tomography, and operative reports.
Results: Four types of tracheobronchial morphology were identified: Type-1, which included normal tracheobronchial arborization (Type-1A, n = 29) and tracheal bronchus (Type-1B, n = 22); Type-2 (tracheal trifurcation; n = 49), and Type-3 (typical bridging bronchus; n = 47). Type-4 (bronchus with an untypical bridging pattern) was divided into Type-4A (involving bronchial diverticulum; n = 52) and Type-4B (absent bronchus; n = 55). Carinal compression and tracheomalacia were significantly more frequent in Type-4 patients than in the other patients (P < 0.01). CVDs were common in patients with CTS, especially in patients with Type-3 and Type-4 (P < 0.01). Persistent left superior vena cava was most common among patients with Type-3 (P < 0.01), and pulmonary artery sling was most frequent among those with Type-4 (P < 0.01). Outflow tract defects were most likely to occur in Type-1B. Early mortality was detected in 12.2% of all patients, and young age (P = 0.02), operation in the early era (P < 0.01), and bronchial stenosis (P = 0.03) were proven to be risk factors.
Conclusions: We demonstrated a useful morphological classification for CTS. Bridging bronchus was most closely linked with vascular anomalies, while tracheal bronchus was frequently associated with outflow tract defects. These results may provide a clue to CTS pathogenesis.
Congenital tracheal stenosis (CTS) is a rare but potentially life-threatening disease in children. The clinical presentation can vary from almost asymptomatic to recurrent dyspnea, stridor, or even near-fatal airway obstruction. Most children with CTS are characterized by complete cartilaginous rings and are usually associated with various congenital malformations, particularly cardiovascular defects (CVDs) and tracheobronchial anomalies (TBAs) (1–3). Although slide tracheoplasty has achieved satisfactory airway reconstruction results, the overall clinical outcomes remain strongly influenced by concurrent tracheobronchial and cardiovascular malformations (4–7). Thus, it is essential to identify these anomalies prior to surgical intervention, especially concurrent cardiac surgery and tracheoplasty, to avoid complications.
In our experience, several CVDs, including pulmonary artery sling (PAS), ventricular or atrial septal defects, and tetralogy of Fallot or pulmonary atresia, are frequently associated with CTS. The association of PAS and CTS is often referred to as a “ring–sling complex” (RSC) owing to the prevalence of complete cartilaginous rings (8–10). However, the relationship between cardiovascular defects and specific tracheobronchial morphologies in children with CTS has not been thoroughly explored.
To the best of our knowledge, various abnormalities of the tracheobronchial tree have been identified using postmortem specimens and radiological findings. In the Wells classification, CTS is commonly associated with tracheal bronchus (TB) and bridging bronchus (BB) (9, 11). In the descriptions of Great Ormond Street Hospital, tracheal trifurcation was identified normally, and the extension of the stenosis into one or both bronchi was also common (12). Currently, there are no uniform classification systems that comprehensively describe the numerous tracheobronchial morphologies in children with CTS. Therefore, we proposed the addition of two new subgroups to the four CTS categories formulated by Wells and colleagues. The objective of this study was to describe different tracheobronchial patterns and various cardiovascular anomalies in patients with CTS and determine whether an association exists between TBAs and concurrent CVDs.
We conducted an observational, single-center, retrospective study. All 254 patients who underwent surgical repair for CTS between November 1, 2009 and December 30, 2018 at Shanghai Children's Medical Center were included. Data were obtained by reviewing medical records from admission until the first follow-up at 1 month after discharge. This study was approved by the hospital's ethics committee (SCMCIRB-W2022008), and the requirement of informed consent was waived. To compare mortality rates throughout the study period, the surgical era was classified into two timeframes: 2008–2014 and 2015–2018, according to the operative quantity.
Airway anatomy was assessed and defined by a multidisciplinary tracheal team, based on a combination of bronchoscopy, computed tomography (CT), and intraoperative findings. Tracheobronchial arborization types include (9, 13) Type-1A [normal tracheobronchial arborization in which the trachea divides at the carina into the left and right main bronchi, and the right upper lobe bronchus (ULB) originates from the right main bronchus], Type-1B (TB; which is described as a right ULB originating from the right side of the trachea, above the carina), Type-2 (tracheal trifurcation; which involves an ectopic right ULB directly arising at the level of the carina), Type-3 (typical BB; which is a malformation in which the right main bronchus ends in the right ULB and the right intermediate bronchus abnormally arises from the left main bronchus, forming a pseudocarina), and Type-4 (atypical BB; which is similar to Type-3, but the right main bronchus is present only as a short “bronchial diverticulum” [Type-4A] or is absent [Type-4B], and the lower bifurcation (pseudocarina) shows a typical inverted “T” pattern). The specific airway anatomical types are shown in Figure 1.
