
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 02 March 2023
Sec. Pediatric Critical Care
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1116166
Background: Stevens-Johnson syndrome/toxic epidermal necrolysis has a severe impact on patients' eyes, genital mucosa, and many other organs. Bronchiolitis obliterans is a rare complication of Stevens-Johnson syndrome/toxic epidermal necrolysis.
Data sources: We report a case of bronchiolitis obliterans associated with toxic epidermal necrolysis in our department. Furthermore, we examined the patients with bronchiolitis obliterans induced by Stevens-Johnson syndrome/toxic epidermal necrolysis and summarized the clinical characteristics, treatment, and prognosis. Databases available online in English including PubMed, Medline, and Web of Science were consulted.
Results: We report one case and review 23 published case reports. Of the 24 patients, 13 were female, the oldest patient was 59 years old and the youngest was 5 years old. The time of bronchiolitis obliterans onset after Stevens-Johnson syndrome/toxic epidermal necrolysis varied from 5 days to 5 months. Bronchoscopy examination showed ulceration, exudative lesions, occlusion, and inflammation. The CT of lung manifestation included mosaic perfusion, bronchiectasis, consolidation, air trapping, pneumatocele, pleural thickening, lung collapse, larger central airway dilatation, lung overinflation, oligemia, and pneumomediastinum. Most cases indicated pulmonary function tests with obstructive ventilation dysfunction. The prognosis was poor; six of the patients died.
Conclusions: Patients with Stevens-Johnson syndrome/toxic epidermal necrolysis may develop bronchitis obliterans at different stages, so all patients with Stevens-Johnson syndrome/toxic epidermal necrolysis should be followed up for possible respiratory complications.
Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) are life-threatening dermatologic diseases characterized by the eruption of mucocutaneous blistering and epithelial sloughing (1). SJS and TEN are rare but are associated with many potential multisystem complications (2). Bronchitis obliterans(BO) causes obstruction and/or obliteration of the small airways, which is a chronic and irreversible obstructive lung disease (3). BO induced by severe lower respiratory tract infection is the most common form of BO in children (3). However, clinical reports about SJS/TEN complicated with BO are rare.
Herein, we report a case of BO associated with TEN in our department. Furthermore, we examined the patients with BO induced by SJS/TEN and summarized the clinical characteristics, treatment, and prognosis. This case report was approved by the Ethics Committee of First Hospital of Jilin University, China (2019-314). Informed consent was obtained from the parents of the patient. We reviewed relevant English literature from the online available databases, including PubMed, Medline, and Web of Science using the keywords “Stevens-Johnson syndrome”, “toxic epidermal necrolysis”, and “bronchiolitis obliterans”. The clinical characteristics, treatment, and prognosis of the participants in each study were summarized.
A 6-year-old previously healthy boy presented to the emergency department of our hospital due to fever for three days, rash for two days, and lethargy for one day. The patient took oral antipathetic before admission and developed a rash before taking antipathetic. He was transferred to the pediatric intensive care unit for further treatment. Upon admission, vital signs revealed temperature at 39°C, pulse rate at 182 beats/min, respiratory rate at 40 breaths/min, and blood pressure at 93/53 mm Hg. He also had a diffuse dark red rash and vesiculobullous lesions involving his face, ear, trunk, and extremities (>30% of the body surface area) (Figure 1). Parts of the rash and blister were