- 1Department of Rheumatology, National Medical Research Centre for Children's Health, Moscow, Russian Federation
- 2Department of Paediatrics and Paediatric Rheumatology, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation
- 3N.F. Filatov Clinical Institute for Children's Health, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation
- 4Association of Paediatric Rheumatologists, Moscow, Russian Federation
Background: A significant number of systemic juvenile idiopathic arthritis (sJIA) patients discontinue biologic disease-modifying antirheumatic drugs (bDMARDs) due to lack of efficacy or safety concerns. Studies of biologic therapy switch regimens in sJIA are required.
Methods: Patients with sJIA who switched from tocilizumab (due to lack of efficacy or safety) to canakinumab (4 mg/kg every 4 weeks) and were hospitalized at the rheumatology department from August 2012 to July 2020 were included. Primary efficacy outcomes were 30% or greater improvement based on the paediatric criteria of the American College of Rheumatology (ACR30), achievement of inactive disease (JADAS-71 = 0) and clinical remission (ACR sJIA clinical inactive disease criteria). Follow-up from time first canakinumab dose administered was 12 months or the closest time point (not less than 6 and not more than 18 months). Data were extracted from electronic outpatient medical records.
Results: During the study period, 46 patients with sJIA switched from tocilizumab to canakinumab. Median age at baseline was 8.2 [interquartile range (IQR) 4.0–12.9] years, with the median sJIA duration being 1.8 (IQR 0.8–5.8) years; 37 (80%) patients received at least one conventional DMARD (cDMARD; oral corticosteroids, methotrexate and/or cyclosporine A). Study outcomes were followed up in 45 patients (one patient did not attend the follow-up for an unknown reason); median follow-up was 359 (IQR 282–404) days. During the follow-up, 1 patient discontinued canakinumab due to tuberculosis detection and the dose was reduced or the injection interval increased in 4 (9%) patients. In total, 27 (60%) patients continued to receive at least one cDMARD. Improvement according to the ACR30 criteria was achieved in 43 patients [96%; 95% confidence interval (CI) 85–99], inactive disease in 42 (93%; 95%CI 82–98), and remission in 37 (82%; 95% CI 69-91); after adjustment for actual time-at-risk, the rates were 83, 85 and 73 events per 100 person-years, respectively. During follow-up, 23 AEs (most frequently infections) were reported in 19/45 (42%) patients; 5/45 (11%) patients developed macrophage activation syndrome, with a favorable outcome in all cases.
Conclusions: One-year canakinumab therapy was found to be potentially effective as second-line biologic therapy after discontinuation of tocilizumab in patients with sJIA.
1. Background
The efficacy of interleukin-1 (IL-1) inhibitors (anakinra, canakinumab) and interleukin-6 (IL-6) inhibitors (tocilizumab) as first-line biologic therapy for children with systemic juvenile idiopathic arthritis (sJIA) has been practically assured. However, a significant number of patients with sJIA discontinue biologic therapy due to lack of efficacy or safety concerns (1–3). As a result, the patient must be switched to another biologic. Based on real-world observational studies, 21%–47% of patients with sJIA switch to a second, or subsequent, biologic disease-modifying antirheumatic drug (bDMARD) (1–3), within 7–16 months (2, 3). According to the Russian Register of patients with sJIA (2002–2015; n = 384), 68% of patients received at least one dose of at least one biologic (most often tocilizumab), with every third patient (32%) requiring a switch to a second or subsequent bDMARDs (4).
Current guidelines developed by American College of Rheumatology (ACR, 2013) (5) and National Health Service (NHS, 2015) (6) state that a biologic with a different mechanism of action should be prescribed when considering a second or subsequent biologic in patients with sJIA who have continued disease activity with, or an intolerance to, first-line biologic therapy. National Russian guidelines (2021) contain similar recommendations (7). To support this expert opinion, efficacy and safety studies of switch regimens in patients with sJIA are required (e.g., tocilizumab with a subsequent switch to canakinumab or any other dual regimen in line with current guidelines). In addition, when conducting such studies, it is important to consider the local/regional specificity of rheumatology care. Only two small-scale, open-label extension studies have demonstrated high efficacy of switching from tocilizumab to canakinumab in patients with sJIA at week 28 (8) [subsequently confirmed for 48 weeks (9)] and at month 12 (10).
This study aimed to evaluate the one-year efficacy and safety of canakinumab as a second-line biologic after tocilizumab treatment failure in children with sJIA in routine clinical practice.
