- 1Specialist Children’s Services, NHS Greater Glasgow and Clyde, Glasgow, United Kingdom
- 2Princess Royal Maternity, NHS Greater Glasgow and Clyde, Glasgow, United Kingdom
- 3College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, United Kingdom
- 4Department of Clinical Physics and Bioengineering, Royal Hospital for Children, NHS Greater Glasgow and Clyde, Glasgow, United Kingdom
Aim: The aim of this study was to examine executive function and emotional and behavioural difficulties of children aged between 8 and 10 years who had been prenatally exposed to methadone, compared to non-exposed peers.
Methods: Prospective study: third follow-up of an original cohort of 153 children born to methadone-maintained opioid-dependent mothers 2008–2010: previous investigations were at 1–3 days and at 6–7 months of age. Carers completed the Strength and Difficulties Questionnaire (SDQ) and the Behaviour Rating Inventory of Executive Function, Second Edition (BRIEF®2). Results were compared between exposed and non-exposed groups.
Results: Carers of 33 of 144 traceable children completed the measures. SDQ responses showed no group differences on subscales of emotional symptoms, conduct problems, or peer relationship problems. A marginally higher proportion of exposed children had a high or very high hyperactivity subscale score. Exposed children scored significantly higher on BRIEF®2 behavioural, emotional, and cognitive regulation indices, and on the global executive composite. After controlling for potentially confounding higher reported maternal tobacco use in the exposed group via regression modelling, the effect of methadone exposure reduced.
Interpretation: This study supports evidence that methadone exposure in utero is associated with adverse neurodevelopmental outcomes in childhood. Challenges in studying this population include difficulties with long-term follow-up and controlling for potentially confounding factors. Further investigation of the safety of methadone and other opioids in pregnancy must include consideration of maternal tobacco use.
1. Introduction
Opioid use in pregnancy has been widely reported to cause significant harm to children, evident both in the neonatal period and in later childhood (1, 2). In the neonatal period, children may suffer from neonatal abstinence syndrome/neonatal opioid withdrawal syndrome (NAS/NOWS) with prolonged hospital admission and/or maternal/infant separation and necessity for pharmaceutical treatment. The development of overt NAS/NOWS is not a prerequisite for adverse childhood outcome(s) (1), but the association of illicit opioid use with multiple obstetric complications may further impact longer-term outcomes (2, 3). Methadone is commonly used to manage opioid misuse in pregnancy with current guidelines stating that this practice is safe other than the risk of NAS/NOWS (4, 5). This advice does not concur with increasing evidence that prenatal opioid exposure is associated with increased risk of adverse neurodevelopmental outcomes, specifically impaired infant cognition and psychomotor performance, impaired early childhood internalising and externalising behaviour, and attention problems (6–9). Difficulties with executive functioning, vision (8), language, and regulation (9) are also reported.
Neurodevelopmental outcomes in later childhood and adolescence are less well understood although it would be predicted that lower cognitive performance in children aged over 2 years would carry a risk of longer-term difficulties (10). Indeed, in a longitudinal study of children prenatally exposed to opioids, group differences in cognition, attention, and behaviour had widened by 8 years of age (11, 12). Lower cognitive function compared to non-opioid-exposed controls has been described in 17- to 21-year-old youths although their performance was within normal limits (13). Unfortunately, studies in this field are limited methodologically because of the challenges of identifying polydrug and other licit [including tobacco (14) and alcohol] exposures, and the potentially confounding effects of these additional drug exposures as well as adverse pregnancy or neonatal illness, ill-health associated with poor socioeconomic status, and suboptimal childhood environment.
A prospective cohort study of infants born to methadone-maintained opioid-dependent (MMOD) mothers established polydrug exposures via both maternal and infant toxicology and recruited a comparison group matched for major confounding factors. The study was designed to investigate visual outcomes and found impaired neonatal visual evoked potentials (15) and significant visual problems at 6 months (16) and at 8–10 years. A subgroup of the cohort attended at 8–10 years for detailed visual investigation and both neurodevelopmental and behavioural enquiry. The aim of this study arm was to compare results of neurodevelopmental/behavioural carer-completed questionnaires at 8–10 years between exposed and comparison children.
