- Department of Pediatrics, National Hospital Organization Saitama Hospital, Saitama, Japan
Immune thrombocytopenia (ITP) is an autoimmune disorder that is sometimes triggered by a preceding viral infection and is characterized by a transient or persistent decrease in the platelet (Plt) count. Herein, we report the first pediatric case of severe ITP that developed immediately after the diagnosis of coronavirus disease 2019 (COVID-19) in a school-aged girl. A previously healthy six-year-old girl was diagnosed with COVID-19 a day before experiencing a high fever, sore throat, and headache. She also presented with gingival hemorrhage, petechiae around both eyes and on the chest, and ecchymosis on her right leg. Based on the mucosal hemorrhage and a very low Plt count of 3 × 103/µl, we diagnosed her with severe ITP and urgently treated her with intravenous immunoglobulin (IVIG) to prevent life-threatening hemorrhage. The Plt count increased to 266 × 103/µl one week after treatment with IVIG. Given the possibility of severe ITP secondary to COVID-19, patients with COVID-19 should be carefully examined for the signs of ITP, such as mucosal hemorrhage. Their Plt counts should also be monitored.
1. Introduction
Immune thrombocytopenia (ITP), which is relatively well-described in children, is an autoimmune disease characterized by idiopathic thrombocytopenia (1). ITP sometimes develops a day to a few weeks after a preceding viral infection, and severe ITP increases the risk of life-threatening hemorrhage (2). The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is likely to be mild or asymptomatic in most children. Among symptomatic cases, fever and cough are the major manifestations (3). Herein, we describe the first case of severe pediatric ITP that developed immediately after a COVID-19 diagnosis.
2. Case presentation
A previously healthy six-year-old girl, with no family history of autoimmune or hematologic diseases, developed purpura associated with one day of gingival hemorrhage. She also presented with fever, sore throat, headache, mild cough, and rhinorrhea on the same day. Her father had tested positive for SARS-CoV-2, based on reverse transcriptase-polymerase chain reaction (RT-PCR) two days prior, and her test result was likewise positive the day before she visited our hospital. Her anti-SARS-CoV-2 antibodies were not measured, as her SARS-CoV-2 RT-PCR test was positive. She had no preceding infection or vaccination within the previous month and was not taking any medications. She had never been vaccinated against SARS-CoV-2.
The patient's vital signs were as follows: body temperature, 40.2°C; pulse, 137 beats/min; respiratory rate, 32 breaths/min; and blood pressure, 115/55 mmHg. On physical examination, the patient looked pale but lively, was able to walk without any help, and spoke clearly. Her palpebral conjunctivas were not pale and had no hemorrhagic spots. She had gingival hemorrhage and redness of the pharynx without a white coat. On auscultation, her respiratory sounds were clear and without any rales bilaterally. She further presented with petechiae around both eyes, on her chest, and at the base of her neck and ecchymoses on her right leg.