2. Methods
2.1. Study design
This single-centre retrospective cohort study evaluated treatment outcomes in patients with sJIA who switched from tocilizumab to canakinumab. Data were collected for participants enrolled in one of two clinical trials at the study centre, CACZ885G2301E1 (G2301; NCT00891046) and CACZ885G2306 (G2306; NCT02296424) (11), in addition to those who switched treatment in routine clinical practice.
2.2. Setting
The patients were those who had been hospitalised and followed up at the rheumatology department of the National Medical Research Centre for Children's Health (previously known as the National Centre for Children's Health until November 2016 and then the National Scientific and Practical Centre for Children's Health, Moscow, until July 2017) in Moscow, from August 2012 (start of canakinumab studies at the centre) to July 2020 (uploading of the database with routinely collected health data to generate the study population).
2.3. Patients
Study included data of patients with sJIA aged 2 to <18 years who were prescribed to initiate canakinumab therapy over no more than 2 weeks after discontinuation of tocilizumab (received at least one dose of the drug) due to lack of efficacy or an adverse reaction were included in the analysis. The decision on the need to discontinue tocilizumab was made by the physician, as part of their routine clinical practice. Exclusion criteria not planned.
2.4. Treatment
All patients (both from routine practice and participants of G2301 and G2306) were prescribed canakinumab, a human anti-IL-1β monoclonal antibody, at a dose of 4 mg/kg subcutaneously every 4 weeks, maximum 300 mg. In accordance with the protocol, dose tapering was applied for participants of G2306 providing that they achieved clinical remission (inactive disease for ≥24 subsequent weeks) without the use of corticosteroids or methotrexate. Tapering regimens included either a dose reduction from 4 to 2 mg/kg (no earlier than Week 28), and then to 1 mg/kg (no earlier than Week 52), or increased inter-injection intervals, followed by discontinuation. Dose tapering was not scheduled for participants of G2301 or those in routine clinical practice. Any changes to therapy were made in accordance with the physician's decision.
2.5. Outcomes
Classification of primary and secondary outcomes were not pre-defined for patients switching to canakinumab in the clinical practice setting.
2.5.1. Primary efficacy outcome
The study endpoints were as follows: at least 30% improvement based on the paediatric criteria of the American College of Rheumatology (ACR30) (12), the achievement of inactive disease according to the 71-point Juvenile Arthritis Disease Activity Score (JADAS-71 = 0 points) (13) and clinical remission according to the criteria of ACR sJIA clinical inactive disease (14). Follow-up for treatment outcomes was 12 months or the closest time, but not less than 6 and not more than 18 months from the day of the first dose of canakinumab.
2.5.2. Secondary efficacy outcomes
2.5.2.1. Clinical parameters
The percentage of patients with morning joint stiffness lasting for > 15-20 min, active joints, systemic signs of disease activity (fever, rash, serositis, hepatosplenomegaly and/or lymphadenopathy).
2.5.2.2. Laboratory tests
Erythrocyte sedimentation rate (ESR) > 20 mm/h, C-reactive protein (CRP) levels > 5 mg/l.
2.5.2.3. Assessment criteria
Non-zero values of disease activity as assessed by the treating physician using a 100-mm visual analogue scale (VAS), the patient's wellbeing assessed by the patient's parent using a 100-mm VAS and functional impairment based on the Childhood Health Assessment Questionnaire (CHAQ) (15) using the patient's parent estimate.
Monitoring of the treatment outcomes using additional parameters was conducted within the same time frame as for those using primary endpoints of the study.
2.5.3. Safety outcomes
Safety of canakinumab-based therapy was assessed using the adverse event (AE) rates, i.e., any untoward medical events (unfavourable changes in the patient's health status or parameters reflecting the patient's health status) that occurred immediately after drug administration or during its use.
2.6. Data sources
Data on patients, administered treatment and its outcomes were extracted from electronic outpatient medical records of the rheumatology department and medical record documentation generated in local healthcare facilities during the time between hospital admissions (discharge summaries, outpatient medical records and medical reports). The latter were provided to the treating physicians of the rheumatology department by the parents of patients with sJIA. The analysis of laboratory parameters of the disease activity was carried out in the clinical diagnostic laboratory at the National Medical Research Centre of Children's Health. Health data were accumulated in the 1C:Enterprise application (1C Company, Russia). After uploading the routinely collected health data database (tables with data of all study subjects), the data entered were checked to exclude input errors.