2. Methods
2.1. Participants
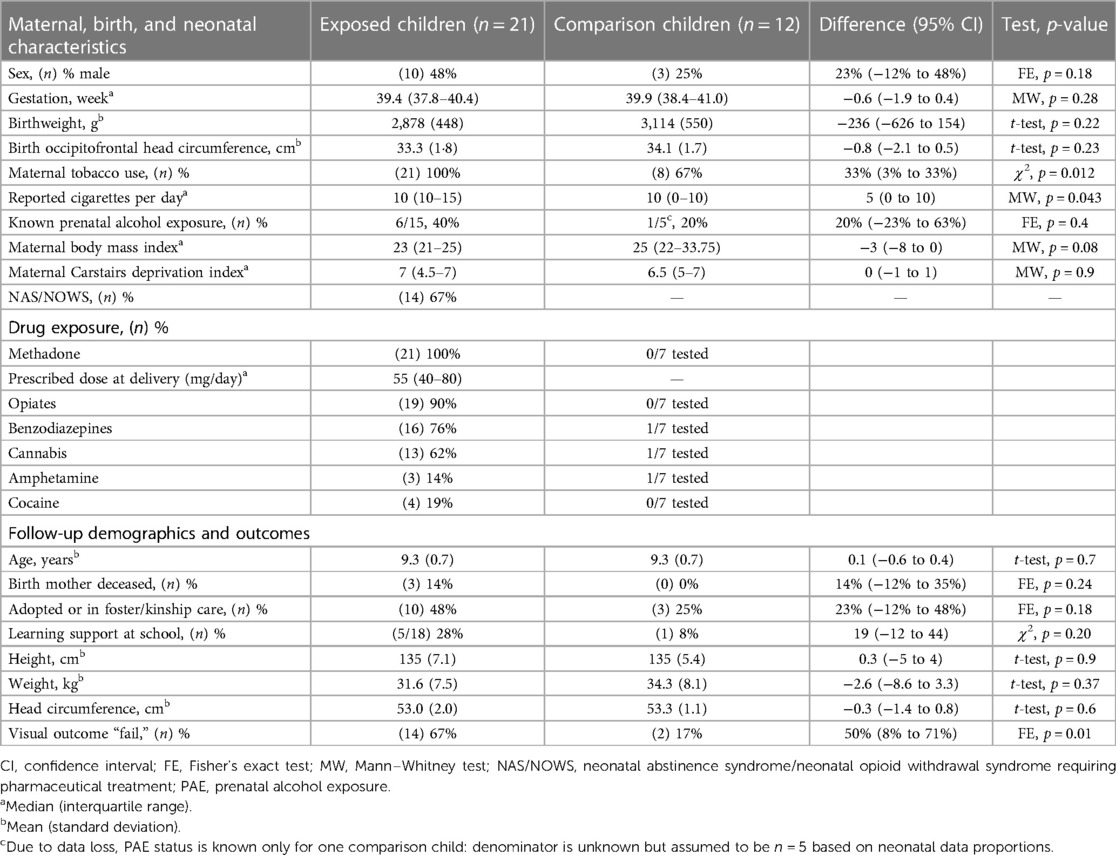
Participants comprised 33 of 144 (98 exposed, 46 comparison) traceable children followed up at ages of 8–10 years. Exposed children (n = 21) were born to MMOD mothers and comparison (non-exposed) children (n = 12) were born contemporaneously (2008–2010) at the same maternity hospital. All were born after 36 weeks’ gestation; none had congenital ocular abnormality or significant neonatal illness. Prenatal drug exposure of infants born to MMOD mothers was established via maternal urine, infant urine and meconium, maternal casenote review, and confidential interview (15). A subgroup of comparison infants had meconium drug analysis. For both exposed and non-exposed newborns, a subset of meconium samples was analysed for prenatal alcohol exposure (PAE), with a fatty acid ethyl ester (FAEEs) concentration ≥10,000 ng/g considered to represent significant PAE (17). Comparison infants were matched at recruitment for completed week of gestation, birthweight (±250 g) and socioeconomic status [Carstairs deprivation index using postcode of residence (±1)] (18) and partially matched for maternal tobacco use. Selection bias was likely to be low due to the high consent rate (98%) at recruitment (19). Characteristics of the 33 children are detailed in Table 1. The 33 attending children closely matched the non-attending traceable children (n = 111) for birth characteristics and drug exposure.
Exposed children were considered to have developed NAS/NOWS if they received pharmaceutical treatment according to the well-established hospital protocol. Oral morphine replacement was commenced (60 μg/kg × 6 per day) and weaned (usually by 10 μg/kg/day as symptoms diminished) when two consecutive 12-h scores >5 on a modified Lipsitz scale (20) were recorded in conjunction with poor feeding/weight gain. Second line phenobarbital was added when morphine treatment was unsuccessful (minority of babies). The median length of morphine treatment was 10 days; phenobarbital, if required, was generally weaned and discontinued by 6 weeks of age. All children had been prenatally exposed to methadone; most were exposed to additional drugs (Figure 1). Casenotes were reviewed for any attendance at hospital eye services, care arrangements (birth parent, adopted, or kinship or foster care), supported learning, or diagnosis of autistic spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), and/or foetal alcohol spectrum disorder (FASD). Casenote review was performed by researchers masked to exposure status with limited bias potential as data collected were previously documented, objective findings.
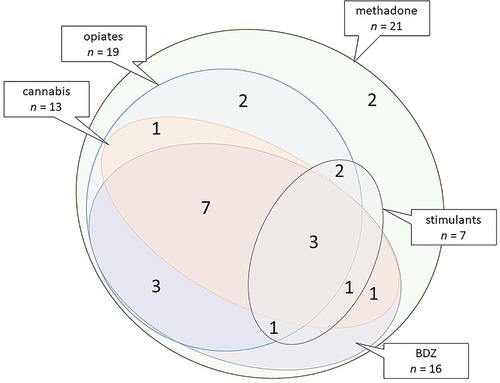
Figure 1. Euler diagram illustrating combinations of polydrug exposure based on combined exposure data for the 21 exposed children. Stimulants: cocaine and/or amphetamines. BDZ, benzodiazepines.
2.2. Assessments
A paediatric research nurse documented care and education status, height, weight, and occipitofrontal head circumference (OFC). Detailed visual assessments were undertaken with predetermined fail criteria (acuity poorer than 0.2 logMAR not attributable to refractive error; any manifest strabismus or any nystagmus; inability to overcome any base-out prisms; or a Frisby stereothreshold >110 arcsec). A researcher applied two child behaviour questionnaires to accompanying adults, assisting where necessary and encouraging completion of all questions.
