- Department of Respiratory Medicine, Children's Hospital of Nanjing Medical University, Nanjing, China
Adenoviral pneumonia in children was an epidemic that greatly impacted children's health in China in 2019. Currently, no simple or systematic scale has been introduced for the early identification and diagnosis of adenoviral pneumonia. The early recognition scale of pediatric severe adenovirus pneumonia was established based on an analysis of the children's community-acquired pneumonia clinical cohort. This study analyzed the clinical data of 132 children with adenoviral pneumonia who were admitted to the Children's Hospital of Nanjing Medical University. The clinical parameters and imaging features were analyzed using univariate and multivariate logistic regression analyses. A nomogram was constructed to predict the risk of developing severe adenovirus pneumonia in children. There were statistically significant differences in age, respiratory rate, fever duration before admission, percentage of neutrophils and lymphocytes, CRP, ALT, and LDH between the two groups. Logistic regression analysis was conducted using the R language, and respiratory rate, percentage of neutrophils, percentage of lymphocytes, and LDH were used as scale indicators. Using the ROC curve, the sensitivity and specificity of the scale were 93.3% and 92.1%. This scale has good sensitivity and specificity through internal verification, which proves that screening for early recognition of severe adenovirus pneumonia can be realized by scales. This predictive scale helps determine whether a child will develop severe adenovirus pneumonia early in the disease course.
1. Introduction
Adenovirus is an important pathogen that causes community-acquired pneumonia (CAP) in children (1). More than 80% of adenovirus pneumonia cases occur in children under the age of four, especially infants and young children <2 years old (2). Adenovirus pneumonia can develop in acute respiratory distress syndrome, critically ill patients with respiratory failure, toxic encephalopathy, hemophagocytic lymphohistiocytosis, post-infectious bronchiolitis obliterans (PIBO), and even death, and is an important cause of infant death and disability (3–6). Adenovirus is highly contagious in schools, hospitals, and other highly enclosed areas, and epidemics in crowded environments can occur (2).
Methods such as the Pneumonia Severity Index (PSI) score and CURB-65 score have been established in adult CAP to predict the severity and prognosis of adult CAP at an early stage and guide treatment (7, 8). Whereas, given the age limitations and practicality of these scales, they cannot be directly applied to children.
We previously developed a scale for the early prediction of refractory Mycoplasma pneumoniae pneumonia in hospitalized children based on big data analysis (9). Predictors of refractory Mycoplasma pneumoniae pneumonia have been studied. Studies have shown that clinically relevant risk factors for refractory Mycoplasma pneumoniae pneumonia are extrapulmonary complications, large area lung morphogenesis, and elevated C-reactive protein (CRP) and lactate dehydrogenase (LDH) levels (10, 11).
However, there are currently no predictive methods for severe adenovirus pneumonia. In some studies, the results were complications from severe adenovirus pneumonia rather than predictors (3, 5, 6). Early and accurate identification, treatment, and prognosis of children with adenovirus pneumonia have positive significance.
To develop a scale for the early prediction of severe adenovirus pneumonia in hospitalized children, we analyzed the clinical data of 132 children with adenoviral pneumonia admitted to the Children's Hospital Affiliated with Nanjing Medical University in the past three years and provided evidence-based medical opinions.
2. Materials and methods
2.1. Ethics
The study was approved by the Institutional Ethics Committee of the Children's Hospital Affiliated with Nanjing Medical University (approval number: 202205067-1). All procedures were performed in accordance with the principles of the Declaration of Helsinki.
2.2. Patients and grouping
A flowchart of the study is shown in Figure 1A. This retrospective observational study was conducted at the Children's Hospital of Nanjing Medical University from January 2017 to December 2019. This was followed by a prospective cohort study conducted at the Children's Hospital of Nanjing Medical University. Adenovirus infection was confirmed by polymerase chain reaction testing of nasopharyngeal swab specimens. The inclusion criterion was no severe pneumonia on admission.
