
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr., 26 July 2023
Sec. Pediatric Neurology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1120728
Background: Gastrointestinal (GI) symptoms are frequently experienced by children with autism spectrum disorder (ASD), and these symptoms cause difficulties for these children and their families. However, studies of GI symptom prevalence differ significantly. This meta-analysis aimed to analyze the prevalence of GI symptoms in children with ASD.
Methods and findings: PubMed, Scopus, Web of Science, EMBASE were electronically searched to collect all literature on gastrointestinal symptoms of children with ASD collected through questionnaires or scales from January 2012 to May 2021. Four researchers independently scanned the literature and extracted information on general characteristics. First author name, year of publication, geographical location, type of study, sample sizes of ASD and control (if any) children, sex and average age, number of GI cases, number of GI symptoms, GI assessment tools (gastrointestinal symptoms scale), autism diagnosis methods, and other necessary data were collected and analyzed using Stata V16. The questionnaires included the Rome, 6-GSI, GIQ, GSRS, GSIQ, ADI-R, PedsQL-GI, parent-report, GI-related, and self-administered questionnaires. Compared with typically developing (TD) children, the odds ratio for In children with ASD with at least one GI symptom was 3.64, and the total prevalence was 55%. The cumulative prevalence rates of various symptoms were summarized, showing that 37% of children with ASD had constipation, 21% had abdominal pain, 19% had diarrhea, 8% had vomiting, and 23% had abdominal distension.
Conclusions: The results of this meta-analysis on GI symptoms in ASD show that patients with ASD are more likely to develop symptoms than TD children. The prevalence of GI symptoms in In children with ASD was 55%.
Systematic Review Registration: www.crd.york.ac.uk/PROSPERO, identifier, #CRD42017080579.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by communication or social difficulties, persistent and circumscribed repetitive behavior, interest, or activity patterns that limit or impair daily function; it is characterized by symptoms in early childhood (1). The prevalence of autism spectrum disorder has shown an upward trend. In the United States in 2018, the overall prevalence rate of ASD in men and women was 3.4 per 1,000 whereas; early identification of 8-year-old children indicated an overall prevalence of 23.0 per 1,000 (2–4).
Children with ASD are often reported to have gastrointestinal (GI) symptoms and other complications (5). GI symptoms in individuals with ASD can include, GI symptoms can include constipation, diarrhea, nausea, abnormal defecation, abdominal pain, and vomiting. Although GI symptoms are not clearly identified as core symptoms or comorbidity of autism, associated GI dysfunction is often mentioned (6–8). GI dysfunction has also been related to sleep disorders and food allergy, which lead to other metabolic disorders, physical and intellectual disability, and endocrine dysfunction. These, in turn, may lead to higher risks of overweight or obesity in children (5, 7, 9). GI symptoms play a critical role in complications found in children with ASD. Ames and coworkers showed that adolescents with autism had more complex needs for psychiatric and health care, which meant they had to pay more money or effort for interventions (10); children with ASD and gastrointestinal symptoms paid more money or effort for interventions. Children and adolescents with autism have a higher rate of hospitalization for gastrointestinal disorders than the general population (11). In 2014, McElhanon et al. reported that children with ASD have higher prevalence of GI symptoms than TD children, through a study investigating gastrointestinal symptoms in In children with ASD. However, this article did not calculate the specific prevalence of GI in children with ASD. In recent years, with the increase of related research reports, the results may change and need to be further updated (12).
Since then, several reviews have explored the GI profile of ASD. One review in 2018 of the ASD-related gastrointestinal literature by Holingue et al. found a prevalence of at least one GI symptom ranging from 4.2% to 96.8% (median 46.8%) (13). A 2021 systematic review of prevalence and comorbidities in children with ASD noted a significant difference in the prevalence of GI symptoms, ranging from 0.00 to 67.80 (5). No new meta-analysis has been conducted since 2014.
At present, the collection of data and investigation of GI symptoms in children with ASD come mostly from parents’ reports by questionnaires, medical records, or physician's diagnosis. One study has shown that the ratio of agreement between physician diagnosis and parental report for GI symptoms in children with ASD is 92.1% (14). Compared with other sources, questionnaire surveys are widely used for data collection in health services research and can be administered through the mail, and face-to-face or telephone interviews. They have the advantages of being cost-effective and easy to administer on a large scale.
The current research on GI symptoms in children with ASD is insufficient. The prevalence of GI symptoms in In children with ASD varies greatly among different studies. There are different assessment methods for symptoms in these children, and reliable and effective GI evaluation tools for ASD research are lacking. As studies are updated, more attention is being paid to GI problems in children with ASD, and this provides an opportunity for better assessments. The purpose of this meta-analysis was to integrate studies on the GI conditions of children with ASD that have been collected using questionnaires or scales, most of which were cross-sectional studies, to determine the relationship between ASD and the prevalence of GI symptoms. To integrate the prevalence of ASD combined with GI, and update the meta-analysis of GI in ASD after 2014.
This systematic evaluation and meta-analysis followed the recommendations of the Preferred Report Project (PRISMA) reporting guidelines for systematic evaluation and meta-analysis. The protocol for this study was registered in PROSPERO (#CRD42017080579, www.crd.york.ac.uk/PROSPERO).
We used a four-pronged, systematic approach to identify relevant publications. We included PubMed, Scopus, Web of Science, and EMBASE with a combination of the following keywords and Medical Subject Headings (if applicable) and we limited the search to title, summary, and keywords. The retrieval formula was (stomach OR intestines OR abdomen OR gastrointestinal OR alimentary OR belly OR digestive) AND (infantile autism) OR autism OR (autism spectrum disorder) OR (child autism) OR (Kanner syndrome) OR (Asperger syndrome). The search was further limited by the period from January 1, 2012, to October 1, 2021, to keep the focus on recent research.
Rayyan (https://www.rayyan.ai/) was used to screen the retrieved literature by title and abstract in a collaborative online manner, enabling blinding to ensure that researchers were screened independently; the blinding was removed once all the studies were screened. Differences in opinion between researchers about study eligibility were resolved through discussion and finally adjudicated by the lead researcher (HZG), as required.
We included children and adolescents (age <18 years) diagnosed with autism. Participants were diagnosed with ASD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Classification of Diseases (ICD) classification and/or based on structured and valid diagnostic tools [the Autism Diagnostic Observation Sheet (ADOS) and/or the Autism Diagnostic Interview (ADI)]. The method used in the literature was questionnaire assessment of gastrointestinal symptoms. There are specific symptom frequency statistics.
In addition to articles that do not qualify for inclusion, we will exclude animal models or other model studies, systematic reviews or meta-analyses, letters, notes and conference abstracts, and studies published in non-peer-reviewed journals. Articles with lack of data regarding specific symptoms, were also excluded.
We extracted the following information from each study: first author's name, year of publication, geographical location, study type, sample sizes of children with ASD and controls (if any), sex and mean age, number of GI cases, number of GI symptoms, GI assessment tool (Gastrointestinal Symptom Scale), and autism diagnosis modality. Three investigators (HZG, YHS, GLH) retrieved, selected the literature, and did the data extraction from the literature independently. For continuous outcomes, the data were pooled as mean (M) or Standard Deviation (SD) with 95% confidence intervals (95% CI) and for dichotomous outcomes, as a odds ratio (OR) with 95% CI.
Three researchers (YHS, GLH, LLZ) independently assessed the quality of the included studies and selected appropriate scales according to the types and characteristics of the included literature. Disagreements among review researchers regarding the risk of bias in particular studies was to be resolved through discussion and ultimately adjudicated by the lead researcher (HZG).
MINORS (15) was used in clinical trials, NOS (16) was used in observational studies including cohort and case-control studies, AHRQ (17) was used in cross-sectional studies, and ROB2 was used in RCTS.