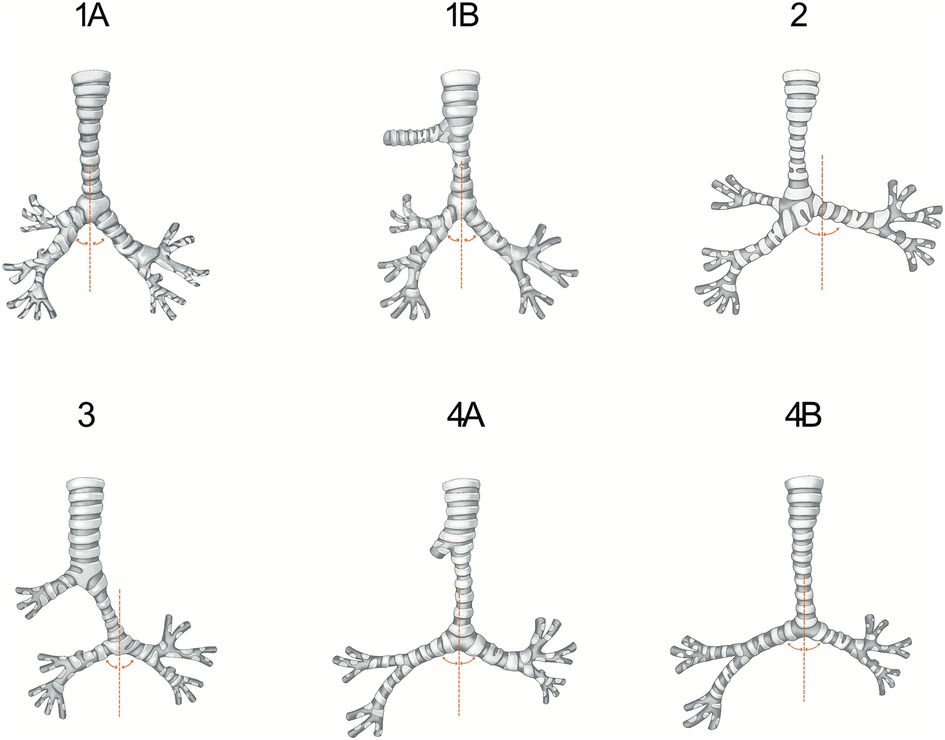
Figure 1. This figure illustrates the types of tracheobronchial arborization included in our study. Type-1A refers to a normal tracheobronchial pattern. In Type-1B, the right upper lobe is supplied by a right tracheal bronchus. In Type-2, the trachea directly divides into three bronchi. The right lower lobe is supplied by a typical bridging bronchus in Type-3, and the right upper lobe bronchus is present only as a short diverticulum in Type-4A or absent in Type-4B. The curved lines represent the right and left bronchial angles in relation to the midline.
Bronchial stenosis was defined as the presence of complete tracheal rings in at least one bronchus beyond the carina, with greater than 30% narrowing. Carinal compression was defined as focal narrowing (>30% decrease in cross-sectional area) at the level of the carina. Tracheomalacia was defined as a dynamic collapse of the trachea that exceeded 50% of the diameter on expiration, as seen on bronchoscopy.
All patients had symptoms of tracheal compression, ranging from mild stridor to severe respiratory distress. Diagnosis of CVD was routinely confirmed by a review of echocardiographic and CT reports before the operation. The surgical procedure was performed through a median sternotomy, with cardiopulmonary bypass. Where necessary, intracardiac repair was performed first and PAS repair was completed subsequently. Tracheal repair techniques included slide tracheoplasty, patch tracheoplasty (pericardial patch and tracheal autograft patch repair), and resection with end-to-end anastomosis (14). Early mortality was defined as death occurring within 30 days of surgery or prior to hospital discharge.