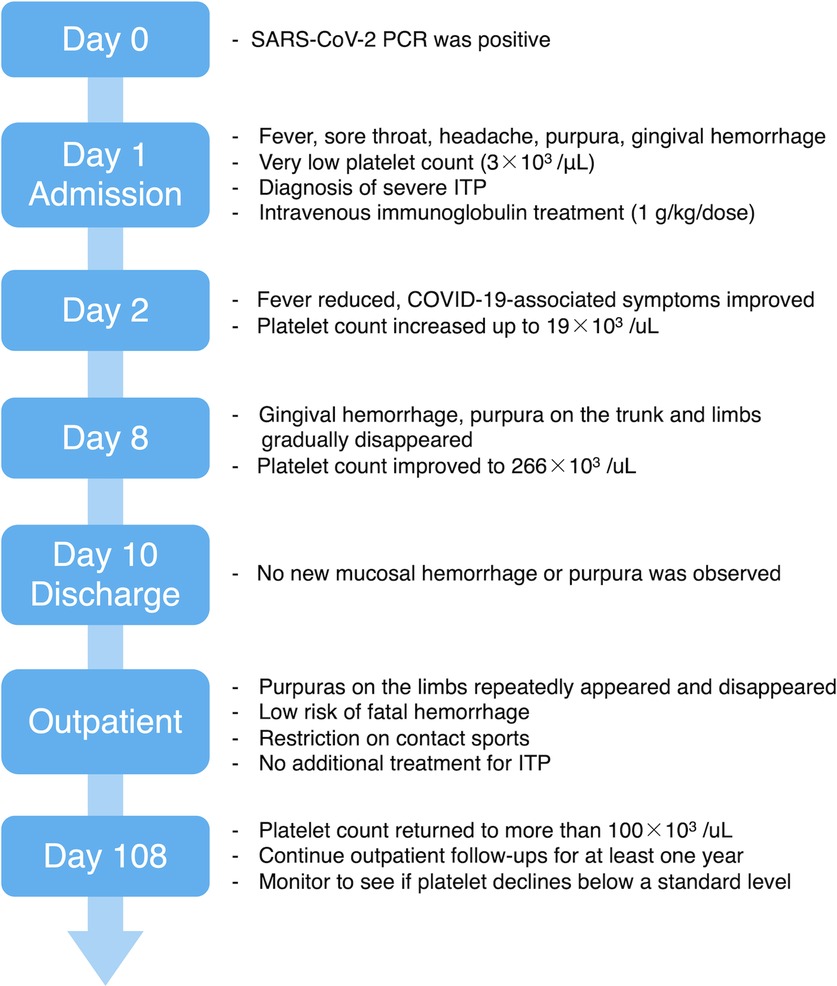
Given that mucosal hemorrhage is a sign of life-threatening hemorrhage, she was hospitalized (day 1). On admission, she had a very low platelet (Plt) count (3 × 103/µl), a normal white blood cell count [5.1 × 103/µl (segmented cells 79%, lymphocytes 16%, monocytes 3%, eosinophils 1%, stab cells 1%)], and a normal hemoglobin level (12.5 g/dl). No blasts and no coagulation abnormalities were observed in her blood examination. Thus, we diagnosed her with severe ITP and treated her with 17.5 g (1 g/kg/dose) of intravenous immunoglobulin (IVIG). Due to her age, the absence of pneumonia, and no risk factors for severe COVID-19, she was not eligible for antivirals or monoclonal antibodies in Japan (4, 5). Therefore, we chose to closely monitor her COVID-19 symptoms without administering any treatments. The day after admission (day 2), her body temperature resolved to 37.1°C, and her cough, headache, sore throat, and rhinorrhea improved. Afterward, her respiratory symptoms did not worsen, and she did not require any respiratory support or oxygen administration. No new hemorrhagic spots emerged, and the existing purpura gradually disappeared. Her Plt count improved slightly on day 2 (19 × 103/µl) and was within the normal range (266 × 103/µl) on day 8. Both a rapid antigen test for group A beta-hemolytic streptococci and a stool antigen EIA test for Helicobacter pylori were negative. An antinuclear antibody test was non-reactive, and her Plt-associated IgG was within the normal reference range (Plt count: 24.3 ng/107, day 17). No possible causes of ITP other than COVID-19 were identified. During hospitalization, the gingival hemorrhagic spots and purpura on the patient's trunk and limbs gradually disappeared. No new mucosal hemorrhage or purpura were observed, and the patient was discharged on day 10 (Figure 1).
During the outpatient follow-up, the patient's Plt count reduced to 25 × 103/µl on day 24 and remained between 15 × 103/µl and 32 × 103/µl for the next two months. Purpura on the patient's lower legs appeared repeatedly and disappeared; however, no mucosal hemorrhage was observed. Owing to the low risk of life-threatening hemorrhage, we restricted the patient from contact sports and kept observing her without any additional treatment. On day 108, her Plt count improved to more than 100 × 103/µl (121 × 103/µl), but we did not suspend the follow-up procedure and planned to continue outpatient follow-ups for at least one year. We intend to carefully monitor her Plt count to see if it drops below a standard level, or if it turns into chronic ITP (Figure 1).