Data from qualified studies were used in the meta-analysis. The prevalence of GI in ASD was pooled by single-group rate meta-analysis. The odds ratio (OR) was used as an effect analysis statistic for the counting data. Point estimates and 95% CI of each effect are given. Heterogeneity among the studies was measured by the heterogeneity index I2 Assessment. I2 ≤ 50% indicated that there was no obvious heterogeneity, and the fixed effect model was adopted; I2 > 50% indicated that there was heterogeneity. The source of heterogeneity was determined by gradually removing a single study; if I2 > 50% remained, the random effect model was used. The results of the meta-analysis are represented by forest maps. Subgroup analysis was conducted to explore the potential heterogeneity of the publication region, publication year, study type, and questionnaire type. Funnel plotting and Egger's test were used to assess for publication bias; p < 0.01 was considered sufficient evidence of no publication bias. When the influence deviation of a study on the overall results was p > 0.05, that study was excluded. Documents with missing data were excluded from both the summary and the itemized analysis. Stata V16 was used for statistical analysis, and a p value <0.05 was considered significant.
A total of 4,824 studies were retrieved from the four databases, with 2,157 studies remaining after re-checking. A manual re-check yielded 1,891 studies. Ultimately, 25 studies (18–41) were screened (Figure 1). Nine were from Asia, five from Europe, nine from the Americas, and two from Africa. The articles included cross-sectional studies (17), clinical trials (3), cohort studies (2), case-control studies (2), and randomized control trials (1). The questionnaires included the Rome, 6-GSI, GIQ, GSRS, GSIQ, ADI-R, PedsQL-GI, parent-report, GI-related, and self-administered questionnaires (see Table 1 for details). A total of 4,723 children with ASD were included, ranging in age from 0.5 to 18 years. The mean and standard deviation of age were calculated in 18 articles, and the overall mean [standard deviation, SD] age was 5.82 [3.09] years. Twenty articles provided the sex differential of study subjects; 81.9% were male. We included 16 medium-quality, one low-quality, and eight high-quality studies (Table 2).
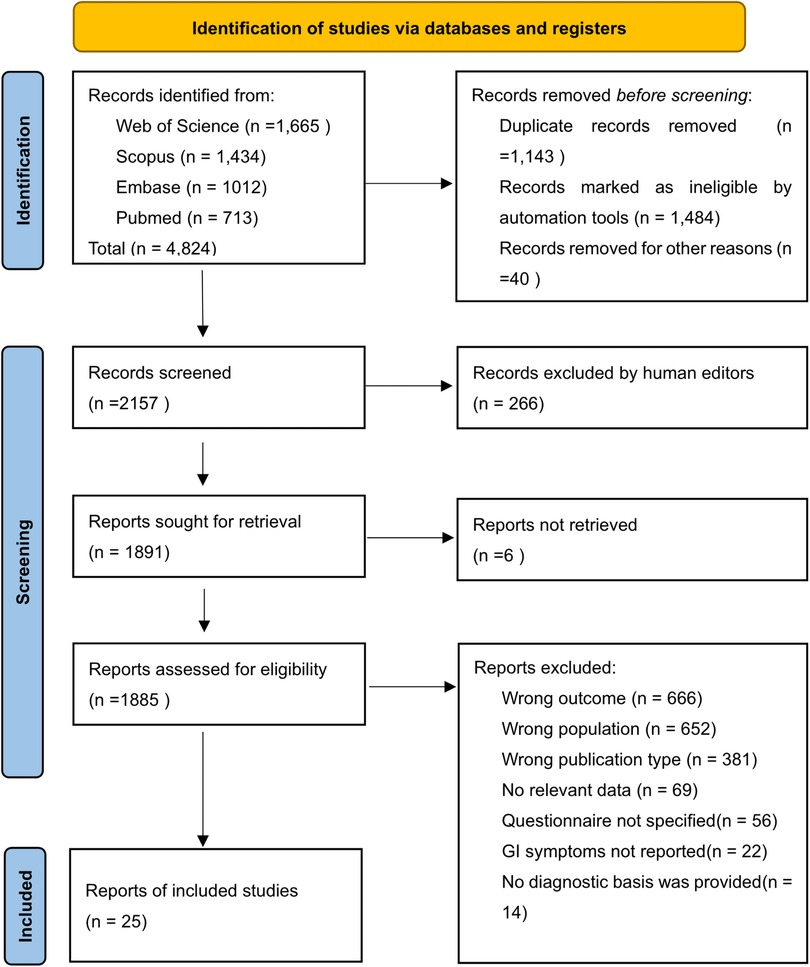
Figure 1. PRISMA flow chart of study selection in this review. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71.
This study explored the prevalence of GI symptoms in children with ASD. Therefore, the publication bias test was performed on 11 studies (Table 3) (18–27) that recorded GI symptoms in the ASD and typically developing (TD) groups and 19 studies (Table 4) (18–35) that collected the total number of GI cases in children with ASD.
Different questionnaires were used in the included studies, which can be roughly divided into the Rome, 6-GSI, Gastrointestinal symptoms, parent-report, GI section of the child behavior checklist (CBCL), and self-made questionnaires. Among these, the Rome IV questionnaire was used in four studies and the Rome III questionnaire in another five. The 6-GSI was selected by two studies; the CBCL was used in two. One study used a self-made questionnaire, and two were parent-report questionnaires. There were eight gastrointestinal symptom questionnaires including the SEED self-built Gastrointestinal questionnaire, the Gastrointestinal System Rating Scale (GSRS), a daily stool record, the 35-item Gastrointestinal symptom checklist developed by the autism treatment network (ATN), and the Pediatric Quality of Life Inventory Gastrointestinal Symptoms Module (PEDSQL-GI). There were 21 symptoms mentioned in all studies, and the symptoms of constipation, abdominal pain, diarrhea, nausea, and vomiting were the GI symptoms included in most of the questionnaires.
Constipation was mentioned in all questionnaires. Abdominal pain was recorded in all except for the parent-administered health history forms, the self-made 18- and 36-month forms, and the mealtime behavioral questionnaires. Diarrhea was not recorded in the Gastrointestinal Symptom Rating Scale and the daily stool records (DSRS + DSR) questionnaire or in two studies using the Rome 3 questionnaire. The self-made 18- and 36-month questionnaires, the 35-item Gastrointestinal Symptom Inventory, and the 6-GSI did not record nausea and vomiting. The collection of other symptoms is scattered (see Table 1, Figure 2).
Eleven studies (18–27) provided the case numbers of gastrointestinal symptoms in ASD versus TD children. The meta-analysis showed that the OR of children with ASD for having GI symptoms was 3.64; that is, children with ASD were about twice as likely as TD children to have GI symptoms. I2 was 58.3% (p = 0.008), indicating great heterogeneity (Figure 3). With subgroup analysis (Figure 4) according to study type with I2 = 30.8% and p = 0.182, heterogeneity decreased significantly, suggesting that study type may be the cause of heterogeneity, with an OR of 3.65 (95% CI: 3.07, 4.34). At the same time, our meta-analysis of the prevalence of total GI symptoms in TD children showed that the prevalence was 26%, I2 = 99.9%, p < 0.05 (Figure 5). After removing the articles that differed significantly from the others, the prevalence was 19%, I2 = 83.49%, p < 0.05 (Supplementary Figure S1, see the Supplementary Material).