Continuous variables are presented as median with interquartile range for skewed variables. Continuous data were compared using the Kruskal–Wallis test. Categorical variables are described as numbers with percentages and were compared using the χ2 test. Fisher's exact test was applied when the expected frequency was less than 5. Univariate and multivariate logistic regression analyses were performed to identify possible risk factors for early mortality. All analyses were performed using SPSS statistical software version 25 (SPSS Inc., Chicago, USA).
Baseline characteristics are presented in Table 1. Among the 254 patients with CTS, 51 (20.1%) patients had Type-1, including 29 with Type-1A and 22 with Type-1B; 49 (19.3%) patients had Type-2, 47 (18.5%) patients had Type-3, and 107 (42.1%) patients had Type-4 (52 with Type-4A and 55 with Type-4B). The prevalence of carinal compression (54/107, 50.5%) or tracheomalacia (58/107, 54.2%) was significantly higher in the Type-4 group than in the other groups (P < 0.01). Bronchial stenosis was present in 60 patients (23.6%). However, there was no significant difference between the four groups.
Among the four CTS groups, the Type-3 group had a lower proportion of male (20/47, 42.6%) than the other three groups (P = 0.03). CVDs were common in all groups and were most predominant in the Type-3 (46/47, 97.9%) and Type-4 (103/107, 96.3%) (P < 0.01) groups. There were various types of extracardiac defects in 20 (7.9%) patients, but no significant difference was found between the four groups. Similarly, there was no significant difference between the four groups in the prevalence of cardiac malposition and pulmonary hypoplasia.
Two hundred and thirty-four patients (92.1%) had various types of cardiovascular malformations (Table 2). The major types of CVDs were PAS (n = 168, 66.1%), persistent left superior vena cava (PLSVC) (n = 82, 32.3%), atrial septal defect (n = 69, 27.2%), patent ductus arteriosus (n = 39, 15.4%), and ventricular septal defect (VSD) (n = 36, 14.2%). Right/double aortic arch (n = 18, 7.1%) and tetralogy of Fallot (n = 15, 5.9%) were also common in the entire cohort. However, left heart obstructive lesions (n = 3, 1.2%), including mitral stenosis, aortic stenosis, and coarctation of the aorta, were rare among all patients.
We analyzed the major concurrent cardiovascular anomalies that could be associated with different tracheobronchial subtypes. Overall, CVDs were more frequent in patients with BB anomaly (Type-3 and 4, P < 0.01). PLSVC was common in all subtypes of CTS but was significantly correlated with Type-3 (P < 0.01). Compared to Type-1A and 1B, PAS was more frequent in the other four subtypes and was markedly associated with Type-4A and 4B (P < 0.01). VSD was most correlated with the presentation of Type-1B (P < 0.01). When combined with conotruncal defects, the correlation between these two types of outflow tract defects and Type-1B was further strengthened. However, outflow tract defects were uncommon in Type-2 and 4B. Type-2 was not significantly correlated with the main types of CVDs, excluding PAS.
The distribution of patients according to CTS type, operative era, and early outcomes are outlined in Table 3. There were 31 (12.2%) early deaths, with 6 (11.8%), 6 (12.2%), and 19 (17.8%) cases in the Type-1, 2, and 4 groups, respectively. No early deaths (0%, 0/47) occurred among Type-3 patients. Early mortality was significantly higher in patients with atypical BB (Type-4, P = 0.02). In univariate analysis, age at operation, surgical technique, operative era, carinal compression, and tracheomalacia were predictors of early mortality (for all comparisons, P < 0.05) (Supplementary Table S1). Furthermore, young age [odds ratio (OR) = 1.00, P = 0.02], operation in the early era (OR = 4.10, P < 0.01), and bronchial stenosis (OR = 2.80, P = 0.03) were proven to be risk factors for early death in the multivariate model (Supplementary Table S2).
The median postoperative length of hospital stay was 15 days [interquartile range (IQR), 13–24 days]. Following tracheoplasty, children with atypical BB anomaly (Type-4) had longer length of hospital stay (17 days; IQR, 14–27 days) compared to the other patients (P = 0.03). Preoperative variables that were significantly associated with postoperative length of hospital stay in univariate analysis included carinal compression (P < 0.01), tracheomalacia (P < 0.01), concurrent CVDs (P = 0.03), and weight at surgery (P < 0.01) (Supplementary Table S3). Nonetheless, no risk factors were associated with postoperative length of hospital stay in the multivariate analysis.