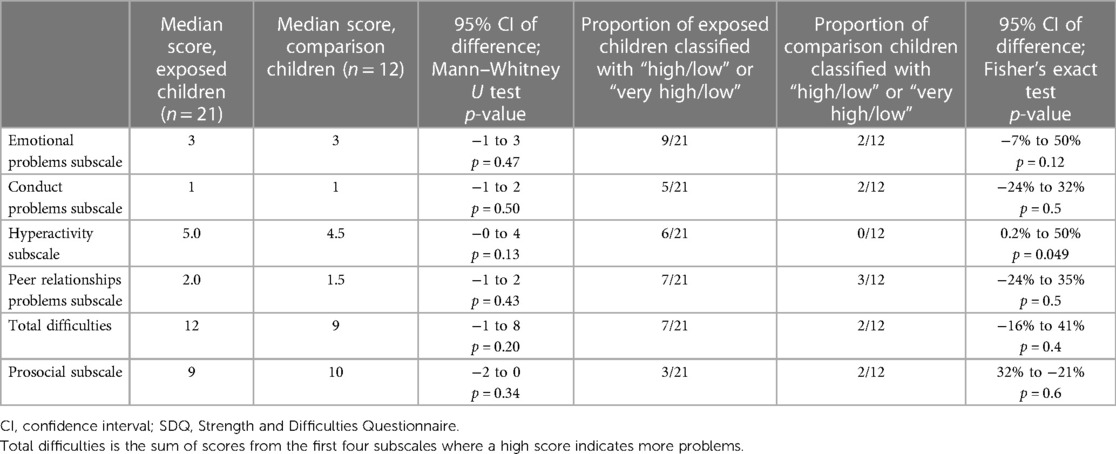
The Strengths and Difficulties Questionnaire (SDQ) (21) is a 25-item emotional and behavioural screening questionnaire with five subscales: emotional problems, conduct problems, hyperactivity and peer problems where high scores indicate more problems, and a prosocial subscale where high scores indicate fewer problems. Each item is scored on a Likert scale (not true = 0; somewhat true = 1, certainly true = 2) with possible subscales scores of 0–10. The total difficulties score is the sum of scores for the first four subscales (possible values 0–40). Scores are categorised as follows: close to average, slightly raised, high, or very high using “parent-completed” scores relative to a large UK reference population (22). The SDQ has high reliability, validity (23), and good concurrent validity (24, 25).
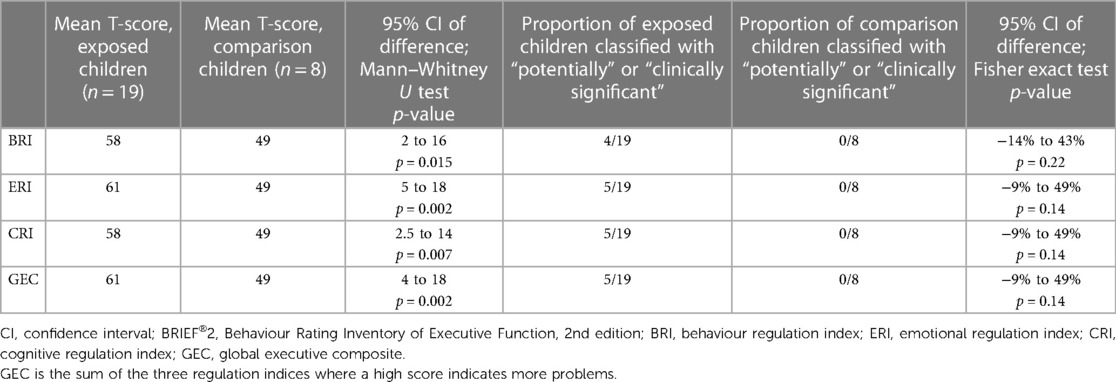
The Behaviour Rating Inventory of Executive Function, 2nd edition (BRIEF®2) (26) is a clinical rating scale of executive function comprising three regulation indices and a global executive composite (GEC). The behavioural regulation index (BRI) measures the child's ability to regulate and monitor their behaviour effectively and consists of “inhibit” and “self-monitor” scales. The emotion regulation index (ERI) measures the child's ability to regulate their emotional responses and to adjust to changes in environment, people, plans or demands, and consists of “shift” and “emotional” scales. The cognitive regulation index (CRI) measures the child's ability to control and manage cognitive processes and to problem solve, and consists of “initiation,” “working memory,” “planning,” “task-monitor,” and “organisation of materials” scales. The GEC is a summed score of all nine scales. The three indices and the GEC are expressed as T-scores. Scores are categorised as follows: mildly elevated (60–64 inclusive), potentially clinically elevated (65–69 inclusive), or clinically elevated (≥70). The test design incorporates checks for inconsistency (respondent answered similar items in an inconsistent manner), infrequency (respondent endorsed unlikely events), and negativity (respondent answered in an unusually negative manner) with criteria for exclusion.
Questionnaires were independently scored by two researchers (RH and KMS) and interpreted and analysed by researchers (KMS and KR) qualified to do so. Researchers were masked to exposure status to limit bias potential. Assessments took place at the paediatric Clinical Research Facility, Queen Elizabeth University Hospital campus, Glasgow, United Kingdom, between January 2018 and February 2020. Any child causing medical or social concern not already being addressed was notified to relevant services after discussion with their carer. Families were offered reimbursement of expenses and the child was given a £20 voucher. Written informed consent was given by the child's legal guardian; children gave written informed assent. The study was approved by West of Scotland Research Ethics Committee 3 (17/WS/0093).