3. Discussion
We describe the first pediatric case of ITP that developed almost simultaneously with the onset of COVID-19. Moreover, the ITP was severe and was associated with mucosal hemorrhage and very low Plt levels (3 × 103/µl).
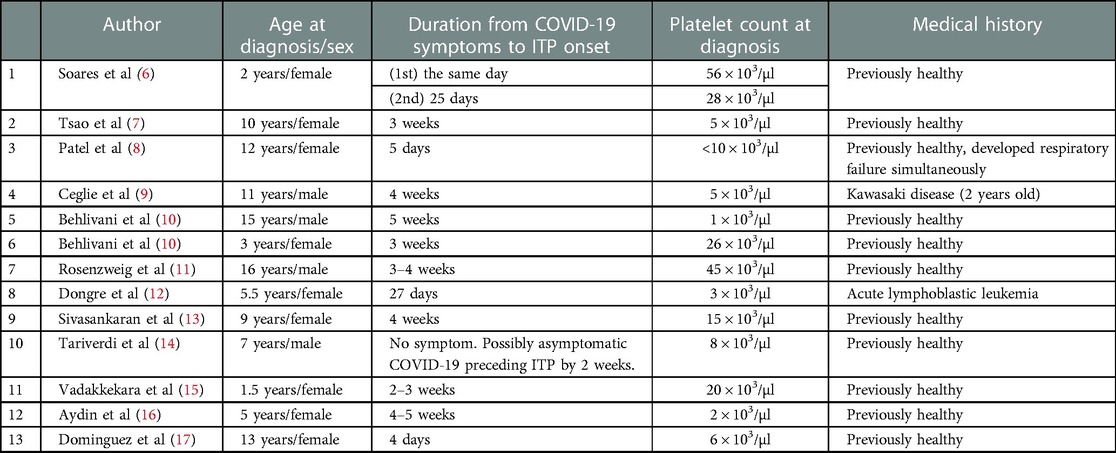
To date, 13 pediatric cases of ITP secondary to COVID-19 have been reported (Table 1) (6–17). Among them, only one patient had developed ITP at almost the same time as the onset of COVID-19 (Case 1 in Table 1) (6). Furthermore, the patient developed ITP associated with COVID-19 twice, once on the day of the onset of COVID-19 (day 1) and again on day 25. The patient's Plt count on day 1 was 56 × 103/µl, which improved without any treatment.
Severe ITP is defined as clinically relevant hemorrhage necessitating treatment or a Plt count of 20 × 103/µl or less (1, 18). The majority of COVID-19 patients with thrombocytopenia show a mild or moderate decrease in Plt count (>50 × 103/µl) without hemorrhagic symptoms (19–21). However, if the Plt count is below 20 × 103/µl or it reduces by >50% within 24–48 h, it is necessary to classify ITP as a differential diagnosis (21). In our case of the six-year-old girl, her Plt count was 3 × 103/µl, and oral mucosal hemorrhage was also observed. Therefore, she was diagnosed with severe ITP with a high risk of life-threatening hemorrhage. In the guidelines from an expert group for adult ITP with COVID-19, IVIG is recommended when a rapid increase in the number of Plts is required due to bleeding (21). We decided to treat her with IVIG, instead of corticosteroids, to restore her Plt count quickly, and she responded to IVIG well. As noted above, the only previously reported patient who developed ITP almost simultaneously with the onset of COVID-19 did not have severe ITP at that time (6). To the best of our knowledge, our patient is the first pediatric case of severe ITP developing immediately after a COVID-19 diagnosis.