Until recently, descriptions of CTS have been primarily limited to rare cases and various tracheobronchial anomalies. Great Ormond Street Hospital and Wells classifications have made good attempts at differentiating morphologic variants of both bronchial arborization and stenotic segments (9, 11, 12). However, neither of these classification systems adequately describe the airway morphological features that are currently associated with CTS patients. Our cohort is unique in that it is the largest single-institutional study of CTS cases. In our experience, all tracheobronchial morphologies could be identified by routine chest CT and classified into four main types and six subtypes. BB is the most common tracheobronchial positional anomaly and is most frequently associated with tracheobronchial malacia and carinal compression. In addition, PLSVC and PAS, which are two typical concurrent vascular malformations, tend to be more frequent in individuals with BB anomalies. Contrarily, VSD and CTD, which are the most common outflow tract defects, have no significant relationship with BB anomalies, especially untypical subtypes.
CTS patients with tracheobronchial abnormalities accounted for approximately 80% of our study population, and the surgeons had to devise various surgical techniques to repair complex forms of tracheal stenosis. In the whole population, the incidence of BB was 60.6% (Type-3 and 4 groups) and that of TB was only 8.7% (Type-1B group). In the cohort of 168 patients with RSC, BB tended to be more frequent (75.6%) than TB (2.4%). Notably, this disparity of coexisting malformation was more obvious in the Type-4 group (56.5% in RSC vs. 42.1% in CTS).
The high frequency of BB in patients with CTS, especially RSC, is a plausible clue to the underlying mechanism. Kishimoto and colleagues revealed that tracheal mesenchymal populations play pivotal roles in tracheal morphogenesis via mechanical characteristics (15). Developmental anomalies of these tissues may result in tracheobronchial malformations, such as tracheal stenosis, agenesis, and malacia. Gonzales-Crussi and colleagues proposed that interactions of ectopic mesenchyme (“exceptional conditions”) with the main bronchi may result in the formation of BB (16). Meanwhile, the development of the tracheobronchial tree is accompanied by parallel development of the pulmonary vasculature that originates from the sixth pair of aortic arches. Chen and colleagues hypothesized that widening of the peritracheal mesenchyme in CTS might provide a roomy space for the left postbranchial vessels to approach the right ventral sixth branchial arch, forming PAS (17). However, current theories do not account for the morphogenesis of BB after the formation of the main bronchi.
The tracheobronchial tree in Type-4 is an extremely rare bronchial malformation, with the right main bronchus present only as a short “diverticulum” in Type-4A or absent in Type-4B. There was no significant difference between these two subtypes in the concomitant intra- and extra-malformations and in early postoperative outcomes. In contrast, tracheomalacia and carinal compression were more common in Type-4B than in Type-4A (Table 1). In young children, Type-4B is occasionally confused with Type-1A when the pseudocarina remains at the T4-T5 level. The bronchial angle of Type-4B is increased and seems to have an inverted T appearance. Moreover, further bronchoscopy often reveals variations in the right bronchial distribution. These two important characteristics are critical in identifying the various subtypes (13).
In both tracheal trifurcation and bridging bronchus subtypes, which are closely related to PAS, the bronchial angle of the pseudocarina increased significantly (Figure 2). Such tracheobronchial features suggest that the aberrant left pulmonary artery, which runs posteriorly from the right pulmonary artery, may exert mechanical resistance to the adjacent tracheobronchial tree during embryonic development (16). Embryonically, the primary bronchial buds enlarge to form the right and left main bronchi, aerating two separate lungs. In contrast, the left pulmonary artery located on the right-posterior side of the trachea prevents the formation of the carina and the right ULB, causing an increase in the bronchial angle.