2.3. Data analysis
SDQ scores were compared between exposed and comparison children using Mann–Whitney U tests; proportions of children with scores classified as “high/low” or “very high/low” were compared using Fisher’s exact test. BRIEF®2 scores were compared between exposed and comparison children using t-tests without the assumption of equal variance; proportions of children with scores classified as “potentially clinical elevated” or “clinically elevated” were compared using Fisher’s exact tests. To assess potential confounders, factors differing meaningfully between exposed and comparison children were treated as predictor variables in regression models of BRIEF®2 scores. SDQ total difficulties and BRIEF®2 GEC scores were compared using linear correlation to investigate whether an elevated score on one questionnaire was associated with an elevated score on the other. Findings were compared qualitatively with neurodevelopmental assessment undertaken at 6 months of age using the Griffiths Mental Development Scales (27). Findings for exposed children were compared for those who had and had not required treatment for NAS/NOWS (SDQ scores, Mann–Whitney U tests; BRIEF®2 scores, unpaired t-tests without assumption of equal variance). The relation between prescribed maternal methadone dose at delivery and questionnaire scores was investigated using scatter plots. Analyses were performed using SPSS® Statistics v24.0 (IBM Corp., Armonk, NY, United States), Minitab® v20.3 (Minitab LLC, PA, United States), and MedCalc® v20.014 (MedCalc Software Ltd, Ostend, Belgium).
3. Results
Exposed and comparison children did not differ in neonatal characteristics except for maternal tobacco use: all MMOD mothers but only two-thirds of comparison mothers smoked tobacco cigarettes (Table 1). Groups did not differ in childhood characteristics except for a greater fail rate on vision assessment for exposed children (Table 1). Individual child characteristics are shown in Table 2.
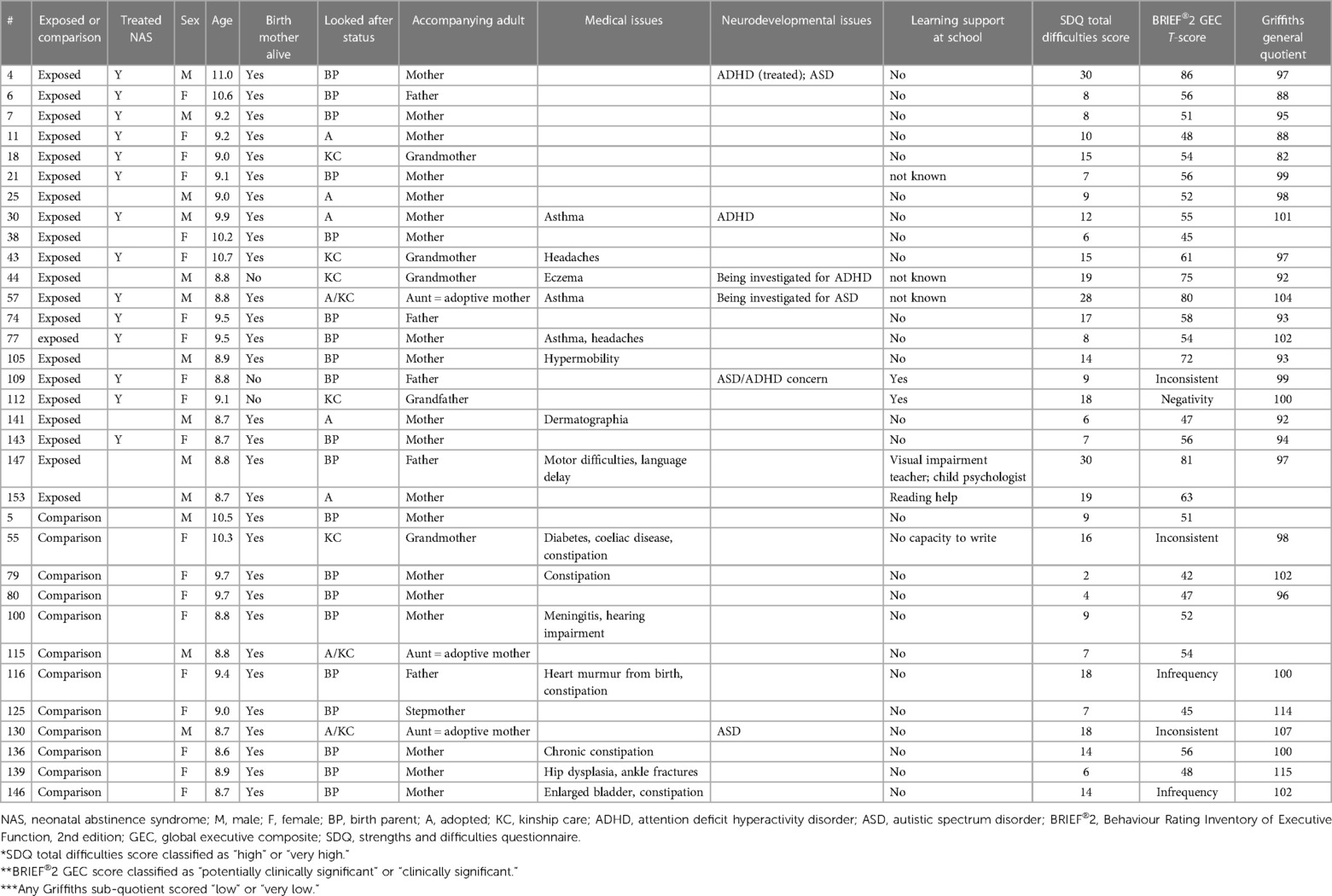
Table 2. Individual subject social, medical, and neurodevelopmental findings (case note review and history) and provision of learning support.