Treatment efficacy measures (primary and secondary), as well as safety measures, were determined using the data contained in electronic outpatient medical records. When assessing treatment safety, information from medical record documentation provided by the patients' parents (cases of AEs in the between admission period) was also considered.
2.7. Statistical analysis
Statistical analysis was performed using SciStat, the online service by MedCalc Software (www.scistat.com). The description of quantitative values was presented as median (interquartile range, IQR). A Wilcoxon test was used to compare quantitative indicators in the dependent samples (before and after values). The event rates for the primary outcome measures were described indicating a 95% confidence interval (CI) without continuity correction (using online service www.vassarstats.net) and the rate per 100 person-years.
2.7.1. Sensitivity analysis
Univariate Cox proportional hazards regression analysis was used to identify any predictors of clinical inactive disease achievement according to ACR sJIA criteria. The ACR30 and JADAS-71 criteria as such were abandoned due to the small number of patients with lack of efficacy. Treatment outcome were analysed taking into account the effect of all baseline clinical, laboratory, and assessment characteristics (at disease onset or before canakinumab treatment initiation).
2.8. Ethical approval
The protocols of G2301 and G2306 trials (dated 10 August 2012 and 5 December 2014, respectively) were approved by the local Ethics Committee of the National Medical Research Centre of Children's Health (at the time of the Ethics Committee opinion issue, referred to as National Centre for Children's Health). The inclusion of data from routine clinical practice in the study was not agreed with the Ethics Committee. At admission, the parents of all patients and patients aged ≥ 15 years provided their written informed consent allowing for the use of examination and treatment results for scientific purposes.
3. Results
3.1. Patient disposition
During the study period, a total of 250 patients with sJIA who received tocilizumab were monitored in the rheumatology department, of whom 65 (26%) discontinued therapy due to lack of efficacy or an adverse reaction. Tocilizumab was switched to canakinumab in 46 patients; 35 patients initiated canakinumab therapy in routine clinical practice, 3 upon entry to the G2301 trial and 8 upon entry to the G2306 trial. The most common reason for tocilizumab withdrawal was secondary treatment failure (defined by physicians as failure to meet the criteria of ACR sJIA clinical inactive disease occurring at any time following initial achievement of clinical remission following initial treatment effect; n = 32, 70%); 12 (26%) patients withdrew due to an adverse reaction, and 2 (4%) due to primary treatment failure (defined by physicians as failure to achieve ACR sJIA clinical inactive disease within the first 6 months after drug initiation).