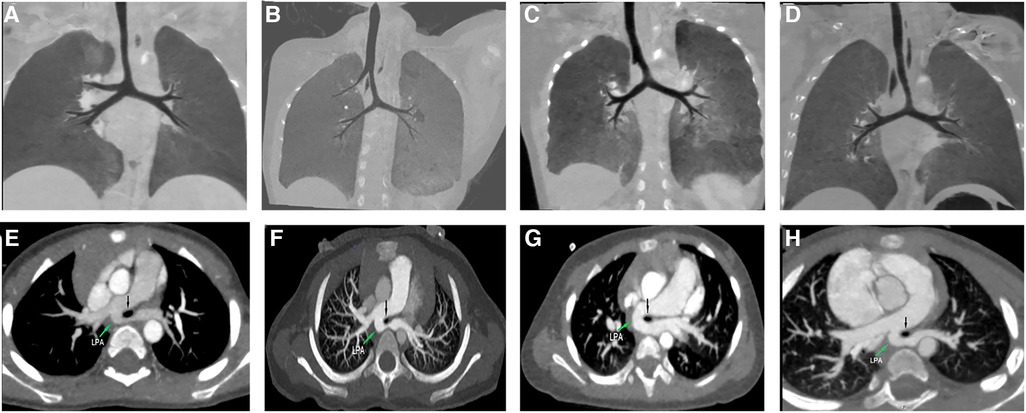
Figure 2. Examples of tracheobronchial branching abnormalities and concurrent pulmonary artery sling (PAS) on computed tomography. (A) Tracheal trifurcation in Type-2 group. (B) Typical bridging bronchus in Type-3 group. (C) The right main bronchus is present as a short “bronchial diverticulum” in Type-4A group. (D) The right main bronchus is absent in Type-4B group. (C,D) Are two subgroups of atypical bridging bronchus. (E–H) Axial images of the corresponding patients showing the left PAS arising from the right pulmonary artery. The PAS is seen compressing the stenotic trachea (black arrow).
Bronchial stenosis, as an extension of tracheal stenosis, is common and is present in almost 25% of the CTS population (4, 12, 18, 19). In our series, bronchial stenosis was noted in 23.6% of the population and was more common in the Type-2 (30.6%) and Type-4 (26.2%) groups, although the difference was not significant. The higher prevalence of bronchial stenosis in these specific morphologies implies that the stenosis may be due to the absence of contribution from the displaced or hypoplastic bronchi in fetal life (19).
Bronchial stenosis is speculated to be associated with a poor prognosis. In our experience with slide tracheoplasty, bronchial stenosis with concomitant carinal stenosis and compression was significantly associated with postoperative tracheomalacia and mortality (4). Our current study further demonstrates that the involvement of bronchial stenosis has an adverse effect on early postoperative outcomes. Coexisting bronchial stenosis reflects the severity of the underlying airway anatomical abnormality and is frequently associated with unilateral pulmonary hypoplasia. Cetrano and colleagues suggested that bronchial mismatches of greater than 20% can identify patients at increased risk for surgical reintervention and chronic respiratory failure (20). Bronchoplasty may be required in this group of patients and should be performed when possible.
PLSVC, the most common venous anomaly, has been considered to be strongly associated with both intra- and extracardiac anomalies. Previous studies demonstrated a high incidence of intracardiac anomalies, including coarctation of the aorta, double-outlet right ventricle, and VSD, and the most common extracardiac anomaly was esophageal atresia (21, 22). Our study is the first to report a special association between PLSVC and TBAs. Besides, PLSVC was found to be more positively associated with an increased risk of BB, even when present as an isolated finding.
During the early embryonic stage, both the esophagus and respiratory tract are derived from the primitive foregut, and the association between digestive and respiratory malformations could be linked to a developmental disorder originating from the division of the primitive foregut into the lung bud and esophagus, which usually affects the right upper and middle lobes. The high prevalence of PLSVC in our series of patients with TBAs is similar to that reported in a series of patients with esophageal atresia (23), suggesting a common etiology. Future embryological studies may provide more evidence for this hypothetical common morphogenesis.
It is also speculated that the combination of PLSVC with intra- and extracardiac defects might be part of a continuum of abnormalities in the secondary heart field. Disorders in the development of this field have been found to be related to complex cardiac phenotypes and possibly to PLSVC (22–24). The correlation between PLSVC and BB anomalies is not surprising because the development of the secondary heart field was found to be closely related to that of the adjacent endoderm, which is the origin of many respiratory, gastrointestinal, and genitourinary organs, during the embryonic period.