broken with serous exudates. The boy could not open his eyes, which were covered with many yellow secretions. The conjunctiva of both lower eyelid, lip, tongue, penile mucosa, and oral mucosa were broken. Auscultation of the two lungs showed some rales. Laboratory tests reflected normal leukocyte count, elevated C reactive protein (112.7 mg/l), and procalcitonin (89.97 ng/ml). Serum cytokine concentration showed that the serum IL-6 was 1,552.24 pg/ml and the serum IL-10 was 135.69 pg/ml. Other laboratory findings were as follows: Mycoplasma pneumoniae (MP) IgM 1.55 COI, MP IgG 237.00 AU/ml, creatine kinase 1,470 U/l, creatine kinase isoenzyme 161.1 U/l, lactate dehydrogenase 778 U/l, aspartate aminotransferase 108 U/l, alanine aminotransferase 35.9 U/l, urinary protein 2+, urinary RBC count 32.0/µl, IgE 764.00 IU/ml, serum ferritin 474.6 ug/l, D-dimer 2.17 ug/ml. The lung CT showed scattered consolidation two days after admission (Figure 2). Upon admission, treatment with invasive mechanical ventilation, systemic steroid therapy, IVIG, azithromycin, vasopressor, and topical medications of eye, and skin dressing were initiated immediately to rescue the patient. Three days after admission, laboratory investigations worsened progressively as follows: creatine kinase 21,098 U/l, creatine kinase isoenzyme 434 U/l, lactate dehydrogenase 1,622 U/l, aspartate aminotransferase 823.5 U/l, alanine aminotransferase 196.2 U/l, amylase 2,069 U/l, C reactive protein (225 mg/l). Due to severe inflammatory reactions and multisystem complications, continuous blood purification was started. Seven days after admission, vital signs and laboratory investigations improved progressively, so continuous blood purification and invasive mechanical ventilation were all removed. The respiratory status was normal without cough, dyspnea, and wheezing, and auscultation of the two lungs showed no rales. The patient's skin and mucosa lesions also improved gradually. Methylprednisolone was gradually decreased from 2 mg/kg/day to 0.5 mg/kg/day and finally stopped. Methylprednisolone was used for 10 days in all. Methylprednisolone was used for 10 days in all. In spite of the improvement of the skin and mucosa lesions, the patient began to suffer from cough and slight tachypnea 26 days after admission. The lung CT showed thickening of the airway wall of two lungs without atelectasis or pneumonic consolidation 29 days after admission (Figure 3). The patient was treated with budesonide, bronchodilators atomization inhalation, and antibiotics. Pulmonary function tests on day 33 revealed extremely severe obstructive dysfunction with forced vital capacity (FVC) of 0.48 L (28.2% predicted), forced expiratory volume in 1 s (FEV1) of 0.28 L (19.4% predicted), FEV1/FVC ratio of 67.8% predicted. Bronchodilation test was negative. The thorax CT showed a widespread mosaic pattern 43 days after admission (Figure 4). The patient developed obvious wheezing and progressive dyspnea with poor response to bronchodilators, so nasal oxygen inhalation and oral prednisone were started. He was discharged home without tachypnea at rest 56 days after admission. After discharge, the patient took prednisone for 2 months and inhaled budesonide for more than 2 years. Four months after discharge, pulmonary function tests revealed severe obstructive dysfunction with FVC of 0.59 L (36.2% predicted), FEV1 of 0.35 L (25.8% predicted), FEV1/FVC ratio of 70.2% predicted. The patient had no wheezing when in a quiet state or undertaking slight activity but could not tolerate intense activities.
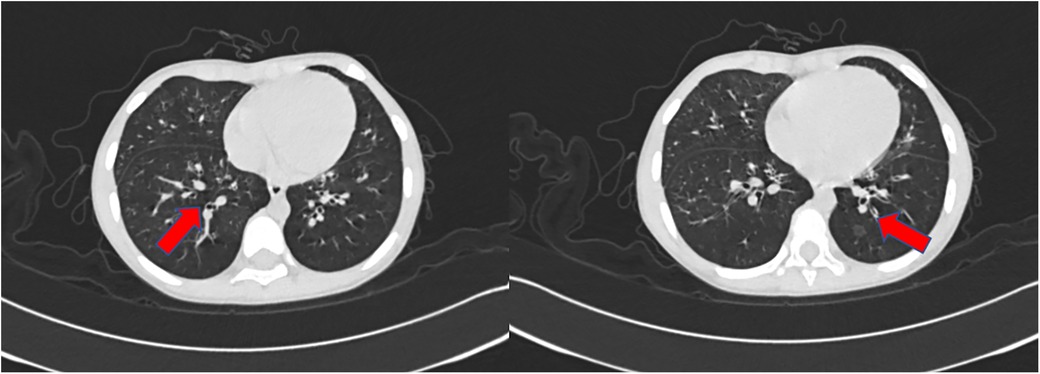
Figure 3. The CT scan of the chest performed 29 days after admission showed a thickening of airway walls.
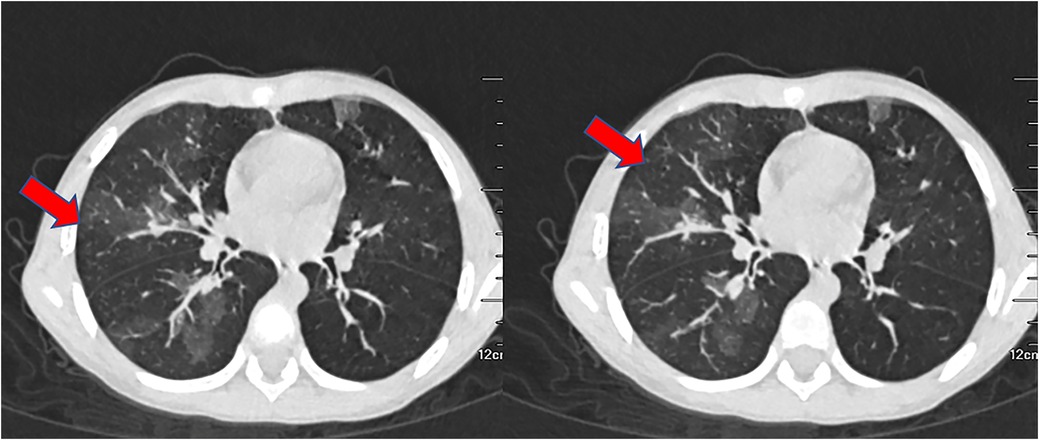
Figure 4. The CT scan of the chest performed 43 days after admission displayed widespread mosaic pattern.