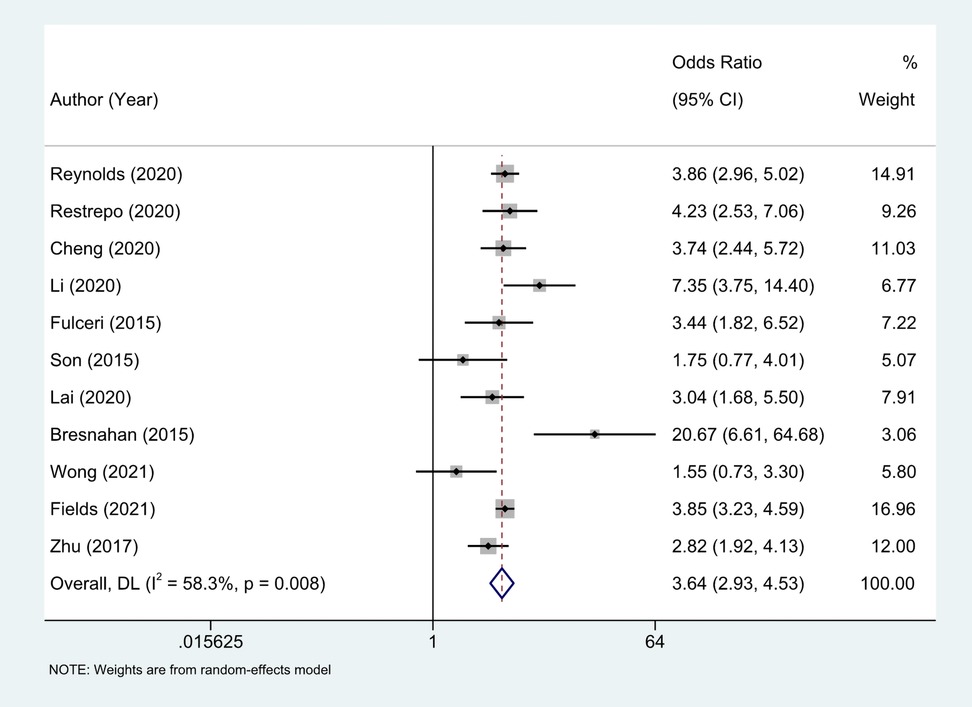
Figure 3. Eleven studies provided the case numbers of gastrointestinal symptoms in ASD versus TD children, OR = 3.64 (2.93, 4.53), Medium heterogeneity. Random effects model.
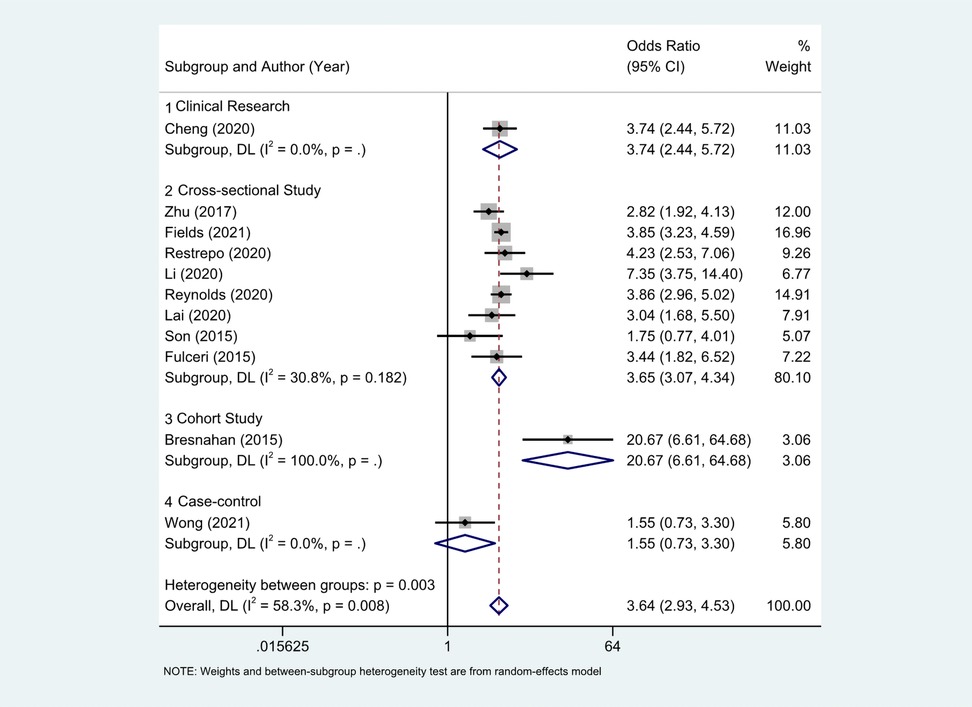
Figure 4. With subgroup analysis according to study type. (1) Clinical research; (2) cross-sectional study; (3) cohort study; (4) case-control.There were 8 cross-sectional studies, I2 = 30.8%, p = 0.182. Other research types just 1 article each, and no heterogeneity statistics. The OR of clinical research, cross-sectional, cohort study, case-control literature were 3.74 (2.44, 5.72), 3.65(3.07, 4.34), 1.55 (0.73, 3.30).
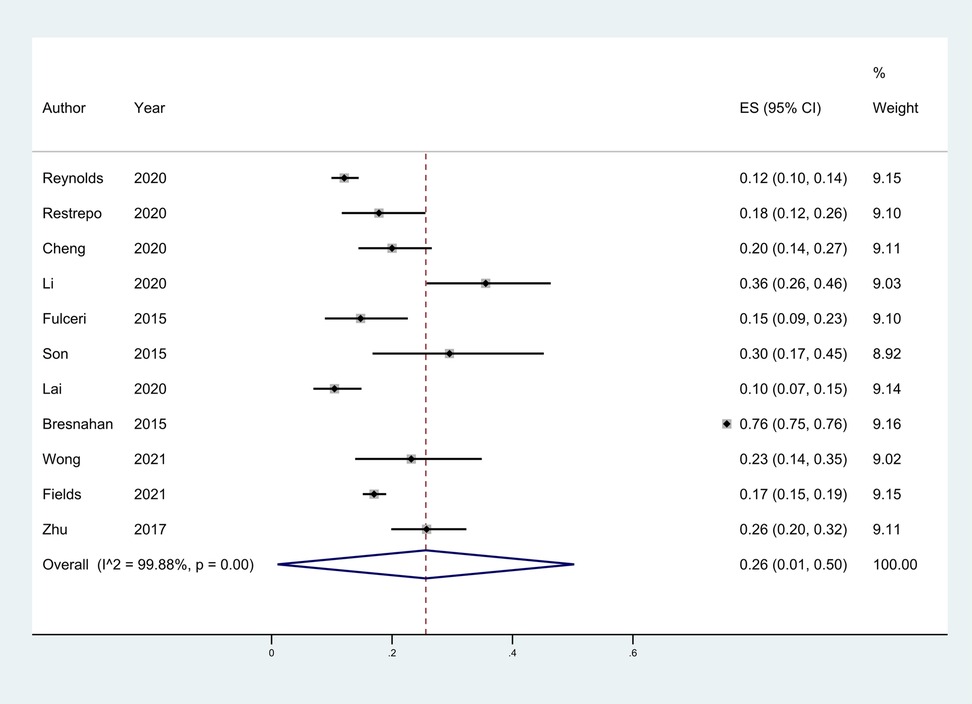
Figure 5. The total prevalence of GI in children in TD group was 26%, 95% CI (0.01, 0.50). Random effects model.
Eleven studies (18–19, 21, 23, 26, 28, 31, 33, 40, 41) reported constipation in children with ASD and TD, with an OR value of 2.83, I2 = 23.6%, p > 0.05. Children with ASD are more likely to experience constipation than TD children (Supplementary Figure S2, see the Supplementary Material). Twelve articles (19, 21, 22, 24, 25, 32, 34–37, 39, 41) recorded cases of children with ASD complaining of simple abdominal pain. Seven studies (18, 18, 20, 23, 29, 30, 38) reported cases of defecation-related abdominal pain, and two (26, 28) found no abdominal pain. Seven studies (18–19, 21, 23, 26, 41) compared abdominal pain in ASD and corresponding TD controls, with an OR of 2.60 (95% CI: 2.07, 3.26). This finding suggests that abdominal pain is more likely to occur in ASD (I2 = 48.7%, p > 0.069). However, heterogeneity in the results was low (Supplementary Figure S3, see the Supplementary Material). The prevalence of diarrhea in ASD and TD groups was reported in nine articles (18–19, 21, 23, 26, 31, 40, 41), and the combined OR was 3.27 (95% CI: 1.81, 5.91); children with ASD were more likely to have diarrhea than TD children (I2 = 71.9%, p = 0.000) (Supplementary Figure S4, see the Supplementary Material). After grouping according to different research types, the OR for cross-sectional literature (18, 18, 21, 23, 26, 40, 41) integration was 3.81 (95% CI: 2.26, 6.42), I2 = 33.3%, p = 0.174. Four studies (18, 19, 26, 41) compared the prevalence of abdominal distension in ASD and TD, and we calculated an OR of 1.45 (95% CI: 0.96, 2.17) (Supplementary Figure S5, see the Supplementary Material). The effect value crosses the invalid line, and the confidence interval includes 1, indicating that ASD has no influence on the occurrence of abdominal distension in children (I2 = 21.2%, p > 0.05). The heterogeneity of the results was low. Combining all nine studies (18–19, 21, 23, 28, 33, 40, 41) providing data on vomiting, we obtained an OR of 3.18 (95% CI: 2.12, 4.77), suggesting that children with ASD were more than twice as likely to experience vomiting as neurotypical children (I2 = 0.0%, p = 0.441) (Supplementary Figure S6, see the Supplementary Material).