Similar to previous reports, patients with TBAs were commonly found to have concurrent CVDs in our study. The most common types were PAS and PLSVC, which are vascular anomalies. In all coexisting intracardiac anomalies, atrial septal defect, patent ductus arteriosus, and VSD were the major simple types, and conotruncal defects were the most prevalent type of the complex type. The association between CVDs, especially conotruncal defects and tracheobronchial branching anomalies, may involve common signaling pathways (25). For example, Shh–null mice may develop a phenocopy of pulmonary atresia, but they are also known to have bilateral hypoplastic lungs attributable to the absence of branching morphogenesis (26, 27).
Although arising from the endoderm, the development of the tracheobronchial epithelium is induced by epitheliomesenchymal interactions that require close proximity of the epithelium and mesenchyme (28). The position of the tracheal primordium is reportedly influenced by the adjacent cardiac tissue for ex vivo culture experiments, which showed that the foregut endoderm can differentiate into Nkx2-1+ respiratory epithelial progenitors in the presence of cardiac tissue (29). Moreover, variant transcription factors and signaling pathways, such as Nkx2-1, Sox2, Shh, Wnt, and bone morphogenetic proteins, play central roles in all dynamic morphogenetic processes (28–31). Sinner and colleagues found that mutations in genes within the Shh and Wnt pathways may lead to complete tracheal ring deformity (31). These transcription factors and signaling pathways are known to be involved in embryonic heart development, including the formation of the heart tube and the septation of the outflow tract.
This study has several limitations. First, because of the observational and retrospective design, there may have been missing data and selection bias. CTS is a rare malformation, and patients without obvious respiratory symptoms were not included in our morphological study of the tracheobronchial tree, especially children with normal bronchial branching. However, in our institution, patients with CTS are likely to undergo echocardiography before CT to evaluate the anatomical features of the cardiovascular system. This may be why patients with CVDs were highly represented in our cohort. Furthermore, CTS patients with unilateral bronchial and lung agenesis are extremely rare compared to those in previous studies (5, 12). Therefore, this special tracheobronchial morphology could not be statistically analyzed in our study. However, evaluating concurrent TBAs and CVDs in children with pulmonary malformations could be of great interest because this presentation may have very important interactions at the embryological origin. Another study limitation was the definition we used for bronchial stenosis, which included diagnosis using CT, because of the difficulty and danger associated with confirming severe tracheal obstruction via bronchoscopy (4). However, this important feature can be overestimated by CT, which was also noted in some cases in our series. Further investigations are needed to precisely evaluate the prevalence of bronchial stenosis and the influence of bronchial stenosis on early postoperative results.
We proposed a morphologic classification for tracheobronchial arborization of CTS and demonstrated that this comprehensive classification is useful for describing congenital anomalies of the tracheobronchial tree. In particular, we observed a high frequency of bridging bronchi in patients with CTS. Our results suggest an interaction between various features of cardiovascular defects and different tracheobronchial branching morphologies and may provide a new clue for pathogenesis. Finally, because it is an important risk factor for early death, bronchial stenosis should be considered essential in making surgical prognosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This study was approved by the hospital's ethics committee (SCMCIRB-W2022008), and the requirement of informed consent was waived.
Guarantor: JH and HC conceived of the analysis. JH: performed all analysis. ZX and HC: supervised the research project. JH and HC: drafted the manuscript. HW, XD, LZ, SW and HZ: provided data collection, substantial review for manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Science and Technology Development Fund of Shanghai Pudong (PKJ2019-Y10).