SJS, SJS/TEN overlap and TEN are different due to the degree of skin detachment: the skin detachment area of SIS < 10%, the skin detachment area of TEN > 30%, the skin detachment area of SJS/TEN overlapping is 10%–30% (4). The causes of SJS/TEN include infection, drugs, and immunity (4). MP infection is associated with SJS/TEN (5). An investigation into outbreaks of MP-associated SJS revealed 3-X-6-2 MP strain is more common in SJS patients than patients with pneumonia only (6). Clinical manifestations of MP-associated SJS include increased erythrocyte sedimentation rate, respiratory infection, and less extensive skin lesions (7, 8). Our patient had a rash before taking medicine, the laboratory data related to infection was significantly increased above normal and the IgM of MP was positive. In this case, we reported that the infection might be the offending agent of the patient, according to medical history and auxiliary examination.
The interventions in the British Association of dermatologists' guidelines for the management of SJS/TEN include corticosteroids, IVIG, ciclosporin, low molecular weight heparin, biological therapy, granulocyte-colony stimulating factor, calcineurin inhibitors, and antibiotics (9). The symptoms of mucocutaneous blistering and epithelial sloughing gradually improved after the patient was treated with methylprednisolone and IVIG.
SJS/TEN has a severe impact on the eyes, kidney, genital mucosa, and other organs (4). Our patient developed renal injury, myocardium injury, liver injury, pancreatic injury, and ophthalmic complications. In the acute phase, nearly 40% of patients with SJS/TEN developed respiratory complications (1, 10). The respiratory involvements include the exfoliation of bronchial epithelium, pulmonary edema, atelectasis, and infectious pneumonia. The late sequelae of SJS/TEN survivors revealed interstitial lung disease, airway obstruction, bronchiectasis, bronchitis, and BO (1). BO is an uncommon complication of SJS/TEN (11). On reviewing the literature, we discovered 23 cases regarding BO associated with SJS/TEN in children and adults (Table 1). In the 23 published cases, 13 patients were female, the oldest aged 59 years and the youngest aged 5 years. Physical examination of BO shows tachypnoea, crackles, and persisting hypoxemia (3).
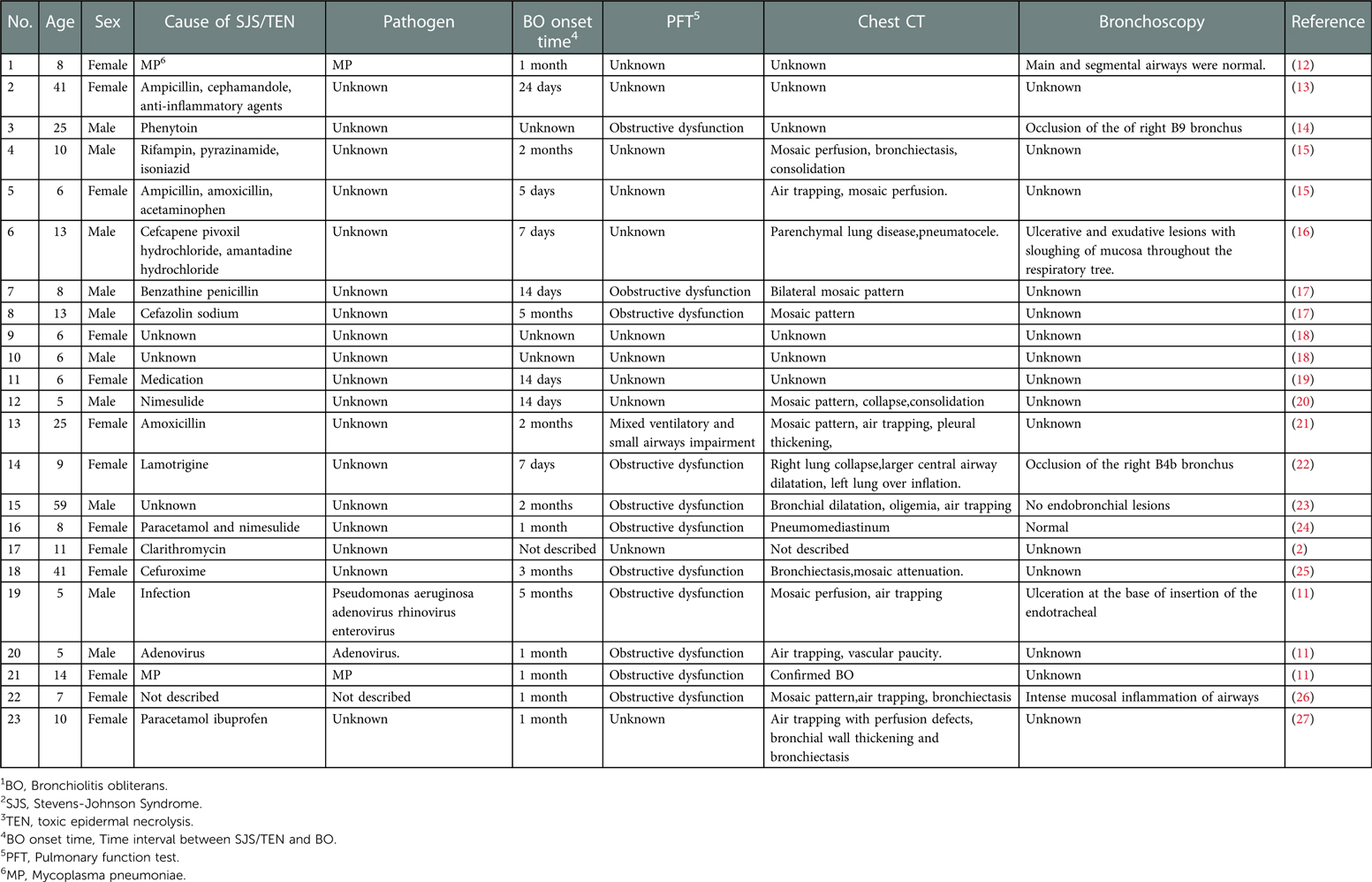
Table 1. Summary of clinical manifestation in BO1 associated with SJS2/TEN3 from the published cases.