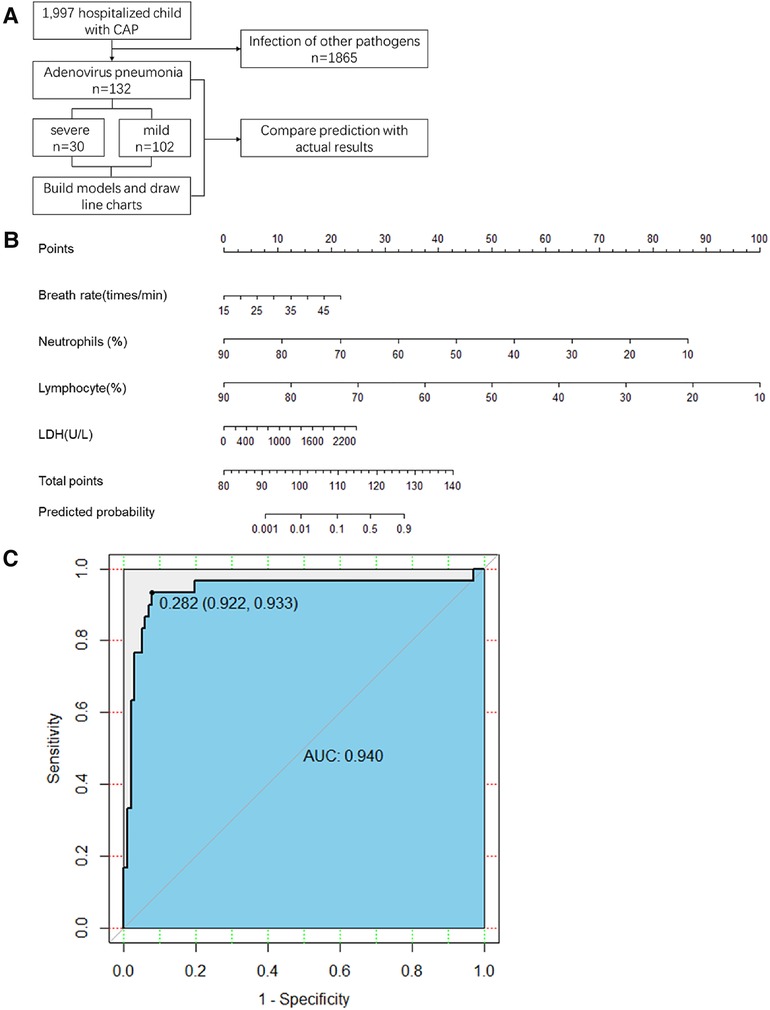
Figure 1. (A) Study flow. CAP, community-acquired pneumonia (B) The first line is the score corresponding to each indicator value. The following lines correspond to the indexes included in the scale, calculated total score, and predicted probability. When used, the table should be scaled up and printed on paper, and the score should be calculated using a tool such as a ruler. LDH, lactate dehydrogenase (C) Scale for predicting severe adenovirus pneumonia by receiver operator characteristic curves in the retrospective cohort. AUC, area under the curve.
Children with a clinical diagnosis and radiographic findings of pneumonia were included. All patients received homogenized treatment. Children with a history of pneumonia within 28 days before admission and those in the convalescent stage of pneumonia were excluded. According to the nasopharyngeal suction adenovirus nucleic acid detection results, patients were divided into two groups: adenovirus-positive and adenovirus-negative groups.
The severe group standard included patients with clinical manifestations of adenovirus pneumonia who met at least one of the following signs: disturbance of consciousness, oxygen saturation < 92%, marked shortness of breath (respiratory rate > 70 breaths per minute in infants and 50 breaths per minute in older children), dyspnea cyanosis, extrapulmonary complications, refusal to eat, dehydration, and severe chest imaging findings (pneumothorax, pleural effusion, atelectasis, or lobular infiltration).
2.3. Data collection and study variables
We collected data on demographic and clinical characteristics, including sex, age,, fever duration before admission, chest imaging findings, presence of underlying diseases, the respiratory rate on admission, laboratory test results, including routine blood tests (white blood cells, % neutrophils, the absolute count of neutrophils, % lymphocytes, CRP and biochemical blood indicators [aspartate aminotransferase, alanine transaminase (ALT), and LDH], and the etiology of available test results. After the preliminary screening of all indicators, statistically significant indicators were selected for regression analysis.