The authors would like to thank Rong Qin, Shanghai Jiao Tong University, for her support in manuscript preparation. Also, the authors would like to thank Editage (https://www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1123237/full#supplementary-material
1. Ho AS, Koltai PJ. Pediatric tracheal stenosis. Otolaryngol Clin North Am. (2008) 41(5):999–1021. doi: 10.1016/j.otc.2008.04.006
2. Chao YC, Peng CC, Lee KS, Lin SM, Chen MR. The association of congenital tracheobronchial stenosis and cardiovascular anomalies. Int J Pediatr Otorhinolaryngol. (2016) 83:1–6. doi: 10.1016/j.ijporl.2016.01.024
3. Sengupta A, Murthy RA. Congenital tracheal stenosis & associated cardiac anomalies: operative management & techniques. J Thorac Dis. (2020) 12(3):1184–93. doi: 10.21037/jtd.2019.10.42
4. Chen H, Shi G, Zhu L, Wang S, Lu Z, Xu Z. Intermediate-term outcomes of slide tracheoplasty in pediatric patients with ring-sling complex. Ann Thorac Surg. (2020) 109(3):820–7. doi: 10.1016/j.athoracsur.2019.06.093
5. DeMarcantonio MA, Hart CK, Yang CJ, Tabangin M, Rutter MJ, Bryant R, et al. Slide tracheoplasty outcomes in children with congenital pulmonary malformations. Laryngoscope. (2017) 127(6):1283–7. doi: 10.1002/lary.26404
6. Butler CR, Speggiorin S, Rijnberg FM, Roebuck DJ, Muthialu N, Hewitt RJ, et al. Outcomes of slide tracheoplasty in 101 children: a 17-year single-center experience. J Thorac Cardiovasc Surg. (2014) 147(6):1783–9. doi: 10.1016/j.jtcvs.2014.02.069
7. Oshima Y, Yamaguchi M, Yoshimura N, Sato S, Muraji T, Nishijima E, et al. Management of pulmonary artery sling associated with tracheal stenosis. Ann Thorac Surg. (2008) 86(4):1334–8. doi: 10.1016/j.athoracsur.2008.04.020
8. Berdon WE, Baker DH, Wung JT, Chrispin A, Kozlowski K, de Silva M, et al. Complete cartilage-ring tracheal stenosis associated with anomalous left pulmonary artery: the ring-sling complex. Radiology. (1984) 152(1):57–64. doi: 10.1148/radiology.152.1.6729137
9. Wells TR, Gwinn JL, Landing BH, Stanley P. Reconsideration of the anatomy of sling left pulmonary artery: the association of one form with bridging bronchus and imperforate anus. Anatomic and diagnostic aspects. J Pediatr Surg. (1988) 23(10):892–8. doi: 10.1016/S0022-3468(88)80379-8
10. Backer CL, Russell HM, Kaushal S, Rastatter JC, Rigsby CK, Holinger LD. Pulmonary artery sling: current results with cardiopulmonary bypass. J Thorac Cardiovasc Surg. (2012) 143(1):144–51. doi: 10.1016/j.jtcvs.2011.09.038
11. Berdon WE, Muensterer OJ, Zong YM, Backer CL. The triad of bridging bronchus malformation associated with left pulmonary artery sling and narrowing of the airway: the legacy of wells and landing. Pediatr Radiol. (2012) 42(2):215–9. doi: 10.1007/s00247-011-2273-2
12. Speggiorin S, Torre M, Roebuck DJ, McLaren CA, Elliott MJ. A new morphologic classification of congenital tracheobronchial stenosis. Ann Thorac Surg. (2012) 93(3):958–61. doi: 10.1016/j.athoracsur.2011.12.019
13. Chassagnon G, Morel B, Carpentier E, Ducou Le Pointe H, Sirinelli D. Tracheobronchial branching abnormalities: lobe-based classification scheme. Radiographics. (2016) 36(2):358–73. doi: 10.1148/rg.2016150115
14. Hofferberth SC, Watters K, Rahbar R, Fynn-Thompson F. Evolution of surgical approaches in the management of congenital tracheal stenosis: single-center experience. World J Pediatr Congenit Heart Surg. (2016) 7(1):16–24. doi: 10.1177/2150135115606627
15. Kishimoto K, Tamura M, Nishita M, Minami Y, Yamaoka A, Abe T, et al. Synchronized mesenchymal cell polarization and differentiation shape the formation of the murine trachea and esophagus. Nat Commun. (2018) 9(1):2816. doi: 10.1038/s41467-018-05189-2
16. Henry BM, Cheruiyot I, Wong LM, Keet K, Mutua V, Chhapola V, et al. The bridging bronchus: a comprehensive review of a rare, potentially life-threatening congenital airway anomaly associated with cardiovascular defects. Pediatr Pulmonol. (2019) 54(12):1895–904. doi: 10.1002/ppul.24488