The study outcomes were followed up in 45 patients, one patient did not attend the follow-up examination and treatment control in the period from month 6 to month 18, for an unknown reason.
3.2. Baseline characteristics
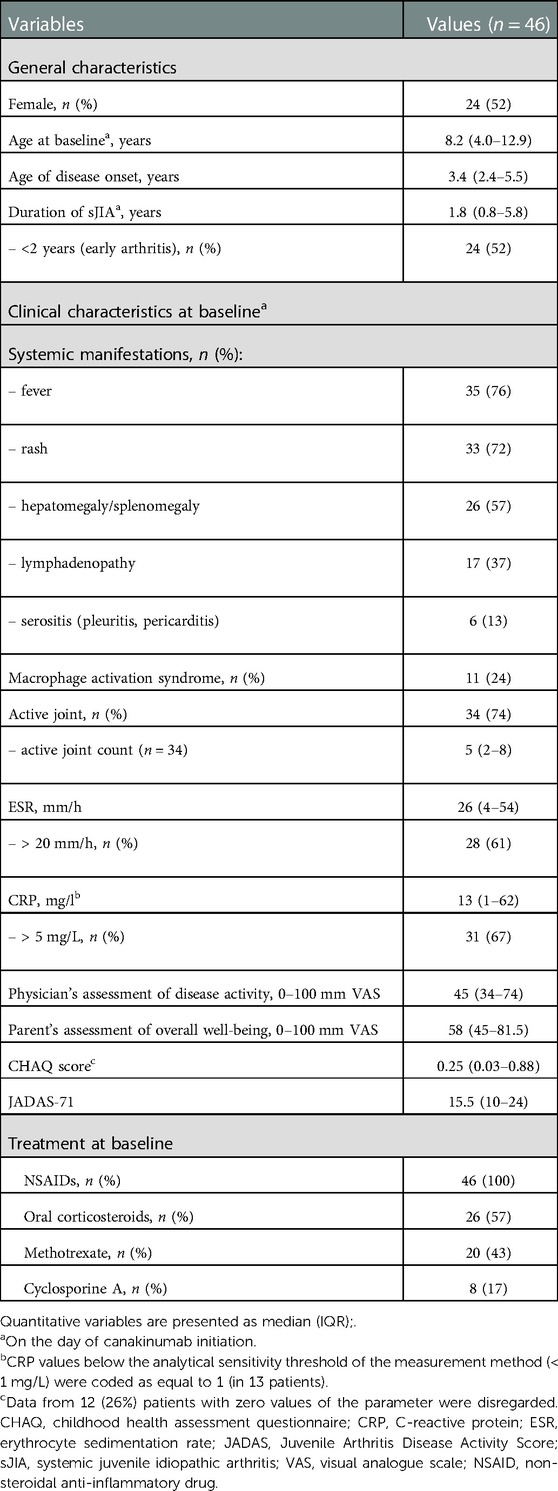
Patient demographics and disease characteristics are presented in Table 1. Approximately half of the patients (52%) were female. the median age at the time of sJIA onset was 3.4 years and median age at the time of canakinumab treatment initiation being 8.2 years. Duration of sJIA before canakinumab initiation did not exceed 2 years in more than half (52%) of the patients. Systemic manifestations (fever, rash, hepatomegaly/splenomegaly and/or lymphadenopathy) were observed in 42 (91%) patients. At the time of canakinumab initiation, all patients received treatment with non-steroidal anti-inflammatory drugs (NSAIDs), more than half received oral corticosteroids, more than received 40% methotrexate; and 17% received cyclosporine A. A total of 37 (80%) patients received at least one immunosuppressant (oral corticosteroids, methotrexate and/or cyclosporine A), of which 16 (43%) patients received concurrently ≥ 2 immunosuppressants.
3.3. Primary efficacy outcomes
Median follow-up for patients with known outcomes (n = 45) was 359 (IQR 282–404) days; range, 184–476 days. Primary efficacy outcomes (paediatric ACR30 criteria, inactive disease based on JADAS-71, and clinical remission) were achieved in most of 45 patients in whom follow-up data was available (Table 2). After adjustment for actual time-at-risk, the rates were 83, 85, and 73 events per 100 person-years for ACR30, inactive disease, and clinical remission, respectively.
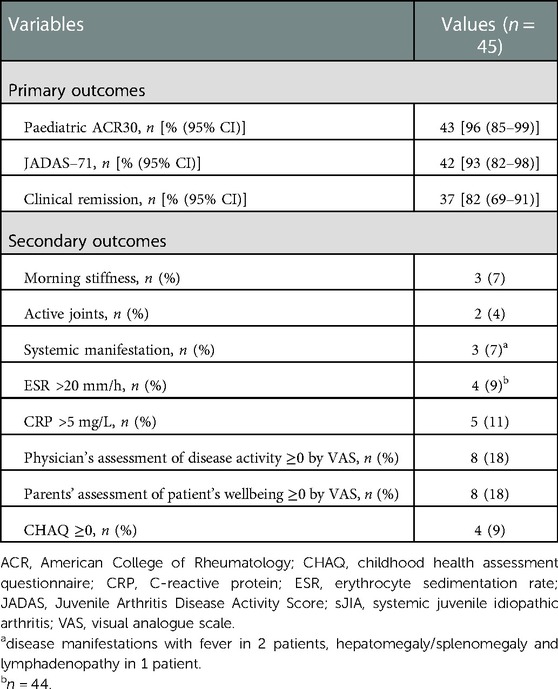
Table 2. Treatment outcomes after 12 months or the closest follow-up time (not less than 6 and not more than 18 months) after the first dose of canakinumab.
Abovementioned outcomes were achieved with some treatment modifications. In particular, during follow-up, canakinumab was discontinued in 1 patient [discontinuation occurring after 14 months due to tuberculosis (TB) detection], the dose was reduced to 2 mg/kg per injection in 3 patients, and the interval between injections was increased up to 8 weeks in 1 patient. In addition, during the follow-up period, NSAIDs were administered in 2 (4%) patients, oral corticosteroids in 20 (44%), methotrexate in 12 (27%) and cyclosporine A in 9 (20%) patients. A total of 27 (60%) patients received at least one immunosuppressant (oral corticosteroids, methotrexate and/or cyclosporine A), of which 13 (48%) patients received concurrently ≥ 2 immunosuppressants.