3.1. SDQ
All SDQ screening questionnaires (n = 33) were completed adequately. Details of accompanying adult who completed the SDQ (e.g., birth mother, grandparent, etc.) are given in Table 2. Exposed children and comparison children scored similarly on all subscales and total difficulties scores. Similar proportions of exposed and comparison children had “high” or “very high” scores in three subscales and in total difficulties. A marginally greater proportion of exposed children had “high” or “very high” scores on the hyperactivity subscale (more children with hyperactive behaviour, Table 3).
3.2. BRIEF®2
Six BRIEF®2 questionnaires were excluded from analysis (two exposed and four comparison children). Three were excluded for inconsistency (respondents: one birth father, one grandparent, and one adoptive mother), two for infrequency (respondents: one birth mother and one birth father). and one for negativity (respondent: grandparent). Data were, therefore, available for 19 exposed children and 8 comparison children. Exposed children scored significantly higher than comparison children on all three regulation indices (behavioural, emotional, and cognitive) and on their total score (GEC) (Table 4). Five of the 19 (26%) exposed children had a clinically elevated GEC. No comparison child had any clinically elevated or potentially clinically elevated index. Three exposed children (#004, #147, and #057) had all three indices clinically elevated, one child (#044) had clinically elevated BRI and CRI, and one child (#105) had clinically elevated ERI and potentially clinically elevated CRI (Tables 2, 4). Of these five children, one had a diagnosis of ADHD and ASD, one was being investigated for ADHD, one was being investigated for ASD, and one was known to have motor and speech difficulties. Statistically significant differences in the proportions of children with potentially clinically elevated and/or clinically elevated indices were not found: large confidence intervals (CIs) indicate a small sample size effect, with only eight comparison children contributing to BRIEF®2 data (Table 4). Regression modelling showed that the effect size (higher BRIEF®2 scores for exposed children) reduced for all indices and for GEC after controlling for maternal tobacco use: methadone exposure no longer predicted higher BRIEF®2 GEC scores after controlling for maternal tobacco use (Table 5). Controlling for maternal tobacco use changed BRI effect size most markedly and had a minimal effect on ERI (Table 5).
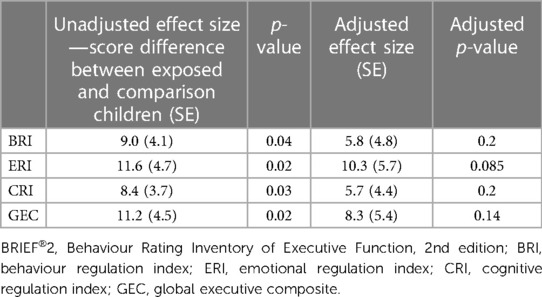
Table 5. Regression parameters: association of methadone exposure with BRIEF®2 scores before (unadjusted) and after (adjusted) controlling for maternal tobacco use.
3.3. SDQ and BRIEF®2 concordance
Considering the 27 children with both questionnaires completed satisfactorily (19 exposed and 7 comparison children), SDQ total difficulty scores and BRIEF®2 GEC scores were highly and positively correlated (r = 0.92, 95% CI 0.82–0.96, p < 0.0005, Figure 2). Treating classifications for each questionnaire as equivalent (SDQ high/very high ≡ BRIEF®2 potentially or clinically elevated; SDQ slightly elevated ≡ BRIEF®2 mildly elevated; SDQ close to average ≡ BRIEF®2 not elevated), 22/27 (81%) children had concordant classifications: 17 children were classified as normal on both, one child was mildly elevated/slightly raised on each, and four children were potentially or clinically elevated and high/very high on each (Figure 2). Of the five children with discordant classifications, one child had a BRIEF®2 score indicating more problems than their SDQ score, and four children had SDQ scores indicating more difficulties than their BRIEF®2 score.
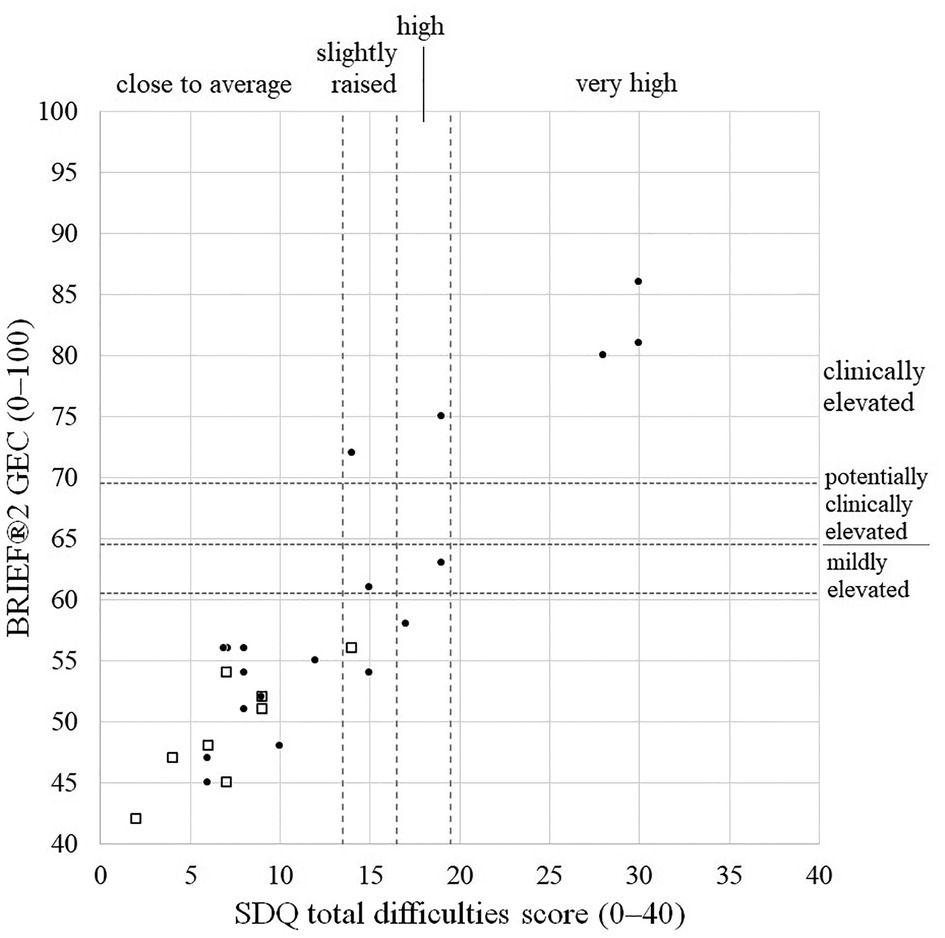
Figure 2. Scatterplot of BRIEF®2 GEC scores vs. SDQ total difficulties scores illustrating degree of concordance. Closed circles, exposed children; Open squares, comparison children. BRIEF®2, Behaviour Rating Inventory of Executive Function, 2nd edition; GEC, global executive composite; SDQ, Strength and Difficulties Questionnaire.