The mechanism of BO secondary to SJS/TEN remains unclear; it may be that the immune complex deposition results in the damage of bronchial epithelial cells and mucosa (21). The combination of abnormal immune response and respiratory infection may play an important role in the occurrence of BO in SJS/TEN patients (21). Table 1 shows four patients infected. Our patient's MP IgM was positive. MP infection has a higher risk of development for post-infectious BO (28). BO may occur following acute MP bronchiolitis due to airway epithelial injury and sloughing (29). Hypoxemia and high level of lactate dehydrogenase are the risk factors for BO in children with MP Bronchiolitis (30, 31). Our patient had hypoxemia and a high level of lactate dehydrogenase. MP infection may be one of the reasons for the development of BO in our patient. Evidence of infection was also identified in patients with BO associated with SJS/TEN (11, 12). It is unclear whether MP infection is a cofactor for BO secondary to SJS/TEN or just an etiological factor in patients with SJS/TEN (1).
Autopsy of BO associated with SJS/TEN showed diffuse epithelial shedding and partial regeneration of the tongue, pharynx, and trachea (13). Eight of the published cases of BO-associated SJS/TEN provided bronchoscopy results. At the early stage of SJS/TEN, bronchoscopy examination showed ulceration and exudative lesions with mucosal detachment in the whole respiratory tract (16). Bronchoscopy of other published cases showed occlusion of the bronchus, no endobronchial lesions, normal main and segmental airways, ulceration at the base of insertion of the endotracheal, and intense mucosal inflammation of airways (11, 12, 14, 22–24, 26). Our patient did not complete a bronchoscopy examination, this is a limitation of this case.
Lung biopsy is regarded as the gold standard for the diagnosis of BO. Due to the patchy distribution of BO, it is difficult to obtain tissue with characteristic pathological changes (3). The clinical diagnosis of BO was made on the basis of clinical characteristics, pulmonary function examination results, and the typical HRCT manifestations (29). According to the persistent respiratory manifestation, pulmonary CT scan, and pulmonary function test of our patient, BO was diagnosed. The CT of lung manifestation included mosaic perfusion, bronchiectasis, consolidation, air trapping, pneumatocele, pleural thickening, lung collapse, larger central airway dilatation, and lung overinflation. oligemia, pneumomediastinum as shown in Table 1. Most of the published cases indicated pulmonary function tests with obstructive ventilation dysfunction. We also summarized the time of BO onset after SJS/TEN in Table 1. Some patients developed a productive cough and dyspnea 5 days after the appearance of SJS (15). So far the longest time between the onset of respiratory symptoms and initial presentation with SJS is 5 months (17). Even if there are no respiratory symptoms in the early stage, we should closely monitor the development of BO for a long time.