2.4. Respiratory pathogens
An adenovirus nucleic acid detection kit was used to detect nasopharyngeal aspiration in the children within 24 h of admission. Nucleic acids were extracted according to the instructions for adenovirus nucleic acid detection, and the conserved region was amplified by Q-PCR. The conserved region was compared to the standard curve and identified using agarose gel electrophoresis.
2.5. Statistical analysis
Epidemiological data were analyzed using SPSS Version 25.0 (IBM Corp, Armonk, NY, USA) and R Version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was considered statistically significant. Normally distributed continuous data were analyzed using t-tests, and non-normally distributed measurement data were analyzed using Mann–Whitney U tests.Different populations were compared using the chi-squared test. A multivariate analysis was performed using a stepwise logistic regression model. The R software was used to transform the final regression model into a nomogram. Receiver operating characteristic (ROC) curves were used to analyze the regression model for predicting severe adenovirus pneumonia. The sensitivity and specificity of the predictive scale were calculated.
3. Results
3.1. Patient characteristics and laboratory findings
We retrospectively enrolled 132 patients with non-severe adenovirus pneumonia. Of these, 30 patients developed severe adenovirus pneumonia, and 102 patients had mild adenovirus pneumonia. The characteristics of patients in the retrospective cohort on admission are summarized in Table 1. There was no significant difference in sex distribution or radiographic chest pneumonia between the two groups. The average age, breathing rate, and fever duration before admission were significantly greater in the critical adenovirus pneumonia group than in the mild adenovirus pneumonia group. Compared to mild adenovirus pneumonia, significantly more patients in the severe adenovirus pneumonia group experienced complications. Compared to the mild group, the severe group showed significantly higher levels of neutrophils, lymphocytes, ALT, and LDH. Other laboratory findings did not differ significantly between the two groups.
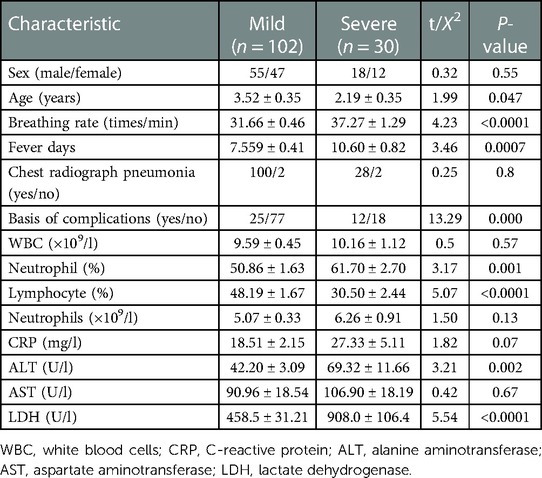
Table 1. Admission characteristics of the children in the retrospective cohorts. Values are presented as mean ± SD.
3.2. Logistic regression and nomogram
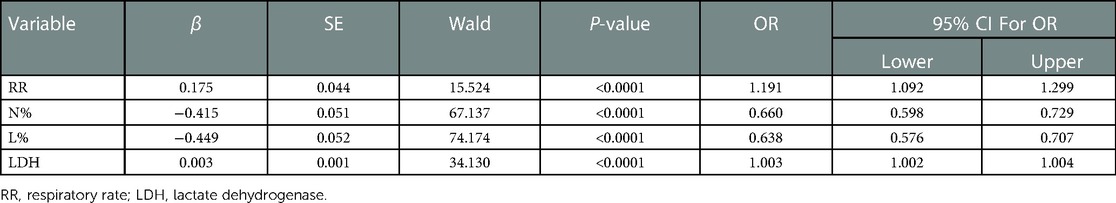
All statistically significant variables in the intergroup comparison were considered for logistic regression analysis. The maximum likelihood ratio forward stepwise regression method was used to screen variables. Finally, breathing rate, % neutrophil, % lymphocyte, and LDH levels were included in the predictive model (Table 2). The final prediction model is shown in Figure 1B.