Only two studies (31, 40) mentioned food allergy in children with ASD and TD, and the OR was 1.76 (95% CI: 1.05, 2.97). Thus, children with ASD were more likely to have food allergy (I2 = 62.6%, p < 0.05). One study (40) offered statistics on picky eating in children with ASD versus TD. According to the article, 41.6% of children with ASD have mild and 26% have severe picky eating. 14.5% of children with ASD have mild resistance and 9% have severe resistance to new foods. For TD children, the comparative percentages were 32.9%, 11%, 20.5%, and 1.4%, respectively. In terms of severe picky eating and resistance to new food, p values of the ASD and TD data were both <0.05, and thus, the differences were statistically significant. This suggests that children with ASD have more serious picky eating behaviors. In addition, the prevalence of ruminant syndrome in children with ASD has been reported as 0%–10% (28), with the prevalence in TD as 0.4%–1.5% (28, 33). The prevalence of gas swallowing in ASD was 1.5%–6.3% (20, 26, 28, 33), and in TD children was 0%–4.5% (26, 28, 33).
A total of 20 studies reported the prevalence of total GI symptoms in children with ASD. One (41) of these included only ASD patients with GI symptoms and was unable to calculate the prevalence of GI disturbance, so it was excluded from the meta-analysis. Thus, data from 19 studies (22–32) were ultimately included. The cumulative prevalence was 55% (95% CI: 0.45, 0.66), and more than half of the children with ASD had at least one gastrointestinal disorder, I2 = 97.93%, p < 0.05 (Figure 6).
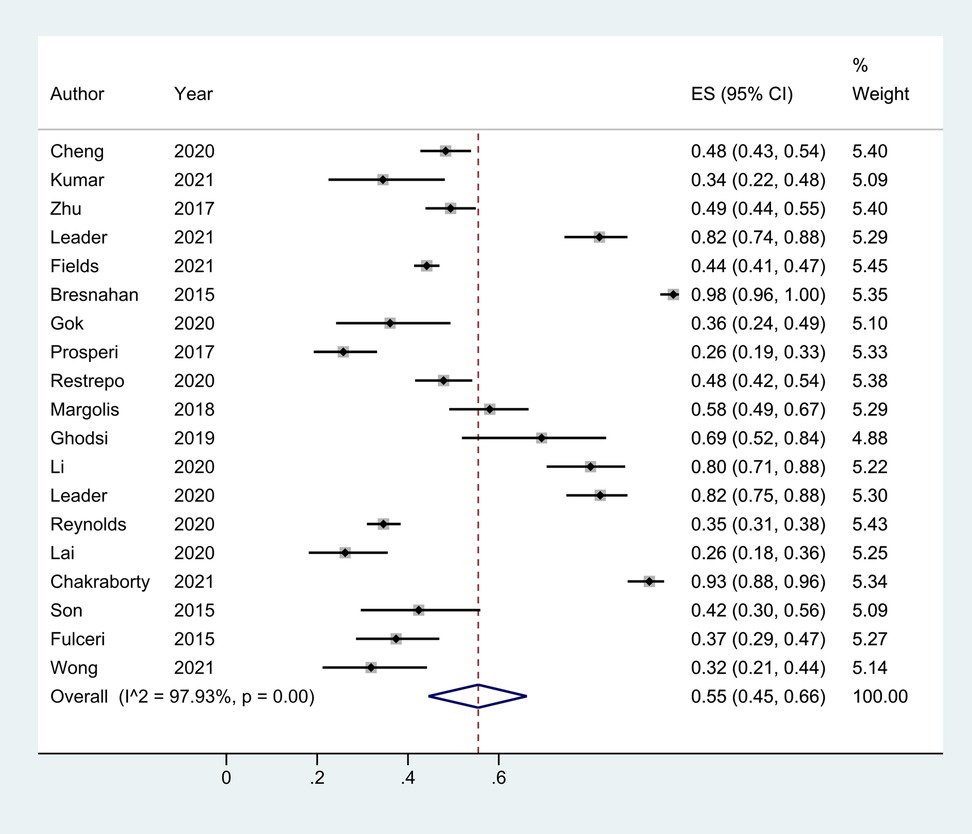
Figure 6. The prevalence of total GI symptoms in children with ASD based on 19 articles. It was 55% (0.45, 0.66).Random effects model.
Meta-regression analysis based on region, year, type of questionnaire, and type of study yielded Tau2 = 0.2041 with I2 = 96.92%; Adj R2 = −17.96%; p values were 0.344 (region), 0.671 (year), 0.959 (questionnaire type), and 0.922 (study type). Thus, the factors listed above were not the main sources of heterogeneity; however, the location of publication had the greatest influence on the prevalence of total GI in children with ASD. We found that the prevalence of GI in children with ASD varied when the literature was grouped by region. The prevalence was 47% (95% CI: 0.36, 0.58) in Asia, 55% (95% CI: 0.39, 0.70) in the Americas, and Europe was the highest at 69% (95% CI:0.35, 0.94) (Figure 7). The children's age, race, family income, parents’ education level, and mothers’ reproductive age could be other factors contributing to heterogeneity; however, as detailed data were not available, further analysis could not be made.
According to the data in the included studies, the cumulative prevalence of various symptoms showed that 37% of the children with ASD had constipation, 21% had abdominal pain, 19% had diarrhea, 8% had vomiting, and 23% had abdominal distention. All 25 articles provided information on constipation in children with ASD, with an prevalence of 37% (95% CI: 0.30, 0.43), I2 = 96.00%, p = 0.00 (Supplementary Figure S7, see the Supplementary Material). A total of 22 articles (18–27, 29–32, 34–37, 39–41) documented diarrhea in children with ASD, with a combined prevalence of 19% (95% CI: 0.14, 0.24), I2 = 96.86%, p = 0.00 (Supplementary Figure S8, see the Supplementary Material). Data on abdominal pain in children with ASD were reported in 21 articles (18–26, 28–30, 32, 34–37, 39, 41), with an prevalence of 21% (95% CI: 0.14, 0.28), I2 = 96.55%, p = 0.00 (Supplementary Figure S9, see the Supplementary Material). Data on vomiting were collected from 17 articles (18–19, 21, 33–25, 28, 29, 33–35, 37–41), with an prevalence of 8% (95% CI: 0.05, 0.12), I2 = 94.11%, p = 0.00 (Supplementary Figure S10, see the Supplementary Material). The prevalence of abdominal distension in the 12 groups (18, 19, 23–26, 30, 32, 34, 36, 41) of children with ASD was 23% (95% CI: 0.12, 0.37), I2 = 97.63%, p = 0.00 (Supplementary Figure S11, see the Supplementary Material).
This meta-analysis and systematic review collected studies on the gastrointestinal symptoms of autism published between 2012 and 2021. Updated and supplemented the meta-analysis of gastrointestinal tract in children with ASD in 2014. Using standardized data collection and rigorous inclusion criteria combined with a variety of statistical tools, the prevalence rate of gastrointestinal symptoms of autism was calculated. This review included 25 studies involving 4,720 children with ASD.