The modalities that have been used in the treatment of BO include azithromycin (32), steroids (33), extracorporeal photopheresis (34), rituximab (35), lung transplantation (36), and so on. Our patient showed improvement in respiratory symptoms and daily activities, after using systemic steroids and azithromycin. We summarized the treatment and outcome of the previous cases as shown in Table 2. All patients received steroid therapy, four patients underwent lung transplants, some patients received bronchodilators, some used macrolides, and some patients received immunosuppressive agents. Owing to post-SJS/TEN, BO is progressive and irreversible; azathioprine can be used in refractory cases (37). There is no clear treatment strategy for airway mucosal diseases and long-term steroid therapy may cause secondary pulmonary infection; therefore, further research is needed (38). A case report showed that the SJS patient who developed severe symptoms of BO did not receive immunomodulatory or systemic immunosuppressive therapy in the acute phase (11). Continuous blood purification could ameliorate the inflammatory response (39). A retrospective cohort study revealed continuous venovenous hemofiltration combined with hemoperfusion might be an effective and safe adjuvant therapy for TEN (40). A drastic decrease was observed in the level of IL-6 and IL-10 after continuous blood purification therapy in our patient. Although we started systemic corticosteroids, continuous blood purification, and IVIG in the acute stage, we still can not protect our patient from developing BO. Whether early intervention would have an impact on the development of BO in SJS/TEN is unclear. More research is needed to reduce the risk of SJS/TEN leading to BO.
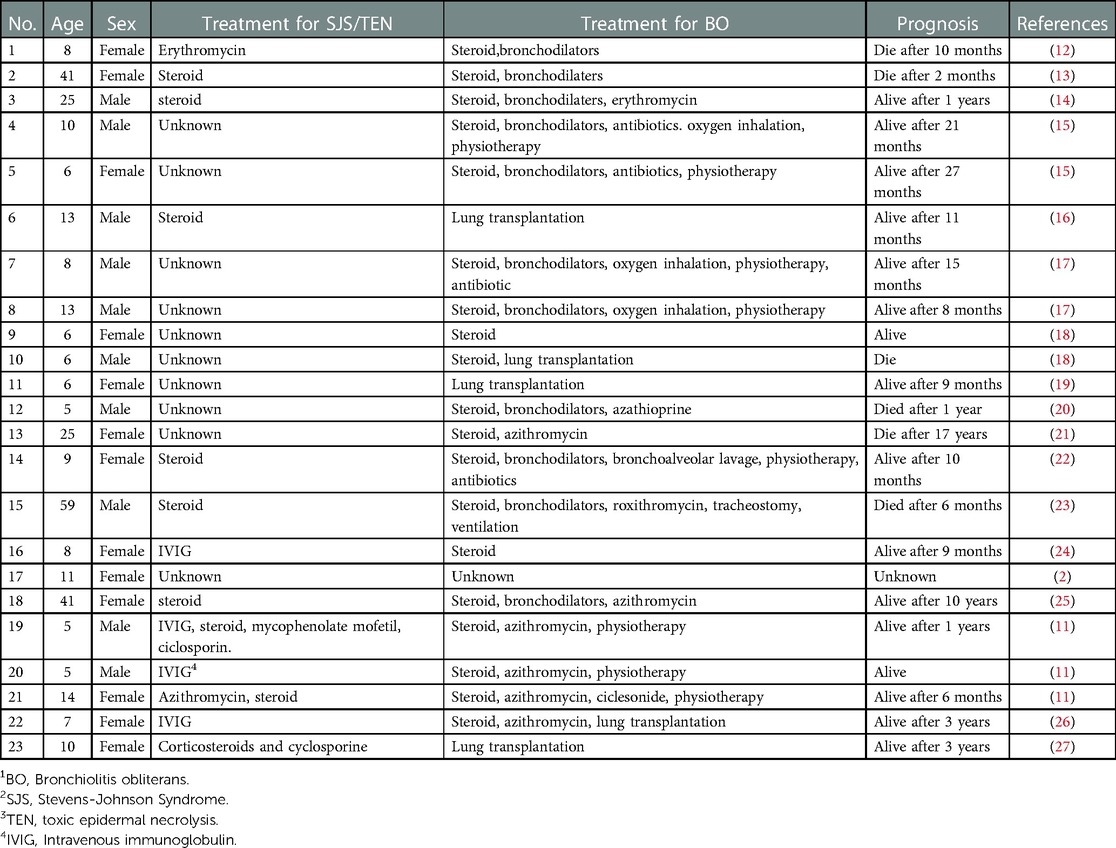
Table 2. Summary of treatment and prognosis in BO1 associated with SJS2/TEN3 from the published cases.