3.3. ROC curve analysis
In the retrospective cohort, the area under the curve for the predictive scale was 0.94 [95% confidence interval (CI) 0.922–0.933], as determined by ROC curve analysis (Figure 1C). The optimal cut-off of the scale for predicting severe pneumonia was 0.282, with a sensitivity of 93.3%, specificity of 92.1%, and consistency rate of 92.42% in the retrospective cohort (Table 3).
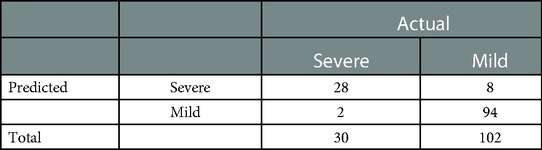
Table 3. Predictive results of the early recognition scale for critically ill children with adenovirus pneumonia in a historical cohort. Sensitivity and specificity were 93.3% and 92.1%, and the diagnostic consistency was 92.42%.
4. Discussion
In this study, based on a clinical cohort of children with CAP, 30 patients developed severe adenovirus pneumonia, and 102 patients had mild adenovirus pneumonia. There were statistically significant differences in age, respiratory rate, fever duration before admission, percentage of neutrophils and lymphocytes, ALT, and LDH between the two groups. Logistic regression analysis was conducted using the R language, and respiratory rate, percentage of neutrophils, percentage of lymphocytes, and LDH were used as scale indicators. The preliminary establishment of a critical early recognition scale for adenovirus pneumonia in children has been completed. Using the ROC curve, the sensitivity and specificity of the scale were 93.3% and 92.1%, respectively, and the critical early recognition scale was presented in the form of a rosette diagram. The scale for preliminary early recognition of severe cases has good specificity and sensitivity, which proves that it is feasible to establish a scale for early recognition of critical cases of adenovirus pneumonia in children based on big data from a clinical cohort.
Early and accurate identification of severe cases is vital for the treatment and prognosis of adenovirus pneumonia in children. Studies have shown that early administration of cidofovir is important in improving respiratory failure caused by adenovirus pneumonia (12, 13). Methods such as PSI and CURB-65 scores have been established to predict the severity and prognosis of adult CAP and guide treatment at an early stage. The PSI score scale is a common clinical CAP assessment tool that can guide the initial treatment mode and help inform patients of expected clinical outcomes (14). The CURB-65 scoring system, developed in 2003 to assess the severity of CAP and predict short-term mortality, is highly regarded by the American Society for Infectious Diseases and the American Thoracic Society. An analysis of the diagnostic efficacy of CURB-65 found that when CURB-65 was 3, the sensitivity, specificity, and area under the curve of CAP were 56%, 74%, and 0.69, respectively, indicating good diagnostic efficacy (8). However, CAP caused by different pathogens in children of different ages has distinct clinical characteristics. Establishing a predictive tool for all etiology-induced CAP in children, such as the adult PSI or CURB-65 scores, is impossible. Therefore, it is necessary to establish a simple, accurate, and quantitative early recognition scale for severe disease in children with adenovirus pneumonia to predict early and guide treatment.
Some clinical indicators of adenovirus pneumonia are correlated with prognosis. Adenovirus pneumonia in adults is usually accompanied by a decrease in peripheral lymphoid count (15), and there is a correlation between the number of monocytes in the peripheral blood of adults and the occurrence of respiratory failure in adenovirus pneumonia (16). Adenovirus viral load and type are associated with severe adenovirus pneumonia (17). However, there is a lack of a scale for the early recognition of acute adenovirus pneumonia in children, and the early recognition of acute pneumonia depends on the experience of clinicians. Therefore, it is necessary to establish a simple, accurate, and quantifiable early identification scale for severe cases of adenovirus pneumonia in children.