Regarding ITP associated with COVID-19, numerous potential mechanisms of hematopoietic dysfunction resulting from SARS-CoV-2 infection have been postulated. These mechanisms include: (1) the production of antibodies to megakaryocytes, and the initiation of apoptosis of their precursors due to viral infection of megakaryocytes; (2) the inhibition of differentiation and maturation of megakaryocytes by cytokines such as interleukin-1β, tumor necrosis factor-α and -β, and interferon-α; (3) the reduction in thrombopoietin production due to viral infection of hepatocytes; and (4) the decreased fragmentation of megakaryocytes and Plt production, and the increased consumption of Plts in pulmonary vessels due to viral lung damage (21, 22). However, no published paper has elucidated the timing of these proposed mechanisms after the onset of COVID-19. A review of the etiology of virus-associated ITP, not limited to COVID-19, presented two patterns based on the onset of viral infection: (1) ITP occurring during the viremic phase of acute virus illness and (2) ITP developing days to weeks following viral infection (2). During the viremic phase of an acute viral infection, it is hypothesized that Plt clearance depends on a viral antigen in the blood circulation (2). In our case, thrombocytopenia occurred almost simultaneously with the onset of COVID-19 and persisted for approximately three months, suggesting that both patterns could be functional.
In conclusion, we reported a pediatric case of severe ITP that developed simultaneously with COVID-19. COVID-19 could cause severe ITP with life-threatening hemorrhage immediately after its onset. Routine physical examination for purpura and mucosal hemorrhage should be performed in patients with COVID-19 to detect severe ITP earlier.
Data availability statement
The original contributions presented in the study are included in the article further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the patient and her parents for the publication.
Author contributions
KS: conceptualized and designed the case report, supervised data collection, and drafted the initial manuscript. YB: mainly revised the manuscript. IK: critically reviewed, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Acknowledgments
The patient and her parents in this report kindly provided informed consent. Thank you to the staff of the Department of Pediatrics at the National Hospital Organization Saitama Hospital for their review of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. (2009) 113:2386–93. doi: 10.1182/blood-2008-07-162503
2. Rand ML, Wright JF. Virus-associated idiopathic thrombocytopenic purpura. Transfus Sci. (1998) 19:253–9. doi: 10.1016/s0955-3886(98)00039-3
3. Ma X, Liu S, Chen L, Zhuang L, Zhang J, Xin Y. The clinical characteristics of pediatric inpatients with SARS-CoV-2 infection: a meta-analysis and systematic review. J Med Virol. (2021) 93:234–40. doi: 10.1002/jmv.26208
4. Japan Pediatric Society. Views on COVID-19 therapeutics in children 1st edition. Remdesivir. In Japanese (2022). Available at: https://www.jpeds.or.jp/uploads/files/20220325_covid-19_1.pdf (Accessed January 16, 2023).
5. Ministry of Health, Labour, and Welfare. Distribution of neutralizing antibody drugs to medical institutions in COVID-19 infections. In Japanese (2022). Available at: https://www.mhlw.go.jp/content/000996090.pdf (Accessed January 16, 2023).
6. Soares ACCV, Loggetto SR, Manga FCM, Faustino LR, Braga JAP. Outcome of SARS-CoV-2 and immune thrombocytopenia in a pediatric patient. Hematol Transfus Cell Ther. (2021) 43:101–3. doi: 10.1016/j.htct.2020.09.145
7. Tsao HS, Chason HM, Fearon DM. Immune thrombocytopenia (ITP) in a pediatric patient positive for SARS-CoV-2. Pediatrics. (2020) 146:e20201419. doi: 10.1542/peds.2020-1419