3.4. Secondary efficacy outcomes
At the time of the follow-up examination, some signs of disease persisted in 4%–18% of patients (Table 2). At least one clinical sign of the active disease (morning stiffness, active joints, systemic disease manifestations) was noted in 7 of 45 (16%) patients. At the end of follow-up, median ESR was 3 (IQR 2–7) mm/h (data presented for 44 patients) (p < 0.001; hereinafter vs. the baseline), median CRP (n = 45) was 1.0 (IQR 1.0–1.9) mg/l (p < 0.001). At least one laboratory sign of the disease was noted in 6/45 (13%) patients. Nonzero scores for at least one estimated parameter of disease activity (activity assessment by the physician, parents' assessment of patient's wellbeing, functional insufficiency according to CHAQ) were identified in 8/45 (18%) patients.
In total, at the end of follow-up, any of the above clinical, laboratory and/or estimated parameters of disease activity was observed in 11 (24%) of 45 patients, of which 5 had only clinical, laboratory or estimated sign of active disease.
3.5. Safety
During follow-up, 23 AEs were reported in 19/45 (42%) patients, of whom 11 had an acute respiratory or intestinal infection, 4 had leukopenia/neutropenia, 4 had elevated liver transaminase levels and 4 had TB infection (including one case of a positive M. tuberculosis test result). Electronic medical records of 5/45 (11%) patients contain the history of macrophage activation syndrome (MAS) during the use of canakinumab, with a favourable outcome in all patients.
3.6. Predictors of remission
According to the criteria of ACR sJIA clinical inactive disease, none of the sample characteristics (see Table 1) were associated with achievement of clinical remission during follow-up.
4. Discussion
4.1. Summary of primary findings
Most sJIA patients treated with canakinumab after discontinuation of tocilizumab (74% due to lack of efficacy, either primary or secondary) achieved remission (82%), inactive disease (93%) and/or improvements in disease signs (ACR30 – 96%) within a year. In addition, the use of c DMARDs, such as oral corticosteroids, methotrexate and/or cyclosporine A, also decreased. The “survival” of canakinumab therapy during follow-up was high, with only one case of therapy withdrawal due to TB infection. At least one AE (most frequently infection) was reported in more than 40% of patients.
4.2. Study limitations
4.2.1. Representativeness of the study sample
The study involved patients who were followed up at a single centre only. In these cases, the patient management does not reflect all specific aspects of routine clinical practice in the study region and the extent to which these findings can be generalised to other healthcare systems is unknown. Moreover, the rheumatology department, whose patients were enrolled in the study, is a subdivision of the largest Russian paediatric centre, the capabilities of which (expert, diagnostic, therapeutic) can significantly exceed those of regional clinical centres. This is reflected by the enrolment of every fourth patient (n = 11; 24%) we analysed in the international clinical trial (G2301 and G2306). In these trials, therapy is pre-determined by the protocol and treatment control and disease outcomes monitoring are more stringent than in routine clinical practice. In addition, this study enrolled patients from the Russian Federation only; it is not known to what extent the findings can be generalised to patients in other countries with other healthcare systems.
4.2.2. Small-scale sample
One of the important advantages of large-scale cohort studies is the heterogeneity of its sample, helping to gain insight into the results of medical interventions in a setting as close to real-world clinical practice as possible. In small-scale trials, the description of relatively rare treatment outcomes (both favourable and unfavourable, including AEs) is obviously impossible.
4.2.3. Variability of follow-up period
Many of our paediatric patients were from remote regions of Russia, which limited their ability to attend regular follow-ups. Attending the clinical centre for at least one assessment per year was recommended for all patients, but some were unable to do this routinely. As a result, the spread of the actual follow-up values varied from month 6 to month 16. Although the median period was 12 months, the distribution of the follow-up duration in the study sample was shifted to the left (coefficient of skewness −0.271). This limits the interpretation of the study results.
4.2.4. sRoutinely collected health data
We analysed the data obtained from electronic health records. The quality of such data may give rise to reasonable doubts (16), particularly with regards to safety information. Furthermore, missing data is often a limitation for the use of medical records. In this study, there were only isolated cases of missing data, which, in our opinion, did not significantly affect the results obtained.