17. Chen SJ, Lee WJ, Lin MT, Wang JK, Chang CI, Chiu IS, et al. Left pulmonary artery sling complex: computed tomography and hypothesis of embryogenesis. Ann Thorac Surg. (2007) 84(5):1645–50. doi: 10.1016/j.athoracsur.2007.05.094
18. Beierlein W, Elliott MJ. Variations in the technique of slide tracheoplasty to repair complex forms of long-segment congenital tracheal stenoses. Ann Thorac Surg. (2006) 82(4):1540–2. doi: 10.1016/j.athoracsur.2005.11.001
19. Doolittle AM, Mair EA. Tracheal bronchus: classification, endoscopic analysis, and airway management. Otolaryngol Head Neck Surg. (2002) 126(3):240–3. doi: 10.1067/mhn.2002.122703
20. Cetrano E, Trezzi M, Secinaro A, Di Chiara L, Trozzi M, Bottero S, et al. Bronchial mismatch as a predictor of respiratory failure after congenital tracheal stenosis repair. Ann Thorac Surg. (2018) 105(4):1264–71. doi: 10.1016/j.athoracsur.2017.10.046
21. Nagasawa H, Kuwabara N, Goto H, Omoya K, Yamamoto T, Terazawa A, et al. Incidence of persistent left superior vena cava in the normal population and in patients with congenital heart diseases detected using echocardiography. Pediatr Cardiol. (2018) 39(3):484–90. doi: 10.1007/s00246-017-1778-3
22. Postema PG, Rammeloo LA, van Litsenburg R, Rothuis EG, Hruda J. Left superior vena cava in pediatric cardiology associated with extra-cardiac anomalies. Int J Cardiol. (2008) 123(3):302–6. doi: 10.1016/j.ijcard.2006.12.020
23. Lejeune S, Le Mee A, Petyt L, Hutt A, Sfeir R, Michaud L, et al. Bronchopulmonary and vascular anomalies are frequent in children with oesophageal atresia. Acta Paediatr. (2020) 109(6):1221–8. doi: 10.1111/apa.15086
24. Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, et al. Conotruncal myocardium arises from a secondary heart field. Development. (2001) 128(16):3179–88. doi: 10.1242/dev.128.16.3179
25. Chassagnon G, Lefort B, Meot M, Carpentier E, Sirinelli D, Chantepie A, et al. Association between tetralogy of Fallot and tracheobronchial branching abnormalities: a new clue for pathogenesis? J Am Heart Assoc. (2017) 7(1):e006921.29288155
26. Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. (2004) 66:625–45. doi: 10.1146/annurev.physiol.66.032102.135749
27. Parisot P, Mesbah K, Théveniau-Ruissy M, Kelly RG. Tbx1, subpulmonary myocardium and conotruncal congenital heart defects. Birth Defects Res A Clin Mol Teratol. (2011) 91(6):477–84. doi: 10.1002/bdra.20803
28. Kishimoto K, Morimoto M. Mammalian tracheal development and reconstruction: insights from in vivo and in vitro studies. Development. (2021) 148(13):dev198192. doi: 10.1242/dev.198192
29. Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. (2005) 132(1):35–47. doi: 10.1242/dev.01570
30. Nikolić MZ, Sun D, Rawlins EL. Human lung development: recent progress and new challenges. Development. (2018) 145(16):dev163485. doi: 10.1242/dev.163485
Keywords: congenital, tracheal stenosis, classification, children, cardiovascular anomalies
Citation: Hu J, Wang H, Du X, Zhu L, Wang S, Zhang H, Xu Z and Chen H (2023) Morphologic classification of tracheobronchial arborization in children with congenital tracheobronchial stenosis and the associated cardiovascular defects. Front. Pediatr. 11:1123237. doi: 10.3389/fped.2023.1123237
Received: 13 December 2022; Accepted: 28 April 2023;
Published: 23 May 2023.
Edited by:
Yusei Ohshima, University of Fukui, JapanReviewed by:
Ruchonnet-Metrailler Isabelle, Hôpitaux universitaires de Genève (HUG), Switzerland© 2023 Hu, Wang, Du, Zhu, Wang, Zhang, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Chen Y2hlbmhhb290ekAxMjYuY29t
Abbreviations CTS, congenital tracheal stenosis; TBAs, tracheobronchial anomalies; CVDs, cardiovascular defects; RSC, “ring–sling complex”; BB, bridging bronchus; CT, computed tomography; ULB, upper lobe bronchus; PAS, including pulmonary artery sling; PLSVC, persistent left superior vena cava; VSD, ventricular septal defect.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.