Although the understanding of the pathogenesis, diagnosis, and treatment of BO have made some progress in the past years, the overall mortality is still very high (41). The prognosis of BO is variable and depends on the initial cause.
BO associated with SJS/TEN is progressive and has a poor prognosis (27). Six of the case reports ended in death, as shown in Table 2. A case report described BO complicating a pneumothorax after SJS (22). Some reported cases showed that death occurs due to respiratory failure, and the longest recorded survival time was 17 years after SJS (21). The shortest recorded survival time was 2 months after SJS (13).
BO secondary to SJS /TEN is a rare but devastating disorder. Continuous monitoring and timely treatment of SJS/TEN contribute to preventing the progression of the disease. Patients with SJS/TEN may develop BO at different stages, so we should follow all patients with SJS/TEN for possible persistent respiratory complications for as long as possible even after they recover from SJS/TEN. In this area, further research is required to explore potential mechanisms and develop better monitoring pathways and treatment options.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The study project has been approved by the ethics committee of The First Hospital of Jilin University. Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
YL concepted and designed the study. JL and HY acquireed and analyzed data. JL drafted the article. CY, HY and YL revised the article. All authors contributed to the article and approved the submitted version.
This work was supported by the Jilin provincial department of science and technology (Grant No. 20210204134YY). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lee HY, Walsh SA, Creamer D. Long-term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow-up. Br J Dermatol. (2017) 177(4):924–35. doi: 10.1111/bjd.15360
2. Sato S, Kanbe T, Tamaki Z, Furuichi M, Uejima Y, Suganuma E, et al. Clinical features of Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr Int. (2018) 60(8):697–702. doi: 10.1111/ped.13613
3. Kavaliunaite E, Aurora P. Diagnosing and managing bronchiolitis obliterans in children. Expert Rev Respir Med. (2019) 13(5):481–88. doi: 10.1080/17476348.2019.1586537
4. Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Current perspectives on Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. (2017) 54(1):147–76. doi: 10.1007/s12016-017-8654-z
5. Liew YCC, Choo KJL, Oh CC, Pang SM, Yeo YW, Lee HY. Mycoplasma-induced Stevens-Johnson syndrome/toxic epidermal necrolysis: case-control analysis of a cohort managed in a specialized center. J Am Acad Dermatol. (2022) 86(4):811–17. doi: 10.1016/j.jaad.2021.04.066
6. Watkins LKF, Olson D, Diaz MH, Lin X, Demirjian A, Benitez AJ, et al. Epidemiology and molecular characteristics of Mycoplasma pneumoniae during an outbreak of M. pneumoniae-associated Stevens-Johnson syndrome. Pediatr Infect Dis J. (2017) 36(6):564–71. doi: 10.1097/INF.0000000000001476
7. Olson D, Watkins LKF, Demirjian A, Lin X, Robinson CC, Pretty K, et al. Outbreak of Mycoplasma pneumoniae-associated Stevens-Johnson syndrome. Pediatrics. (2015) 136(2):e386–94. doi: 10.1542/peds.2015-0278
8. Kurata M, Kano Y, Sato Y, Hirahara K, Shiohara T. Synergistic effects of Mycoplasma pneumoniae infection and drug reaction on the development of atypical Stevens-Johnson syndrome in adults. Acta Derm Venereol. (2016) 96(1):111–3. doi: 10.2340/00015555-2180
9. McPherson T, Exton LS, Biswas S, Creamer D, Dziewulski P, Newell L, et al. British Association of Dermatologists’ guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in children and young people, 2018. Br J Dermatol. (2019) 181(1):37–54. doi: 10.1111/bjd.17841
10. Stitt VJ Jr. Stevens-Johnson syndrome: a review of the literature. J Natl Med Assoc. (1988) 80(1):104. PMID: 3276904 PMCID: PMC2625691