4.2.5. Absence of control group
We cannot directly attribute the effects of therapy (both beneficial and adverse) solely to the effect of canakinumab, since most patients (80% at baseline and 60% at follow-up) received at least one more immunosuppressant agent, such as oral corticosteroids, methotrexate and/or cyclosporine A. Similarly, it should be noted that approximately three-quarters of patients were prescribed canakinumab in view of lack of efficacy of tocilizumab (mainly secondary failures) and at the same time, while on canakinumab, a decrease in the proportion of patients receiving oral corticosteroids (from 57% to 44%) and methotrexate (from 43% up to 27%) was reported. The results described with canakinumab treatment were therefore achieved against a less intense background regimen of cDMARDs. Moreover, this does not take into account a change in the dose of cDMARDs, the reduction of which has been shown in randomised clinical trials with canakinumab (9, 17).
4.3. Interpretation of study results
4.3.1. Efficacy of switching from tocilizumab to canakinumab
In our study, the minimum significant improvement according to the paediatric ACR30 criteria was reported in 96% (43/45) of patients (median follow-up of 12 months or 359 days) or in 85% in terms of the rate per 100 person-years. Similar results were demonstrated in clinical trials by Brunner et al., 2015 (10) and Nishimura et al., 2021 (9). In the former study, after switching from tocilizumab (withdrawn due to lack of efficacy or safety) to canakinumab, the ACR30 criteria was achieved in 23/24 (96%) patients with sJIA at month 12, in the latter, in 16/19 (84%) at approx. month 11 (median 337 days). A similar result (ACR30 in 86% at the median follow-up of 8 months or 236 days) was obtained in a clinical trial (withdrawal phase) by Ruperto N. et al., 2012, where 80% of patients received tocilizumab before canakinumab (17). Moreover, in the above-mentioned trials, roughly one third of patients discontinued glucocorticoids following initiation of canakinumab (9, 17); in our study, oral glucocorticoids were discontinued in 6 of the 26 patients (23%) who were receiving these drugs at the time of administration of the first dose of canakinumab.
Several hypotheses can explain the lack of efficacy of tocilizumab in patients with sJIA and, accordingly, the efficacy of switching to canakinumab. First, IL-1 but not Il-6, can be considered a primary mediator of autoimmune (in fact, aseptic) inflammation. In particular, in vitro studies demonstrate that sJIA is an IL-1β-mediated disease (18). The pathogenetic role of these cytokines in the development of autoimmune processes and arthritis was confirmed both in IL-1Ra (19) and IL-6-deficient mice (20) as well as the initial clinical studies of IL-1- (18) and IL-6-inhibitors (21). However, it is noteworthy that systemic inflammation in combination with arthritis is observed in IL-1-overexpressing (22), but not IL-6-overexpressing transgenic mice (23, 24). In addition, the aseptic inflammation model showed that IL-6 is not detectable in the blood serum of IL-1β-deficient mice (25), while, on the contrary, IL-1 is induced normally in IL-6-deficient mice (26). It was also found that in mice transgenic for IL-1α (activation of IL-1 signalling) and prone to arthritis, IL-6 deficiency resulting from knockout of the corresponding gene reduces, but does not cancel, pathological changes in the joints (22). Finally, in newly diagnosed untreated patients with sJIA, high expression levels of genes involved in the negative regulation of IL-1R signalling have been observed (27).
Secondly, different sensitivity to tocilizumab in patients with sJIA may be fundamental. For instance, it has been shown that the lack of efficacy of tocilizumab is associated with initially high concentrations of IL-6 (28, 29), sIL-6R (30, 31) and a low concentration of soluble gp130 (29), which is a natural inhibitor of the IL-6 trans-signalling pathway (32). The effect of high levels of IL-6 may be due to the competition of significant amounts of cytokine with tocilizumab for binding sites (IL-6R) on the membranes of target cells, i.e., immunocompetent cells and hepatocytes, but not for soluble forms of the receptor, which is confirmed in studies with IL-6-dependent myeloma cell line and recombinant BaF/IL-6R (33). It can be assumed that in some patients the recommended dose of tocilizumab may not be enough to suppress both a high concentration of soluble IL-6R and significant amounts of the IL-6/sIL-6R complex due to a low concentration of soluble gp130.
Thirdly, there are rare cases of treatment failure and/or hypersensitivity reactions associated with antibodies to biologics and, in particular to tocilizumab (34). In these cases, the efficacy of IL-1 inhibitors can be considered as a therapy for “biologically naïve” patients. The development of antibodies to canakinumab in such patients is unlikely as no anti-drug neutralising antibodies were detected (11, 35).