Various infectious diseases are most susceptible in childhood, and children soon enter a susceptible state after the temporary immunity endowed by maternal antibodies. Childhood infectious diseases have been widely prevalent throughout human history. Morbidity and mortality rates were extremely high in the early 20th century and were an important social and medical problem. With national public health initiatives, remarkable achievements have been made in preventing and controlling infectious diseases. The prevention and treatment of infectious diseases in children is still the focus of global health work in the 21st century. There are even clusters of critically ill cases in individual regions, including multiple outbreaks of adenovirus pneumonia in China, Singapore, and South Korea (18–21). Children are particularly affected by adenoviruses owing to their unique physiological characteristics. The epidemic is endemic in some parts of our country, greatly impacts children's health, and puts great pressure on the medical system (5, 22). Prevention and control measures for nosocomial adenovirus infection and treatment plans for severe adenovirus infection and adenovirus pneumonia in children are major emerging.
A clinical cohort with a longer period, scale generation based on big data, and parallel prospective validation is needed to increase the accuracy and scientificity of the scale.
In summary, we included four off-the-shelf clinical indicators for predicting severe adenovirus pneumonia. This predictive scale helps determine whether a child will develop severe adenovirus pneumonia early in the disease course. In the retrospective cohort, the scale had better discriminative power and higher sensitivity and specificity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of the Children's Hospital Affiliated with Nanjing Medical University (approval number: 202205067-1). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
CX: designed the study. SY: acquired the data. JZ: analyzed the data and drafted the manuscript. DZ and FL: reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Nanjing Health Science and Technology Development Special fund project (YKK21143) and Research project of Jiangsu Maternal and Child Health Association (FYX201905).
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1122589/full#supplementary-material.
References
1. Zhu Y, Xu B, Li C, Chen Z, Cao L, Fu Z, et al. A multicenter study of viral aetiology of community-acquired pneumonia in hospitalized children in Chinese mainland. Virol Sin. (2021) 36:1543–53. doi: 10.1007/s12250-021-00437-0
2. Lynch JR, Kajon AE. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin Respir Crit Care Med. (2016) 37:586–602. doi: 10.1055/s-0036-1584923
3. La Fay C, Bosdure E, Baravalle-Einaudi M, Stremler-Le BN, Dubus JC, Mazenq J. Severe adenovirus pneumonia with hemophagocytic syndrome and respiratory failure. Arch Pediatr. (2020) 27:383–5. doi: 10.1016/j.arcped.2020.07.003
4. Fu Y, Tang Z, Ye Z, Mo S, Tian X, Ni K, et al. Human adenovirus type 7 infection causes a more severe disease than type 3. BMC Infect Dis. (2019) 19:36. doi: 10.1186/s12879-018-3651-2
5. Yu X, Ma Y, Gao Y, You H. Epidemiology of adenovirus pneumonia and risk factors for bronchiolitis obliterans in children during an outbreak in jilin, China. Front Pediatr. (2021) 9:722885. doi: 10.3389/fped.2021.722885
6. Zhang HY, Xiao M, Yan F, Zhang MR, Zhang Y. Risk factors for the development of hemophagocytic lymphohistiocytosis in children with severe adenovirus pneumonia: a single-center retrospective study. Front Pediatr. (2021) 9:654002. doi: 10.3389/fped.2021.654002
7. Aujesky D, Fine MJ. The pneumonia severity index: a decade after the initial derivation and validation. Clin Infect Dis. (2008) 47(Suppl 3):S133–9. doi: 10.1086/591394
8. Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. (2003) 58:377–82. doi: 10.1136/thorax.58.5.377