Based on the statistical analysis of the gastrointestinal questionnaires included in the studies, we concluded that different regions and vary types of questionnaires are the key factors for heterogeneity of the above results. Using the same questionnaire to compare the relevant scores of healthy children and children with autism may be more conducive to assessing the difference in GI symptoms prevalence.
The objectivity and reliability of the data may need to be based on the collection of questionnaires from different ASD populations to reduce the spatial bias; the included literature involves reports of parents as well as self-reported data. In addition, children with ASD generally have heterogeneous neurodevelopmental disorders and thus, differences in their abilities to perceive and talk about the severity of individual GI symptoms. Therefore, a simple GI questionnaire may not be sufficient to reflect the actual situation, and it needs to be supplemented with neurodevelopmental, psychological, behavioral, stool sample, and other evaluations (43). The potential influence of GI symptoms on the severity and progression of ASD has always been concerning. Therefore, a comprehensive evaluation tool for the GI symptom scale needs to be further improved and upgraded.
The review showed that children with autism were more likely to have gastrointestinal symptoms than healthy children. The prevalence rate was 55%, versus 26% in neurotypical children. Thus, among children with gastrointestinal symptoms, the prevalence rate of ASD is approximately twice that of TD children. Sharp mentioned that children with autism are more likely than TD children to have eating issues such as food selectivity, food refusal, and poor oral intake, which indicates that children with autism have feeding problems (44, 45). An important way in which dietary habits may affect behavior or gastrointestinal function is the ability to change the intestinal microbiome. In preclinical and clinical studies, diet rapidly changes the composition of the intestinal microbiota, and the specific microbiota environment is related to changes in behavior, emotion, cognition, and gastrointestinal problems (46, 47). The relationship between breastfeeding and ASD is still controversial. If breastfeeding lasts for 6 months, the risk of autism is lower in some studies ‘report; also the proportion of Clostridium difficile in the intestines of formula-fed infants is higher (48, 49). Some studies have also suggested that ASD is not associated with a history of breastfeeding. However, there was a significant positive relationship between early breastfeeding initiation and less severe core symptoms of autism on Childhood Autism Rating Scale scores (U = 405, p = 0.017) and better intellectual functions on intelligence quotient score (U = 18, p = 0.03) (50, 51) Increased microflora and decreased microbial diversity are characteristics of the ASD intestinal microbiota (52, 53).
At the same time, we found that the prevalence of GI in ASD varies among different regions. In the included literature, the prevalence of GI was the highest in Europe, followed by the Americas and Asia. There were few relevant literatures in Africa, and only one paper was included in this study, so it was not included in the scope of discussion. This may be related to the difference of intestinal flora among people in different regions. Geographical region and dietary intake have previously been shown to independently influence the microbial communities (54). Rinninella suggested that each human's gut microbiota are shaped in early life, but differ between individuals due to enterotypes, body mass index (BMI) level, exercise frequency, lifestyle, and cultural and dietary habits. The European diet with animal protein and lipids mainly formed enterotype Bacteroides, while the African or Asian diet with millet/sorghum and local vegetables principally formed enterotype Prevotella. Shifts in plant-based and animal-based diets will change firmicutes levels quickly and repeatably (55, 56). In a review of children's gut microbiota composition, it was noted that African (31.6%) and Central American children (35.7%) had lower percentages of firmicutes. There were significant differences between firmicutes and Bacteroides in European and Central American children. In European and North American children, firmicutes to Bacteroides ratios were higher than those in African and Central American children, and Asian children were in the between. Asian children have significantly higher rates of actinomycetes than Central American children (54). Studies on the composition of the gut microbiome in populations with ASD comorbid GI symptoms vary, with most suggesting that ASD patients exhibit decreased Bacteroides, elevated Firmicute phylum levels, low levels of enterococcus and Bifidobacteria, and higher abundance of Actinomycete and beta Proteobacteria (57). Iglesias-Vázquez et al. conducted a meta-analysis of intestinal microbial composition in children with ASD, and showed that children with ASD showed a significantly higher abundance of the genera Bacteroides, Parabacteroides, Clostridium, Faecalibacterium, and Phascolarctobacterium and a lower percentage of Coprococcus and Bifidobacterium. By analyzing the data provided in the literature, we found that the abundance of corresponding bacteria genera in the literature reports of different regions was significantly different. The Bacteroides’levels in In children with ASD in Asia (31.44%) was significantly higher than that in America (20.47%) and Europe (9.05%), while the Clostridium in Europe (6.24%) was higher than that in America (0.05%) and Asia (0.65%), and the Bifidobacterium’levels was higher in Europe (7.75%) and Asia (5.87%). It was lowest in the Americas (0.37%) (58). The diet, living habits and consumption level in different regions have a huge impact on the intestinal microbes of people in the region, which may be one of the reasons for the differences in the prevalence of GI symptoms in children with ASD in different regions.
The prevalence rate of gastrointestinal symptoms in individuals diagnosed with ASD is two times higher than that of typical children, possibly due to feeding methods and autoimmune. The reason for the increases in the IgG subclasses, IgG2 and IgG4, in the serum of patients with certain autism remains unclear. Serum levels of γ immunoglobulins are increased in autoimmune diseases. Hypergammaglobulinemia, related to autoimmunity, can be partly attributed to the activation of polyclonal B cells. Therefore, patients with autism exhibit elevated levels of autoimmune changes. The concentrations of IgG2 and IgG4 in the serum of patients with inflammatory bowel disease also increase. At the same time, children with autism are often accompanied by inflammatory bowel-like intestinal symptoms such as diarrhea, constipation, flatulence, and esophageal reflux (59). Children with autism often have food selectivity and inadequate nutrient intake. Different dietary structure affects the composition of intestinal microbes, which in turn causes gastrointestinal problems (42).
We found that the most common GI symptom in children with ASD was constipation, followed by food intolerance, and the prevalence of abdominal distension and pain was more than 20%. A cross-sectional study by Harris (60) found that ASD in children was positively correlated with constipation, and the indirect effects of food selectivity (pickiness) on both of these cannot be ignored. In addition, there are multidirectional and complex interactions between changes in intestinal microbial function, ASD, and constipation. The low content of Bacteroides, Firmicutes, and six other diet-related bacteria may increase the prevalence of constipation in ASD patients (61).
Constipation is also significantly related to the toilet resistance of In children with ASD. At the same time, it is related to delayed language expression and low social motivation. However, these can be improved through toilet training and other social skills training (62). As In children with ASD show more difficulties completing the above training than typical children, the vicious cycle of constipation and toilet resistance makes In children with ASD even more prone to constipation. There is a significant two-way relationship between gastrointestinal symptoms and internalization symptoms such as anxiety and sleep disorders in children with ASD (63). Lower GI symptoms, such as constipation, correlate significantly with decreases in parasympathetic tone (64). Anxiety caused by parasympathetic activity at baseline is closely associated with lower gastrointestinal symptoms (65). It can be inferred that the probability of anxiety in children with ASD is also significantly higher than that in TD children. The prevalence of constipation in ASD is positively correlated with anxiety severity and age. The likelihood of constipation among older children with increased anxiety increased by 11% (66). A case-control study on gastrointestinal function and anxiety in autistic children showed that the anxiety of In children with ASD may be partly explained by the presence of gastrointestinal symptoms, and that the occurrence of abdominal pain and vomiting in children with ASD may be more related to anxiety (30).