Finally, tocilizumab does not affect the concentration of IL-1β (28), i.e., does not have an indirect effect on the key mechanism of sJIA pathogenesis. In this regard, it is important to note that high baseline levels of IL-1 in serum is associated with a lower efficacy of tocilizumab after 16 (29) and 52 weeks (36) of therapy. In contrast, down-modulation of IL-6 has been reported as a result of therapy with IL-1 inhibitors, occurring as early as 1 week after anakinra initiation (37) and 3 days after the administration of canakinumab (38). Down-modulation of IL-6 in patients with sJIA on canakinumab therapy (switching from tocilizumab) has been shown to persist for 24 and 48 weeks (9).
Therefore, the benefits of switching from an IL-6 inhibitor to an IL-1 inhibitor and, in particular from tocilizumab to canakinumab can potentially be explained by: IL-1-mediated links in the pathogenesis of sJIA; unequal sensitivity to tocilizumab in patients and the effect exerted by IL-1 inhibitors on the sJIA mechanisms associated both with IL-1 and IL-6.
4.3.2. Safety of switching from tocilizumab to canakinumab
In our study, AEs were reported in more than 40% of patients. According to the findings from clinical studies in patients with sJIA who received canakinumab after tocilizumab, AEs were reported in 78%–100% of patients (9, 17). This difference can be explained by the study design and, in particular, using medical records to estimate the AEs. There is no doubt that routinely collected health data include only the most remarkable deviations in the health status, which, in the physicians' opinion, are obviously related to the prognosis. In this regard, our data on the frequency of AEs are consistent with the rates of serious AEs reported in the study by Nishimura K. et al. (42%) (9). According to the German BiKeR Registry, the rate of serious adverse events with canakinumab was even lower, 20 cases per 100 patient-years (39).
It should be separately noted that in our study, 11% of patients developed MAS while using canakinumab and that this is higher than the rate observed in clinical trials of canakinumab (5.3% or 2.8 per 100 patient-years) (40) where the incidence rate of (probable) MAS observed in canakinumab-treated patients was comparable to the incidence reported in patients in the placebo group. In part, the higher incidence of MAS in our study may be due to the limited diagnostic accuracy of the criteria for this condition (over-diagnosis) (41). Indeed, in small-scale studies [with < 100 subjects (27, 42, 43)] the frequency of reported cases of MAS significantly exceeds that reported in large-scale studies [> 100 patients (44–46)] (12%–20% vs. 4%–7%, respectively).
5. Conclusion
One-year canakinumab therapy was found to be potentially effective as second-line biologic therapy after discontinuation of tocilizumab in patients with sJIA. Efficient treatment with canakinumab was achieved along with a decrease in the use of cDMARDs, such as oral corticosteroids and methotrexate. The “survival” of canakinumab therapy during the follow-up was high, with only one case of drug withdrawal. At least one AE (most frequently – infections) was reported in more than 40% of patients. Long-term data needs to be evaluated to see the benefit of canakinumab in patients with sJIA over a longer period.
Data availability statement
The datasets presented in this article are not readily available because Open study data sharing is not applicable due to local regulations. Requests to access the datasets should be directed to Ekaterina Alexeeva,YWxla2F0eWFAeWFuZGV4LnJ1.
Ethics statement
The studies involving human participants were reviewed and approved by Approved by the local Ethics Committee of the National Medical Research Centre of Children's Health. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
CRediT author statement: Conceptualization: KA, RS, Data curation: RS, Formal analysis: RS, Funding acquisition: EA, Investigation: TDKI, AC, EC, OL, RD, AM, AF, MG, DV, Methodology: RS, EA, Project administration: EA, Resources, Software, Supervision: EA. Validation. Visualization: Writing – original draft: EA, EK, RS. Writing – review & editing: RS, EA, EK. All authors contributed to the article and approved the submitted version.
Funding
Funding for medical writing support in the development of the manuscript was provided by Novartis Pharma AG. The funder of medical writing support was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
Medical writing support, under the direction of the authors, was provided by Sarah Johnston of Ashfield MedComms, an Ashfield Health company, Yakov Pakhomov, and Nikolay Tabakaev of SARL MAG.