9. Bi Y, Zhu Y, Ma X, Xu J, Guo Y, Huang T, et al. Development of a scale for early prediction of refractory Mycoplasma pneumoniae pneumonia in hospitalized children. Sci Rep. (2021) 11:6595. doi: 10.1038/s41598-021-86086-5
10. Saraya T, Watanabe T, Tsukahara Y, Ohkuma K, Ishii H, Kimura H, et al. The correlation between chest x-ray scores and the clinical findings in children and adults with Mycoplasma pneumoniae pneumonia. Intern Med. (2017) 56:2845–9. doi: 10.2169/internalmedicine.8500-16
11. Zhu Z, Zhang T, Guo W, Ling Y, Tian J, Xu Y. Clinical characteristics of refractory mycoplasma pneumoniae pneumonia in children treated with glucocorticoid pulse therapy. BMC Infect Dis. (2021) 21:126. doi: 10.1186/s12879-021-05830-4
12. Ko JH, Lim JU, Choi JY, Oh HS, Yoo H, Jhun BW, et al. Early cidofovir administration might be associated with a lower probability of respiratory failure in treating human adenovirus pneumonia: a retrospective cohort study. Clin Microbiol Infect. (2020) 26:646.e9–646.e14. doi: 10.1016/j.cmi.2019.10.012
13. Siew JX, Seah X, Chew YR, Thoon KC, Chong CY, Yung CF, et al. Epidemiology of adenovirus infections and outcomes of cidofovir treatment in severely ill children. Pediatr Infect Dis J. (2020) 39:907–13. doi: 10.1097/INF.0000000000002726
14. Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. (1997) 336:243–50. doi: 10.1056/NEJM199701233360402
15. Clark TW, Fleet DH, Wiselka MJ. Severe community-acquired adenovirus pneumonia in an immunocompetent 44-year-old woman: a case report and review of the literature. J Med Case Rep. (2011) 5:259. doi: 10.1186/1752-1947-5-259
16. Yoon H, Jhun BW, Kim SJ, Kim K. Clinical characteristics and factors predicting respiratory failure in adenovirus pneumonia. Respirology. (2016) 21:1243–50. doi: 10.1111/resp.12828
17. Xie L, Zhang B, Zhou J, Huang H, Zeng S, Liu Q, et al. Human adenovirus load in respiratory tract secretions are predictors for disease severity in children with human adenovirus pneumonia. Virol J. (2018) 15:123. doi: 10.1186/s12985-018-1037-0
18. Ng OT, Thoon KC, Chua HY, Tan NW, Chong CY, Tee NW, et al. Severe pediatric adenovirus 7 disease in Singapore linked to recent outbreaks across Asia. Emerg Infect Dis. (2015) 21:1192–6. doi: 10.3201/eid2107.141443
19. Gu L, Liu Z, Li X, Qu J, Guan W, Liu Y, et al. Severe community-acquired pneumonia caused by adenovirus type 11 in immunocompetent adults in Beijing. J Clin Virol. (2012) 54:295–301. doi: 10.1016/j.jcv.2012.04.018
20. Lee E, Kim CH, Lee YJ, Kim HB, Kim BS, Kim HY, et al. Annual and seasonal patterns in etiologies of pediatric community-acquired pneumonia due to respiratory viruses and Mycoplasma pneumoniae requiring hospitalization in South Korea. BMC Infect Dis. (2020) 20:132. doi: 10.1186/s12879-020-4810-9
21. Tsou TP, Tan BF, Chang HY, Chen WC, Huang YP, Lai CY, et al. Community outbreak of adenovirus, Taiwan, 2011. Emerg Infect Dis. (2012) 18:1825–32. doi: 10.3201/eid1811.120629
Keywords: adenovirus, pneumonia, early recognition scale, respiratory infection, risk facors
Citation: Zhang J, Xu C, Yan S, Zhang X, Zhao D and Liu F (2023) A nomogram for predicting severe adenovirus pneumonia in children. Front. Pediatr. 11:1122589. doi: 10.3389/fped.2023.1122589
Received: 13 December 2022; Accepted: 14 February 2023;
Published: 1 March 2023.
Edited by:
Ting Fan Leung, The Chinese University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Quan Wang, Capital Medical University, ChinaYibing Cheng, Children's Hospital Affiliated of Zhengzhou University, China
© 2023 Zhang, Xu, Yan, Zhang, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deyu Zhao emhhb2RleXU5OEAxMjYuY29t Feng Liu YXhzbGl1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Pediatric Pulmonology, a section of the journal Frontiers in Pediatrics
 Jiamin Zhang
Jiamin Zhang Changdi Xu†
Changdi Xu† Feng Liu
Feng Liu