3.4. Comparison with infant Griffiths scores, NAS/NOWS, and maternal methadone dose
Twenty-eight of these 33 children had attended for investigation at age of 6 months and had completed the Griffiths MD neurodevelopmental assessment (Table 2). Only 2 of these 28 had problematic infant scores: child #011 had a low eye-hand sub-quotient in infancy, but normal results on SDQ and BRIEF®2 at 8–10 years; child #018 had a low performance and eye-hand sub-quotients in infancy and also had normal SDQ and BRIEF®2 scores. Conversely, nine children with either an abnormal SDQ total difficulties score or an abnormal BRIEF®2 GEC at age 8–10 years had normal Griffiths scores in infancy.
Considering only exposed children, neither SDQ scores (subscales, total difficulties score) nor BRIEF®2 scores (subscales, indices, GEC) differed between children who did (n = 14) or did not (n = 7) require treatment for NAS/NOWS. Scatter plots of questionnaire scores and maternal methadone dose at delivery were random by visual inspection, indicating no relation between these two factors.
4. Discussion
The main aim of this study was to expand current literature on the developmental impact of prenatal opioid exposure for older children. We found that, at mid-elementary school age, children prenatally exposed to methadone and/or other drugs were not significantly different from their non-exposed peers in terms of carer reports on the SDQ screening questionnaire. On the BRIEF®2 measure, methadone-exposed children scored significantly higher than their non-exposed peer group on behaviour, emotional, and cognitive regulation indices as well as GEC, indicating that methadone-exposed children were significantly less able to cognitively regulate, control, and manage cognitive processes and problem solve in various contexts. After controlling for maternal tobacco use, however, methadone exposure was no longer a predictor of higher BRIEF®2 scores, with the BRI and CRI indices showing the largest adjustments. This may represent a type II error with this relatively small study as a large study of 92 methadone-exposed 2-year-old children and 108 unexposed control children found problems with motor function, cognitive development, and emotional/behaviour dysregulation persisted after controlling for confounding licit (including tobacco) and illicit drug use in pregnancy (9).
Prenatal tobacco exposure is known to be detrimental to brain development and function (14) with a long-term follow-up study linking tobacco exposure to reduced cognition (28). In a large US study of teenagers using teacher-reported BRIEF questionnaires, tobacco exposure was found to predict impaired behavioural regulation but not meta-cognition after controlling for multiple confounders including cocaine, alcohol, and cannabis (but not opioid exposure) (29). Our data support this finding by suggesting that tobacco exposure particularly exacerbates problems with behaviour regulation. Maternal smoking is significantly associated with childhood ADHD after adjusting for parental psychiatric history and socioeconomic status, but other confounders—such as opioid exposure—could not be included in the meta-analysis (30). It remains uncertain, therefore, whether tobacco and opioids act independently, exacerbate the other's teratogenic effect, or act as a marker for more extensive use. Studies investigating the safety of opioids in pregnancy must therefore control for maternal tobacco use (31).
Griffiths scores at 6 months were poorly predictive of 8–10 year outcomes. NAS/NOWS requiring treatment was not related to the presence or extent of any difficulties, suggesting that any prenatal exposure to opioids is a better risk factor for surveillance than a history of treated NAS/NOWS (1). The SDQ and BRIEF®2 tests correlated well across a wide range of scores, suggesting that non-significant SDQ findings may relate to the lower sensitivity of non-parametric testing used to compare SDQ scores between groups. The preponderance of children with difficulties highlighted by the SDQ but not by BRIEF®2 (n = 4) rather than vice versa (n = 1) is in keeping with the SDQ's design as a screening questionnaire.