Autism is often accompanied by autoimmune abnormalities. A large-sample, multi-ethnic, and national cross-sectional study showed that allergic diseases, especially food allergy, are more closely related to autism, and their mechanism may be related to the gut brain behavior axis, involving changes in the gut microbiome, allergic immune activation, neurodevelopmental abnormalities, and other biological processes (67). A national health survey report from 2011 to 2015 showed that the prevalence of food allergy reported by the parents of autistic children (13.1%) was nearly 2.5 times that of non-autistic children (5.4%) (68). A meta-analysis showed that food hypersensitivity was positively associated with ASD, whereas girls and children under 12 years of age were more likely to present this association (69). Another report gives a contrasting conclusion that young men with autism are more likely to develop food allergy, indicating that sex is an uncertain factor. Sex hormones affect the number of T lymphocytes, and immune-related genes show characteristics of immune dimorphism (70). In addition, food allergy in ASD (especiallyβ-lactoglobulin sensitization, the main allergen in milk) is closely related to the mechanism of “brain allergy,” which includes the loss of the intestinal barrier, imbalance of intestinal flora, abnormal morphology of astrocytes, and brain TNF-α-related inflammation. This also increases the risk of anxiety and depression in autism (71).
The symptoms of GI tract involvement such as constipation, food allergy, abdominal pain, abdominal distension, and diarrhea are mostly considered to be related to the metabolic disorder of “microbiota gut brain axis” (73). Their severity is closely related to the repetitive and stereotypical behavior of ASD, but not significantly related to social and communication difficulties (40). At the same time, they are also related to increased self-mutilation (such as arm biting, head banging, and hair pulling, that occur without an apparent intent of willful self-harm but may pose significant risk of harm to self) (59), aggressive behavior, sleep disorders, and attention deficits (23); therefore, improving the symptoms of ASD by treating gastrointestinal symptoms would be a good idea. Clinicians and parents should take the high prevalence of gastrointestinal symptoms in patients with autism very seriously.
This study mainly contributes to estimating the prevalence of GI symptoms among those children with autism, as well as adding to the evidence of its high prevalence. However, there is also a defect in this study where the factors leading to heterogeneity, such as children's age, race, family income, parent's education level, and mother's childbearing age, etc., are unfortunately not able to be considered and further discussed because of the limited data within the references included.
The results of this meta-analysis on GI symptoms related to ASD demonstrate that children with ASD are more prone to develop GI symptoms than TD children, in spite of the data discrepancy in its prevalence reported in the literature, which may be accounted for, in a way, by the variety in respondents of surveys conducted in different regions. Another hard nut to crack in this study is that, the deviation of expression of children with ASD, the difficulty in communication with them, and the varieties of questionnaires that have been used, can all be the factors leading to an unsatisfactory scenario where the current mainstream GI symptom scale may not comprehensively reflect the true GI symptoms of children with ASD. In conclusion, this study does provide a foundation for further researches in such fields, as well as contributing to better comprehension and treatment towards gastrointestinal symptoms in individuals diagnosed with AsD, but still has limitations remained to be overcome in the future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
HG and YS designed the study and wrote the first draft of the manuscript. HG, YS, LZ, and GH performed the study search. LZ, GH, and CL extracted the data from the studies. HG, YLv, and YLi checked the data for consensus agreement. All authors contributed to the article and approved the submitted version.
This work was supported by the Study on the mechanism of regulating intestinal flora by invigorating spleen and stomach in the treatment of children with autism, [2019B026]; the Clinical randomized controlled study of Shengmai turmeric powder on improving radiotherapy sensitivity of nasopharyngeal carcinoma [20213009].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1120728/full#supplementary-material
SUPPLEMENTARY FIGURE S1
The total prevalence of GI in children in TD group. Removing the articles that differed significantly from the others, the prevalence was 19%, I2 = 83.49%, P <0.05.
SUPPLEMENTARY FIGURE S2
Constipation in children with ASD and TD, with an OR value of 2.83, I2 = 23.6%, p > 0.05. Fixed effects model.
SUPPLEMENTARY FIGURE S3
Seven studies compared abdominal pain in ASD and corresponding TD controls, with an OR of 2.60 (95% CI: 2.07, 3.26). Fixed effects model.
SUPPLEMENTARY FIGURE S4
The prevalence of diarrhea in ASD and TD groups. The combined OR was 3.27 (95% CI: 1.81, 5.91). Random effects model.
SUPPLEMENTARY FIGURE S5
The prevalence of abdominal distension in ASD and TD. The OR was 1.45 (95% CI: 0.96, 2.17). Fixed effects model.
SUPPLEMENTARY FIGURE S6
The prevalence of vomiting in ASD and TD groups. OR of 3.18 (95% CI: 2.12, 4.77). Fixed effects model.
SUPPLEMENTARY FIGURE S7
The cumulative prevalence of various symptoms showed that 37% of the children with ASD had constipation. (95% CI: 0.30, 0.43), I2 = 96.00%, p = 0.00. Random effects model.
SUPPLEMENTARY FIGURE S8
According to 22 articles, diarrhea in children with ASD, with a combined prevalence of 19% (95% CI: 0.14, 0.24), I2 = 96.86%, p = 0.00. Random effects model.
SUPPLEMENTARY FIGURE S9
Abdominal pain in children with ASD with a prevalence of 21% (95% CI: 0.14, 0.28), I2 = 96.55%, p = 0.00. Random effects model.
SUPPLEMENTARY FIGURE S10
Vomiting was collected from 17 articles, with a prevalence of 8% (95%CI: 0.05, 0.12), I2 = 94.11%, p = 0.00. Random effects model.
SUPPLEMENTARY FIGURE S11
The prevalence of abdominal distension in the 12 groups of children with ASD was 23% (95% CI: 0.12, 0.37), I2 = 97.63%, p = 0.00. Random effects model.
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing (2013).
2. Fombonne E, Editorial: the rising prevalence of autism. J Child Psychol Psychiatry, (2018) 59(7): 717–20. doi: 10.1111/jcpp.12941
3. Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ. (2021) 70(11):1–16. doi: 10.15585/mmwr.ss7011a1
4. Shaw KA, Maenner MJ, Bakian AV, Bilder DA, Durkin MS, Furnier SM, et al. Early identification of autism spectrum disorder among children aged 4 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ. (2021) 70(10):1–14. doi: 10.15585/mmwr.ss7010a1
5. Bougeard C, Picarel-Blanchot F, Schmid R, Campbell R, Buitelaar J. Prevalence of autism spectrum disorder and co-morbidities in children and adolescents: a systematic literature review. Front Psychiatry. (2021) 12:744709. doi: 10.3389/fpsyt.2021.744709