11. Seccombe EL, Ardern-Jones M, Walker W, Austin S, Taibjee S, Williams S, et al. Bronchiolitis obliterans as a long-term sequela of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Clin Exp Dermatol. (2019) 44(8):897–902. doi: 10.1111/ced.13969
12. Edwards C, Penny M, Newman J. Mycoplasma pneumonia, Stevens-Johnson syndrome, and chronic obliterative bronchitis. Thorax. (1983) 38(11):867–9. doi: 10.1136/thx.38.11.867
13. Tsunoda N, Iwanaga T, Saito T, Kitamura S, Saito K. Rapidly progressive bronchiolitis obliterans associated with Stevens-Johnson syndrome. Chest. (1990) 98(1):243–5. doi: 10.1378/chest.98.1.243
14. Yatsunami J, Nakanishi Y, Matsuki H, Wakamatsu K, Takayama K, Kawasaki M, et al. Chronic bronchobronchiolitis obliterans associated with Stevens-Johnson syndrome. Intern Med. (1995) 34(8):772–5. doi: 10.2169/internalmedicine.34.772
15. Kim MJ, Lee KY. Bronchiolitis obliterans in children with Stevens-Johnson syndrome: follow-up with high resolution CT. Pediatr Radiol. (1996) 26(1):22–5. doi: 10.1007/BF01403698
16. Date H, Sano Y, Aoe M, Goto K, Tedoriya T, Sano S, et al. Living-donor lobar lung transplantation for bronchiolitis obliterans after Stevens-Johnson syndrome. J Thorac Cardiovasc Surg. (2002) 123(2):389–91. doi: 10.1067/mtc.2002.119331
17. Bakirtas A, Harmanci K, Toyran M, Razi CH, Turktas I. Bronchiolitis obliterans: a rare chronic pulmonary complication associated with Stevens-Johnson syndrome. Pediatr Dermatol. (2007) 24(4):E22–5. doi: 10.1111/j.1525-1470.2007.00433.x
18. Chiu CY, Wong KS, Huang YC, Lin TY. Bronchiolitis obliterans in children: clinical presentation, therapy and long-term follow-up. J Paediatr Child Health. (2008) 44(3):129–33. doi: 10.1111/j.1440-1754.2007.01209.x
19. Shoji T, Bando T, Fujinaga T, Date H. Living-donor single-lobe lung transplant in a 6-year-old girl after 7-month mechanical ventilator support. J Thorac Cardiovasc Surg. (2010) 139(5):e112–3. doi: 10.1016/j.jtcvs.2009.04.015
20. Dogra S, Suri D, Saini AG, Rawat A, Sodhi KS. Fatal bronchiolitis obliterans complicating Stevens-Johnson syndrome following treatment with nimesulide: a case report. Ann Trop Paediatr. (2011) 31(3):259–61. doi: 10.1179/1465328111Y.0000000019
21. Sugino K, Hebisawa A, Uekusa T, Hatanaka K, Abe H, Homma S. Bronchiolitis obliterans associated with Stevens-Johnson syndrome: histopathological bronchial reconstruction of the whole lung and immunohistochemical study. Diagn Pathol. (2013) 8:134. doi: 10.1186/1746-1596-8-134
22. Wang WP, Ni YF, Wei YN, Li XF, Cheng QS, Lu Q. Bronchiolitis obliterans complicating a pneumothorax after Stevens-Johnson syndrome induced by lamotrigine. J Formos Med Assoc. (2015) 114(3):285–9. doi: 10.1016/j.jfma.2012.02.026
23. Park H, Ko YB, Kwon HS, Lim CM. Bronchiolitis obliterans associated with Stevens-Johnson syndrome: a case report. Yonsei Med J. (2015) 56(2):578–81. doi: 10.3349/ymj.2015.56.2.578
24. Milheiro Silva T, Farela Neves J, Casimiro A, Varandas L, Gouveia C. Severe Stevens-Johnson syndrome/toxic epidermal necrolysis overlap syndrome-beyond skin involvement. Pediatr Dermatol. (2018) 35(1):e17–19. doi: 10.1111/pde.13328
25. Shabrawishi M, Qanash SA. Bronchiolitis obliterans after cefuroxime-induced Stevens-Johnson syndrome. Am J Case Rep. (2019) 20:171–74. doi: 10.12659/AJCR.913723
26. Romagnoli V, Amici M, Amici L, Gasparini S, Cazzato S. Alteplase treatment for massive lung atelectasis in a child with severe bronchiolitis obliterans complicating Stevens-Johnson syndrome. Pediatr Pulmonol. (2020) 55(7):1541–3. doi: 10.1002/ppul.24793
27. Matar M, Kessler R, Olland A, Falcoz P, Desprez P, Roche A, et al. End-Stage respiratory failure secondary to bronchiolitis obliterans syndrome induced by toxic epidermal necrosis, also known as lyell syndrome: a case report. Transplant Proc. (2021) 53(4):1371–74. doi: 10.1016/j.transproceed.2021.03.020