Strengths of this study include being the first prospective cohort-based study describing longer-term neurodevelopmental effects of prenatal methadone exposure, uniquely comprehensive information on maternal substance misuse in pregnancy and a comparison group matched for gestation, birthweight, and postcode at delivery as a proxy for socioeconomic status. Limitations include the small sample size which reflects the difficulty of long-term follow-up of families with challenging and/or chaotic lives. The greater imprecision associated with small sample sizes may have masked any methadone effect after controlling for maternal tobacco use. Children in the exposed group had birthweights 236 g lighter on average and had smaller birth OFC by an average of 0.8 cm, in line with expected, corrected differences seen in methadone-exposed children (32), but not reaching significance likely due to the small sample size. A greater proportion of exposed children had poor vision: since vision tests were selected to be easily performed by younger children, visual findings are unlikely to be affected by behaviour or executive difficulties. It is possible that reported behaviour and/or executive difficulties were at least partly due to the presence of visual problems but because prenatal opioid exposure is associated with impaired vision (10), childhood vision outcome was not treated as a confounder. The comparison group had a higher proportion of females than the exposed group (6/8, 75%, vs. 9/19, 47%, with adequately completed BRIEF®2), which may have exaggerated positive findings in the exposed group as ADHD is more prevalent in males. Long-term outcomes for all children are confounded by multiple factors including impaired foetal growth, socioeconomic deprivation, and challenged parenting skills, each of which is more likely to affect those exposed prenatally to opioids, thereby limiting the strength of any association. However, multiple systematic analyses now point to an independent effect of prenatal opioid exposure on developmental outcomes (1, 6, 7, 10).
Opioid exposure in utero, specifically methadone, may at least partly explain adverse neurodevelopmental outcomes at mid-elementary school age, which are currently misunderstood or misdiagnosed, potentially as ASD or ADHD, by professionals. Unfortunately, potentially confounding effects of other illicit and licit drug exposures, challenged parenting and/or multiple placements (33), and socioeconomic deprivation are extremely difficult to control. Given national recommendations (4, 5) and the widespread use of methadone in the treatment of opioid use disorder in pregnancy, establishing whether this practice may contribute to long-term harm for children's developmental outcomes is essential.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by West of Scotland Research Ethics Committee 3 (17/WS/0093). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
RH and HM conceived the study. KMS, RH, and HM contributed significantly to the study design and all authors contributed significantly to data collection or analysis. KR wrote the first version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by a joint grant from Action Medical Research for Children, Horsham, UK, and the Chief Scientist Office, Edinburgh, UK (Ref GN2493) and supported by The RS Macdonald Charitable Trust.
Acknowledgments
We thank the children and their families; the research nursing and administrative team, Helen Bannister, Emily Blyth, Karen Duffy, Annmarie Jordan, Barry Milligan, Leeanne Milne, Ashleigh Neil, Elizabeth Waxman; and Janice Waterson-Wilson, research orthoptists, for help with clinical data collection and SDQ scoring.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hall ES, McAllister JM, Wexelblatt SL. Developmental disorders and medical complications among infants with subclinical intrauterine opioid exposures. Popul Health Manag. (2019) 22:19–24. doi: 10.1089/pop.2018.0016
2. Minozzi S, Amato L, Jahanfar S, Bellisario C, Ferri M, Davoli M. Maintenance agonist treatments for opiate-dependent pregnant women. Cochrane Database Syst Rev. (2020) 2020. doi: 10.1002/14651858.CD006318.pub4
3. Patrick SW, Barfield WD, Poindexter BB, COMMITTEE ON FETUS AND NEWBORN, COMMITTEE ON SUBSTANCE USE AND PREVENTION; James Cummings. Neonatal opioid withdrawal syndrome. Pediatrics. (2020) 146:e2020029074. doi: 10.1542/peds.2020-029074
4. ASAM. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. (2020) 14:1–91. doi: 10.1097/ADM.0000000000000633
5. Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group. Drug misuse and dependence: UK guidelines on clinical management (2017). Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/673978/clinical_guidelines_2017.pdf (Accessed June 10, 2021).
6. Baldacchino A, Arbuckle K, Petrie DJ, McCowan C. Erratum: neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: a systematic review and meta-analysis. BMC Psychiatry. (2015) 15:134. doi: 10.1186/s12888-015-0438-5
7. Lee SJ, Bora S, Austin NC, Westerman A, Henderson JMT. Neurodevelopmental outcomes of children born to opioid-dependent mothers: a systematic review and meta-analysis. Acad Pediatr. (2020) 20:308–18. doi: 10.1016/j.acap.2019.11.005
8. Andersen JM, Høiseth G, Nygaard E. Prenatal exposure to methadone or buprenorphine and long-term outcomes: a meta-analysis. Early Hum Dev. (2020) 143:104997. doi: 10.1016/j.earlhumdev.2020.104997
9. Levine TA, Davie-Gray A, Kim HM, Lee SJ, Woodward LJ. Prenatal methadone exposure and child developmental outcomes in 2-year-old children. Dev Med Child Neurol. (2021) 63:1114–22. doi: 10.1111/dmcn.14808
10. Monnelly VJ, Hamilton R, Chappell FM, Mactier H, Boardman JP. Childhood neurodevelopment after prescription of maintenance methadone for opioid dependency in pregnancy: a systematic review and meta-analysis. Dev Med Child Neurol. (2019) 61:750–60. doi: 10.1111/dmcn.14117
11. Nygaard E, Moe V, Slinning K, Walhovd KB. Longitudinal cognitive development of children born to mothers with opioid and polysubstance use. Pediatr Res. (2015) 78:330–5. doi: 10.1038/pr.2015.95
12. Nygaard E, Slinning K, Moe V, Walhovd KB. Behavior and attention problems in eight-year-old children with prenatal opiate and poly-substance exposure: a longitudinal study. PLoS One. (2016) 11:e0158054. doi: 10.1371/journal.pone.0158054
13. Nygaard E, Slinning K, Moe V, Walhovd KB. Cognitive function of youths born to mothers with opioid and poly-substance abuse problems during pregnancy. Child Neuropsychol. (2017) 23:159–87. doi: 10.1080/09297049.2015.1092509
14. Hofhuis W. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch Dis Child. (2003) 88:1086–90. doi: 10.1136/adc.88.12.1086
15. McGlone L, Mactier H, Hamilton R, Bradnam MS, Boulton R, Borland W. Visual evoked potentials in infants exposed to methadone in utero. Arch Dis Child. (2008) 93:784–6. doi: 10.1136/adc.2007.132985
16. McGlone L, Hamilton R, McCulloch DL, MacKinnon JR, Bradnam M, Mactier H. Visual outcome in infants born to drug-misusing mothers prescribed methadone in pregnancy. Br J Ophthalmol. (2014) 98:238–45. doi: 10.1136/bjophthalmol-2013-303967
17. Gareri J, Lynn H, Handley M, Rao C, Koren G. Prevalence of fetal ethanol exposure in a regional population-based sample by meconium analysis of fatty acid, ethyl esters. Ther Drug Monit. (2008) 30:239–45. doi: 10.1097/FTD.0b013e318167cfe5
18. McLoone P. Carstairs scores for Scottish postcode sectors from the 2001 census (2004). Available at: https://www.gla.ac.uk/researchinstitutes/healthwellbeing/research/mrccsosocialandpublichealthsciencesunit/programmes/inequalities/healthinequalities/determinantsofhealthandhealthinequalitiesinscotland/carstairsscores/ (Accessed October 25, 2021).