6. Holingue C, Poku O, Pfeiffer D, Murray S, Fallin MD. Gastrointestinal concerns in children with autism spectrum disorder: a qualitative study of family experiences. Autism. (2021) 26(7):698–1711. doi: 10.1177/13623613211062667
7. Kang V, Wagner GC, Ming X. Gastrointestinal dysfunction in children with autism spectrum disorders. Autism Res. (2014) 7(4):501–6. doi: 10.1002/aur.1386
8. Valicenti-McDermott MD, McVicar K, Cohen HJ, Wershil BK, Shinnar S. Gastrointestinal symptoms in children with an autism spectrum disorder and language regression. Pediatr Neurol. (2008) 39(6):392–8. doi: 10.1016/j.pediatrneurol.2008.07.019
9. Tkachuk EA, Martynovich NN, Globenko NE. Features of the nutritional status and nutrition of children with autistic disorders. Vopr Pitan. (2021) 90(5):67–76. doi: 10.33029/0042-8833-2021-90-5-67-76
10. Ames JL, Massolo ML, Davignon MN, Qian Y, Croen LA. Healthcare service utilization and cost among transition-age youth with autism spectrum disorder and other special healthcare needs. Autism. (2021) 25(3):705–18. doi: 10.1177/1362361320931268
11. McMaughan DJD, Jones JL, Mulcahy A, Tucker EC, Beverly JG, Perez-Patron M. Hospitalizations among children and youth with autism in the United States: frequency, characteristics, and costs. Intellect Dev Disabil. (2022) 60(6):484–503. doi: 10.1352/1934-9556-60.6.484
12. McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. (2014) 133(5):872–83. doi: 10.1542/peds.2013-3995
13. Holingue C, Newill C, Lee LC, Pasricha PJ, Daniele Fallin M. Gastrointestinal symptoms in autism spectrum disorder: a review of the literature on ascertainment and prevalence. Autism Res. (2018) 11(1):24–36. doi: 10.1002/aur.1854
14. Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Res. (2012) 5(2):101–8. doi: 10.1002/aur.237
15. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
16. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2008). Available at: http://ohri. ca/ programs/clinical epidemiology/oxford
18. Reynolds AM, Soke GN, Sabourin KR, Croen LA, Daniels JL, Fallin MD, et al. Gastrointestinal symptoms in 2- to 5-year-old children in the study to explore early development. J Autism Dev Disord. (2021) 51(11):3806–17. doi: 10.1007/s10803-020-04786-9
19. Restrepo B, Angkustsiri K, Taylor SL, Rogers SJ, Cabral J, Heath B, et al. Developmental-behavioral profiles in children with autism spectrum disorder and co-occurring gastrointestinal symptoms. Autism Res. (2020) 13(10):1778–89. doi: 10.1002/aur.2354
20. Cheng B, Zhu J, Yang T, Guo M, Lai X, Li Q, et al. Vitamin A deficiency increases the risk of gastrointestinal comorbidity and exacerbates core symptoms in children with autism spectrum disorder. Pediatr Res. (2021) 89(1):211–6. doi: 10.1038/s41390-020-0865-y
21. Ferguson BJ, Dovgan K, Severns D, Martin S, Marler S, Gross Margolis K, et al. Lack of associations between dietary intake and gastrointestinal symptoms in autism spectrum disorder. Front Psychiatry. (2019) 10:528. doi: 10.3389/fpsyt.2019.00528
22. Li C, Liu Y, Fang H, Chen Y, Weng J, Zhai M, et al. Study on aberrant eating behaviors, food intolerance, and stereotyped behaviors in autism spectrum disorder. Front Psychiatry. (2020) 11:493695. doi: 10.3389/fpsyt.2020.493695
23. Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. (2017) 5(1):10. doi: 10.1186/s40168-016-0225-7
24. Fulceri F, Morelli M, Santocchi E, Cena H, Del Bianco T, Narzisi A, et al. Gastrointestinal symptoms and behavioral problems in preschoolers with autism spectrum disorder. Dig Liver Dis. (2016) 48(3):248–54. doi: 10.1016/j.dld.2015.11.026
25. Leader G, Hogan A, Chen JL, Maher L, Naughton K, O'Rourke N, et al. Age of autism spectrum disorder diagnosis and comorbidity in children and adolescents with autism spectrum disorder. Dev Neurorehabil. (2021) 25(1):29–37. doi: 10.1080/17518423.2021.1917717
26. Leader G, Tuohy E, Chen JL, Mannion A, Gilroy SP. Feeding problems, gastrointestinal symptoms, challenging behavior and sensory issues in children and adolescents with autism spectrum disorder. J Autism Dev Disord. (2020) 50(4):1401–10. doi: 10.1007/s10803-019-04357-7
27. Son JS, Zheng LJ, Rowehl LM, Tian X, Zhang Y, Zhu W, et al. Comparison of fecal microbiota in children with autism spectrum disorders and neurotypical siblings in the simons simplex collection. PLoS One. (2015) 10(10):e0137725. doi: 10.1371/journal.pone.0137725
28. Margolis KG, Buie TM, Turner JB, Silberman AE, Feldman JF, Murray KF, et al. Development of a brief parent-report screen for common gastrointestinal disorders in autism spectrum disorder. J Autism Dev Disord. (2019) 49(1):349–62. doi: 10.1007/s10803-018-3767-7
29. Lai KYC, Leung PWL, Hung SF, Shea CKS, Mo F, Che KKI, et al. Gastrointestinal problems in Chinese children with autism spectrum disorder. Neuropsychiatr Dis Treat. (2020) 16:1807–15. doi: 10.2147/NDT.S260654
30. Prosperi M, Santocchi E, Balboni G, Narzisi A, Bozza M, Fulceri F, et al. Behavioral phenotype of ASD preschoolers with gastrointestinal symptoms or food selectivity. J Autism Dev Disord. (2017) 47(11):3574–88. doi: 10.1007/s10803-017-3271-5
31. Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One. (2019) 14(1):e0210064. doi: 10.1371/journal.pone.0210064
32. Bresnahan M, Hornig M, Schultz AF, Gunnes N, Hirtz D, Lie KK, et al.. Association of maternal report of infant and toddler gastrointestinal symptoms with autism evidence from a prospective birth cohort. Jama Psychiatry. (2015) 72(5):466–74. doi: 10.1001/jamapsychiatry.2014.3034
33. Khalil M, Azouz HG, Ahmed SA, Gad HA, Omar OM. Sensory processing and gastrointestinal manifestations in autism spectrum disorders: no relation to Clostridium difficile. J Mol Neurosci. (2021) 71(1):153–61. doi: 10.1007/s12031-020-01636-2
34. Wong OWH, Lam AMW, Lai KYC, Ma SL, Hung SF, Chan S, et al. An elevated anxiety level among prepubertal autistic boys with non-treatment-seeking functional gastrointestinal disorders: a case–control study. Autism Res: Off J Inter Soc Autism Res. (2021) 14(10):2131–42. doi: 10.1002/aur.2555
35. Chakraborty P, Carpenter KLH, Major S, Deaver M, Vermeer S, Herold B, et al. Gastrointestinal problems are associated with increased repetitive behaviors but not social communication difficulties in young children with autism spectrum disorders. Autism. (2021) 25(2):405–15. doi: 10.1177/1362361320959503
36. Ghodsi R, Kheirouri S. Positive association between plasma levels of advanced glycation and precursor of lipoxidation end products with gastrointestinal problems in children with autism. Curr Pediatr Rev. (2019) 15(3):184–90. doi: 10.2174/1573396315666190628141333
37. Shaaban SY, El Gendy YG, Mehanna NS, El-Senousy WM, El-Feki HSA, Saad K, et al. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci. (2018) 21(9):676–81. doi: 10.1080/1028415X.2017.1347746
38. Gok S, Ozturk SN, Karaca R, İlbars S, Nogay NH. Evaluation of sleep disturbances, gastrointestinal problems and eating behaviors in turkish children with autistic disorder and PDD-NOS. Advances in Autism. (2020) 7(2):101–13. doi: 10.1108/AIA-12-2019-0049
39. Kumar S, Sharon Ruth SV. Gastrointestinal issues with behavior problems and sleep issues in children and adolescents with autism spectrum disorder (ASD): a preliminary estimate. (2021) 17(3):112–26.