8. Patel PA, Chandrakasan S, Mickells GE, Yildirim I, Kao CM, Bennett CM. Severe pediatric COVID-19 presenting with respiratory failure and severe thrombocytopenia. Pediatrics. (2020) 146:e20201437. doi: 10.1542/peds.2020-1437
9. Ceglie G, De Ioris MA, Mercadante S, Olivini N, Del Bufalo F, Marchesani S, et al. Immune thrombocytopenia in a child with COVID-19: is it the calm after the (cytokine) storm? Pediatr Blood Cancer. (2022) 69:e29326. doi: 10.1002/pbc.29326
10. Behlivani E, Tragiannidis A, Hatzipantelis E, Panagopoulou P. Immune thrombocytopenia secondary to COVID-19 infection: report of two cases. Pediatr Blood Cancer. (2021) 68:e29175. doi: 10.1002/pbc.29175
11. Rosenzweig JD, McThenia SS, Kaicker S. SARS-CoV-2 infection in two pediatric patients with immune cytopenias: a single institution experience during the pandemic. Pediatr Blood Cancer. (2020) 67:e28503. doi: 10.1002/pbc.28503
12. Dongre A, Jameel PZ, Deshmukh M, Bhandarkar S. Immune thrombocytopenic purpura secondary to SARS-CoV-2 infection in a child with acute lymphoblastic leukaemia: a case report and review of literature. BMJ Case Rep. (2021) 14:e245869. doi: 10.1136/bcr-2021-245869
13. Sivasankaran M, Siva Balan S, Munirathnam D. Immune thrombocytopenic purpura in a child following a SARS-CoV-2 infection. J Pediatr Hematol Oncol. (2021) 43:e1268–9. doi: 10.1097/MPH.0000000000002330
14. Tariverdi M, Mohammadzadeh Esini M, Pazarkar H, Naghmehsanj Z, Farahbakhsh N. Immune thrombocytopenic purpura in a child with COVID-19: a case report. Arch Pediatr Infect Dis. (2022) 10:e110428. doi: 10.5812/pedinfect.110428
15. Vadakkekara J, Mathew R, Khera S. COVID-19–associated immune. Thrombocytopenia in a toddler. Indian J Pediatr. (2022) 89:623. doi: 10.1007/s12098-022-04109-z
16. Aydin S, Altinkaynak GG, Kocabaş BA. A pediatric case report with immune thrombocytopenic purpura associated with COVID-19. Arch Pediatr. (2021) 6:196. doi: 10.29011/2575-825X.100096
17. Dominguez Rojas JA, Tello Pezo MV, Coronado Muñoz A, Alvarado G, Murillo KC. Severe immune thrombocytopenic purple in children critical of SARS-CoV-2: case report. Open J Pediatr Child Health. (2021) 6:1–4. doi: 10.17352/ojpch.000029
18. Buchanan GR, Adix L. Grading of hemorrhage in children with idiopathic thrombocytopenic purpura. J Pediatr. (2002) 141:683–8. doi: 10.1067/mpd.2002.128547
19. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
20. Lippi G, Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin Chem Lab Med. (2020) 58:1063–9. doi: 10.1515/cclm-2020-0240
21. González-López TJ, Bárez A, Bernardo-Gutiérrez A, Bernat S, Canaro-Hirnyk M, Entrena-Ureña L, et al. Recommendations on the management of patients with immune thrombocytopenia (ITP) in the context of SARS-CoV-2 infection and vaccination: consensus guidelines from a Spanish ITP expert group. Infect Dis Ther. (2023) 12:303–15. doi: 10.1007/s40121-022-00745-2
Keywords: immune thrombocytopenia, SARS-CoV-2, COVID-19, viremia, children
Citation: Shinno K, Banno Y and Kamimaki I (2023) Severe immune thrombocytopenia that developed immediately after COVID-19 in a school-aged patient: A case report. Front. Pediatr. 11:1120093. doi: 10.3389/fped.2023.1120093
Received: 9 December 2022; Accepted: 1 March 2023;
Published: 22 March 2023.
Edited by:
Giancarlo Castaman, University of Florence, ItalyReviewed by:
Chiara Minotti, University of Modena and Reggio Emilia, ItalyMarco Marietta, Ospedale Universitario di Modena, Italy
© 2023 Shinno, Banno and Kamimaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuma Shinno ay5zLngxOTk2MTEyMkBnbWFpbC5jb20=
†ORCID Kazuma Shinno orcid.org/0000-0003-3999-2687 Yoshinori Banno orcid.org/0000-0002-1526-870X
Specialty Section: This article was submitted to Pediatric Hematology and Hematological Malignancies, a section of the journal Frontiers in Pediatrics
 Kazuma Shinno
Kazuma Shinno Yoshinori Banno
Yoshinori Banno Isamu Kamimaki
Isamu Kamimaki