19. Mactier H, McGlone L. Comment on: infants of opioid-dependent mothers: neurodevelopment at six months reply. Early Hum Dev. (2015) 91:245–245. doi: 10.1016/j.earlhumdev.2015.01.006
20. Lipsitz P. Proposed narcotic withdrawal score for use with newborn-infants—pragmatic evaluation of its efficacy. Clin Pediatr. (1975) 14:592–4. doi: 10.1177/000992287501400613
21. Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. (1997) 38:581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x
22. Goodman A, Goodman R. Strengths and difficulties questionnaire as a dimensional measure of child mental health. J Am Acad Child Adolesc Psychiatry. (2009) 48:400–3. doi: 10.1097/CHI.0b013e3181985068
23. Yao S, Zhang C, Zhu X, Jing X, McWhinnie CM, Abela JRZ. Measuring adolescent psychopathology: psychometric properties of the self-report strengths and difficulties questionnaire in a sample of Chinese adolescents. J Adolesc Health. (2009) 45:55–62. doi: 10.1016/j.jadohealth.2008.11.006
24. Muris P, Meesters C, van den Berg F. The strengths and difficulties questionnaire (SDQ). Eur Child Adolesc Psychiatry. (2003) 12:1–8. doi: 10.1007/s00787-003-0298-2
25. Goodman R, Ford T, Simmons H, Gatward R, Meltzer H. Using the strengths and difficulties questionnaire (SDQ) to screen for child psychiatric disorders in a community sample. Br J Psychiatry. (2000) 177:534–9. doi: 10.1192/bjp.177.6.534
26. Gioia GA, Isquith PK, Guy SC, Kenworthy L. TEST REVIEW behavior rating inventory of executive function. Child Neuropsychol. (2000) 6:235–8. doi: 10.1076/chin.6.3.235.3152
27. McGlone L, Mactier H. Infants of opioid-dependent mothers: neurodevelopment at six months. Early Hum Dev. (2015) 91:19–21. doi: 10.1016/j.earlhumdev.2014.10.006
28. Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. (2003) 25:427–36. doi: 10.1016/S0892-0362(03)00029-1
29. Rose-Jacobs R, Richardson MA, Buchanan-Howland K, Chen CA, Cabral H, Heeren TC. Intrauterine exposure to tobacco and executive functioning in high school. Drug Alcohol Depend. (2017) 176:169–75. doi: 10.1016/j.drugalcdep.2017.02.022
30. Dong T, Hu W, Zhou X, Lin H, Lan L, Hang B, Lv W, Geng Q, Xia Y. Prenatal exposure to maternal smoking during pregnancy and attention-deficit/hyperactivity disorder in offspring: a meta-analysis. Reprod Toxicol. (2018) 76:63–70. doi: 10.1016/j.reprotox.2017.12.010
31. Bann CM, Newman JE, Poindexter B, Okoniewski K, DeMauro S, Lorch SA. Outcomes of babies with opioid exposure (OBOE): protocol of a prospective longitudinal cohort study. Pediatr Res. (2022):1–8. doi: 10.1038/s41390-022-02279-2
32. Mactier H, Shipton D, Dryden C, Tappin DM. Reduced fetal growth in methadone-maintained pregnancies is not fully explained by smoking or socio-economic deprivation: fetal growth in methadone pregnancies. Addiction. (2014) 109:482–8. doi: 10.1111/add.12400
Keywords: prenatal methadone exposure, cognition, behaviour, long-term outcomes, prenatal tobacco exposure
Citation: Spowart KM, Reilly K, Mactier H and Hamilton R (2023) Executive functioning, behavioural, emotional, and cognitive difficulties in school-aged children prenatally exposed to methadone. Front. Pediatr. 11:1118634. doi: 10.3389/fped.2023.1118634
Received: 7 December 2022; Accepted: 22 March 2023;
Published: 18 April 2023.
Edited by:
Eugene Dempsey, University College Cork, IrelandReviewed by:
Balaji Govindaswami, Valley Medical Center Foundation, United StatesPavla Pokorna, Charles University, Czechia
© 2023 Spowart, Reilly, Mactier and Hamilton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruth Hamilton cnV0aC5oYW1pbHRvbkBnbGFzZ293LmFjLnVr
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Katherine M. Spowart1
Katherine M. Spowart1 Kasey Reilly
Kasey Reilly Ruth Hamilton
Ruth Hamilton