40. Fields VL, Soke GN, Reynolds A, Tian LH, Wiggins L, Maenner M, et al. Association between pica and gastrointestinal symptoms in preschoolers with and without autism spectrum disorder, study to explore early development. Disabil Health J. (2020) 14(3):101052. doi: 10.1016/j.dhjo.2020.101052
41. Liu X, Liu J, Xiong X, Yang T, Hou N, Liang X, et al. Correlation between nutrition and symptoms: nutritional survey of children with autism spectrum disorder in Chongqing, China. Nutrients. (2016) 8(5):294. doi: 10.3390/nu8050294
42. Zhu J, Guo M, Yang T, Lai X, Lei YY, He ML, et al. Association between behavioral problems and gastrointestinal disorders among children with autism spectrum disorder. Zhonghua Er Ke Za Zhi. (2017) 55(12):905–10. doi: 10.3760/cma.j.issn.0578-1310.2017.12.007
43. Ristori MV, Quagliariello A, Reddel S, Ianiro G, Vicari S, Gasbarrini A, et al. Autism, gastrointestinal symptoms and modulation of gut microbiota by nutritional interventions. Nutrients. (2019) 11(11):2812. doi: 10.3390/nu11112812
44. Holingue C, Kalb LG, Musci R, Lukens C, Lee LC, Kaczaniuk J, et al. Characteristics of the autism spectrum disorder gastrointestinal and related behaviors inventory in children. Autism Res: Off J Inter Soc Autism Res. 15(6):1142–55. doi: 10.1002/aur.2707
45. Sharp WG, Berry RC, McCracken C, Nuhu NN, Marvel E, Saulnier CA, et al. Feeding problems and nutrient intake in children with autism spectrum disorders: a meta-analysis and comprehensive review of the literature. J Autism Dev Disord. (2013) 43(9):2159–73. doi: 10.1007/s10803-013-1771-5
46. Ahearn WH, Castine T, Nault K, Green G. An assessment of food acceptance in children with autism or pervasive developmental disorder-not otherwise specified. J Autism Dev Disord. (2001) 31(5):505–11. doi: 10.1023/a:1012221026124
47. Murtaza NÓCP, Morrison M. Diet and the microbiome. Gastroenterol Clin North Am. (2017) 46(1):49–60. doi: 10.1016/j.gtc.2016.09.005
48. Parekh PJ, Balart LA, Johnson DA. The influence of the gut microbiome on obesity, metabolic syndrome and gastrointestinal disease. Clin Transl Gastroenterol. (2015) 6:e91. doi: 10.1038/ctg.2015.16
49. Schultz ST, Klonoff-Cohen HS, Wingard DL, Akshoomoff NA, Macera CA, Ji M, et al. Breastfeeding, infant formula supplementation, and autistic disorder: the results of a parent survey. Int Breastfeed J. (2006) 1:16. doi: 10.1186/1746-4358-1-16
50. Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. (2013) 185(5):385–94. doi: 10.1503/cmaj.121189
51. Abd Elmaksoud Marwa, Aly Omneya, Elfatah Magdy, Mahfouz Aml. Breastfeeding and autism spectrum disorder: a cross-sectional study from Egypt. Alexandria J Pediatr. (2022) 35:59. doi: 10.4103/ajop.ajop_10_22
52. Husk JS, Keim SA. Breastfeeding and autism spectrum disorder in the national survey of children’s health. Epidemiology. (2015) 26(4):451–7. doi: 10.1097/EDE.0000000000000290
53. Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe. (2012) 18(2):260–2. doi: 10.1016/j.anaerobe.2011.12.018
54. Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. (2013) 8(7):e68322. doi: 10.1371/journal.pone.0068322
55. Deering KE, Devine A, O'Sullivan TA, Lo J, Boyce MC, Christophersen CT. Characterizing the composition of the pediatric gut microbiome: a systematic review. Nutrients. (2019) 12(1):16. doi: 10.3390/nu12010016
56. Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. (2019) 7(1):14. doi: 10.3390/microorganisms7010014
57. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2014) 505(7484):559–63. doi: 10.1038/nature12820
58. Settanni CR, Bibbò S, Ianiro G, Rinninella E, Cintoni M, Mele MC, et al. Gastrointestinal involvement of autism spectrum disorder: focus on gut microbiota. Expert Rev Gastroenterol Hepatol. (2021) 15(6):599–622. doi: 10.1080/17474124.2021.1869938
59. Iglesias-Vàzquez L, Van Ginkel Riba G, Arija V, Canals J. Composition of gut microbiota in children with autism spectrum disorder: a systematic review and meta-analysis. Nutrients. (2020) 12(3):792. doi: 10.3390/nu12030792
60. Philipsen EK, Bondesen S, Andersen J, Larsen S. Serum immunoglobulin G subclasses in patients with ulcerative colitis and crohn’s disease of different disease activities. Scand J Gastroenterol. (1995) 30(1):50–3. doi: 10.3109/00365529509093235
61. Harris HA, Micali N, Moll HA, van Berckelaer-Onnes I, Hillegers M, Jansen PW. The role of food selectivity in the association between child autistic traits and constipation. Int J Eating Disord. (2021) 54(6):981–5. doi: 10.1002/eat.23485
62. Fu SC, Lee CH, Wang H. Exploring the association of autism spectrum disorders and constipation through analysis of the gut microbiome. Int J Environ Res Public Health. (2021) 18(2):667. doi: 10.3390/ijerph18020667
63. Wiggins LD, Nadler C, Hepburn S, Rosenberg S, Reynolds A, Zubler J. Toileting resistance among preschool-age children with and without autism spectrum disorder. J Dev Behav Pediatr. (2022) 43(4):216–23. doi: 10.1097/DBP.0000000000001036
64. Dovgan K, Gynegrowski K, Ferguson BJ. Bidirectional relationship between internalizing symptoms and gastrointestinal problems in youth with autism spectrum disorder. J Autism Dev Disord. (2022). doi: 10.1007/s10803-022-05539-6
65. Yuan Y, Ali MK, Mathewson KJ, Sharma K, Faiyaz M, Tan W, et al.. Associations between colonic motor patterns and autonomic nervous system activity assessed by high-resolution manometry and concurrent heart rate variability. Front Neurosci. (2019) 13:1447. doi: 10.3389/fnins.2019.01447
66. Barbier A, Chen JH, Huizinga JD. Autism spectrum disorder in children is not associated with abnormal autonomic nervous system function: hypothesis and theory. Front Psychiatry. (2022) 13:830234. doi: 10.3389/fpsyt.2022.830234
67. Ferguson BJ, Dovgan K, Takahashi N, Beversdorf DQ. The relationship among gastrointestinal symptoms, problem behaviors, and internalizing symptoms in children and adolescents with autism spectrum disorder. Front Psychiatry. (2019) 10:194. doi: 10.3389/fpsyt.2019.00194
68. Xu G, Snetselaar LG, Jing J, Liu B, Strathearn L, Bao W. Association of food allergy and other allergic conditions with autism spectrum disorder in children. JAMA Network Open. (2018) 1(2):e180279. doi: 10.1001/jamanetworkopen.2018.0279
69. Tan Y, Thomas S, Lee BK. Parent-reported prevalence of food intolerance in children with autism spectrum disorder: national health interview survey, 2011–2015. Autism Res. (2019) 12(5):802–5. doi: 10.1002/aur.2106
70. Li H, Liu H, Chen X, Zhang J, Tong G, Sun Y. Association of food hypersensitivity in children with the risk of autism spectrum disorder: a meta-analysis. Eur J Pediatr. (2021) 180(4):999–1008. doi: 10.1007/s00431-020-03826-x
71. Smith NA, Germundson DL, Combs CK, Vendsel LP, Nagamoto-Combs K. Anxiety-like behavior and intestinal microbiota changes as strain-and sex-dependent sequelae of mild food allergy in mouse models of cow’s milk allergy. Brain Behav Immun. (2021) 95:122–41. doi: 10.1016/j.bbi.2021.03.002
72. Smith NA, Germundson DL, Gao P, Hur J, Floden AM, Nagamoto-Combs K. Astrogliosis associated with behavioral abnormality in a non-anaphylactic mouse model of cow’s milk allergy. Front Cell Neurosci. (2019) 13:320. doi: 10.3389/fncel.2019.00320
Keywords: autism spectrum disorder, gastrointestinal, children and adolescent, prevalence rate, questionnaire
Citation: Gan H, Su Y, Zhang L, Huang G, Lai C, Lv Y and Li Y (2023) Editorial: the rising prevalence of autism. Front. Pediatr. 11:1120728. doi: 10.3389/fped.2023.1120728
Received: 10 December 2022; Accepted: 11 July 2023;
Published: 26 July 2023.
Edited by:
Roberto Canitano, Siena University Hospital, ItalyReviewed by:
Calliope Holingue, Kennedy Krieger Institute, United States© 2023 Gan, Su, Zhang, Huang, Lai, Lv and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongchun Li aG9sbWVzbHljQHNtdS5lZHUuY24= Ying Lv bHZ5aW5nMTk2NkBzbXUuZWR1LmNu
†These authors contributed equally to this work and share first authorship
Abbreviations ASD, autism spectrum disorder; GI, gastrointestinal; TD, typical development; DD, developmental delays; POP, the general population; DSM, the diagnostic and statistical manual of mental disorders; ADOS, the autism diagnostic observation schedule; ADI-R, the autism diagnostic interview-revised; 6-GSI, six-item GI severity index; OR, odds ratio; 95% CI, 95% confidence intervals.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.