28. Li YN, Liu L, Qiao HM, Cheng H, Cheng HJ. Post-infectious bronchiolitis obliterans in children: a review of 42 cases. BMC Pediatr. (2014) 14:238. doi: 10.1186/1471-2431-14-238
29. Zhao C, Liu J, Yang H, Xiang L, Zhao S. Mycoplasma pneumoniae-associated bronchiolitis obliterans following acute bronchiolitis. Sci Rep. (2017) 7(1):8478. doi: 10.1038/s41598-017-08861-7
30. Huang K, Liu J, Lv W, Chu Y, Li B, Wu P, et al. Analysis of risk factors of bronchiolitis obliterans in children with Mycoplasma pneumoniae bronchiolitis. Comput Math Methods Med. (2022) 2022:9371406. doi: 10.1155/2022/9371406
31. Zheng HQ, Ma YC, Chen YQ, Xu YY, Pang YL, Liu L. Clinical analysis and risk factors of bronchiolitis obliterans after Mycoplasma Pneumoniae pneumonia. Infect Drug Resist. (2022) 15:4101–08. doi: 10.2147/IDR.S372940
32. Gan CT, Ward C, Meachery G, Lordan JL, Fisher AJ, Corris PA. Long-term effect of azithromycin in bronchiolitis obliterans syndrome. BMJ Open Respir Res. (2019) 6(1):e000465. doi: 10.1136/bmjresp-2019-000465
33. Even-Or E, Ghandourah H, Ali M, Krueger J, Sweezey NB, Schechter T. Efficacy of high-dose steroids for bronchiolitis obliterans syndrome post pediatric hematopoietic stem cell transplantation. Pediatr Transplant. (2018) 22(2. doi: 10.1111/petr.13155
34. Lucid CE, Savani BN, Engelhardt BG, Shah P, Clifton C, Greenhut SL, et al. Extracorporeal photopheresis in patients with refractory bronchiolitis obliterans developing after allo-SCT. Bone Marrow Transplant. (2010) 46(3):426–29. doi: 10.1038/bmt.2010.152
35. Brownback KR, Thomas LA, McGuirk JP, Ganguly S, Streiler C, Abhyankar S. Effect of rituximab on pulmonary function in bronchiolitis obliterans syndrome due to graft-versus-host-disease. Lung. (2017) 195(6):781–88. doi: 10.1007/s00408-017-0051-0
36. Gao F, Chen J, Wei D, Wu B, Zhou M. Lung transplantation for bronchiolitis obliterans syndrome after allogenic hematopoietic stem cell transplantation. Front Med. (2018) 12(2):224–28. doi: 10.1007/s11684-017-0538-3
37. Dogra S, Saini AG, Suri D, Rawat A, Sodhi KS, Singh S. Bronchiolitis obliterans associated with Stevens-Johnson syndrome and response to azathioprine. Indian J Pediatr. (2014) 81(7):732–3. doi: 10.1007/s12098-013-1204-7
38. Kaneko Y, Seko Y, Sotozono C, Ueta M, Sato S, Shimamoto T, et al. Respiratory complications of Stevens-Johnson syndrome (SJS): 3 cases of SJS-induced obstructive bronchiolitis. Allergol Int. (2020) 69(3). doi: 10.1016/j.alit.2020.01.003
39. Pan J, Xu B, Yu J. The effect of continuous blood purification on P38MAPK signaling pathway in patients with multiple organ dysfunction syndrome. J Clin Lab Anal. (2019) 33(4):e22849. doi: 10.1002/jcla.22849
40. Bai M, Yu Y, Huang C, Liu Y, Zhou M, Li Y, et al. Continuous venovenous hemofiltration combined with hemoperfusion for toxic epidermal necrolysis: a retrospective cohort study. J Dermatolog Treat. (2017) 28(4):353–59. doi: 10.1080/09546634.2016.1240326
Keywords: stevens-Johnson syndrome, dyspnea, toxic epidermal necrolysis, bronchiolitis obliterans, infection
Citation: Liu J, Yan H, Yang C and Li Y (2023) Bronchiolitis obliterans associated with toxic epidermal necrolysis induced by infection: A case report and literature review. Front. Pediatr. 11:1116166. doi: 10.3389/fped.2023.1116166
Received: 5 December 2022; Accepted: 8 February 2023;
Published: 2 March 2023.
Edited by:
Suyun Qian, Capital Medical University, ChinaReviewed by:
Rujipat Samransamruajkit, Chulalongkorn University, Thailand© 2023 Liu, Yan, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumei Li eW1fbGlAamx1LmVkdS5jbg==
Specialty Section: This article was submitted to Pediatric Critical Care, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.