Conflict of interest
Ekaterina Alexeeva has received grant/research support from Roche, Pfizer, Centocor, Eli Lilly, and Novartis, and has participated in Speakers bureau sessions for Roche, AbbVie, AbbVie, Bristol-Myers, Squibb, MSD, Novartis and Pfizer. Tatyana Dvoryakovskaya has received grant/research support from Roche, Pfizer, Centocor, Eli Lilly, and Novartis, and has participated in Speakers bureau sessions for Roche, AbbVie, Bristol-Myers, Squibb, MSD, Novartis and Pfizer. Ksenia Isaeva has received grant/research support from Roche, Novartis and Sanofi. Olga Lomakina has received grant/research support from Pfizer, Eli Lilly. Rina Denisova has received grant/research support from Roche, Pfizer, Centocor, Sanofi and Novartis, and has participated in Speakers bureau sessions for Roche, AbbVie, MSD, Novartis. Anna Mamutova has received grant/research support from Eli Lilly has participated in Speakers bureau sessions for Novartis. Anna Fetisova has received grant/research support from Amgen and AbbVie. Elizaveta Krekhova, Aleksandra Chomakhidze, Evgeniya Chistyakova, Marina Gautier, Dariya Vankova, Ivan Kriulin, and Ruslan Saygitov confirm that s(he) has no competing interests to declare.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Otten MH, Prince FH, Anink J, Ten Cate R, Hoppenreijs EP, Armbrust W, et al. Effectiveness and safety of a second and third biological agent after failing etanercept in juvenile idiopathic arthritis: results from the Dutch national ABC register. Ann Rheum Dis. (2013) 72(5):721–7. doi: 10.1136/annrheumdis-2011-201060
2. Woerner A, Uettwiller F, Melki I, Mouy R, Wouters C, Bader-Meunier B, et al. Biological treatment in systemic juvenile idiopathic arthritis: achievement of inactive disease or clinical remission on a first, second or third biological agent. RMD Open. (2015) 1(1):e000036. doi: 10.1136/rmdopen-2014-000036
3. Kearsley-Fleet L, Heaf E, Davies R, Baildam E, Beresford MW, Foster HE, et al. Frequency of biologic switching and the outcomes of switching in children and young people with juvenile idiopathic arthritis: a national cohort study. Lancet Rheumatol. (2020) 2(4):e217–26. doi: 10.1016/S2665-9913(20)30025-4
4. Alekseeva I, Lomakina ОL, Valieva SI, Bzarova ТМ, Nikishina IP, Zholobova Ye S, et al. Features of the drug therapy of children with systemic juvenile idiopathic arthritis: analysis results of the all-Russian register of the union of pediatricians of Russia. Voprosy Sovremennoi Pediatrii — Curr Pediatr. (2016) 15(1):59–67. doi: 10.15690/vsp.v15i1.1500
5. Ringold S, Weiss PF, Beukelman T, DeWitt EM, Ilowite NT, Kimura Y, et al. American college of rheumatology. 2013 update of the 2011 American college of rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum. (2013) 65(10):2499–512. doi: 10.1002/art.38092
6. NHS England. Clinical commissioning policy statement: biologic therapies for the treatment of juvenile idiopathic arthritis. 2015. Retrieved March 25, 2021, from https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/10/e03pd-bio-therapies-jia-oct15.pdf
7. Systemic-onset juvenile arthritis. Clinical guidelines, 2021. Retrieved May 26, 2021, from https://aspirre-russia.ru/upload/medialibrary/e44/КР%20сЮИА_АДР.pdf
8. Takei S, Hara R, Umebayashi H, Iwata N, Imagawa T, Shimizu M, et al. THU0599 Evaluation of efficacy and safety of canakinumab in Japanese patients with systemic juvenile idiopathic arthritis in phase iii clinical trial, composed predominantly of patients with prior use of tocilizumab. Ann Rheum Dis. (2018) 77:499–500. doi: 10.1136/annrheumdis-2018-eular.2464
9. Nishimura K, Hara R, Umebayashi H, Takei S, Iwata N, Imagawa T, et al. Efficacy and safety of canakinumab in systemic juvenile idiopathic arthritis: 48-week results from an open-label phase III study in Japanese patients. Mod Rheumatol. (2021) 31(1):226–34. doi: 10.1080/14397595.2020.1783163
10. Brunner HI, Ruperto N, Quartier P, Constantin T, Berkun Y, Calvo-Penedes I, et al. Efficacy of canakinumab in systemic juvenile idiopathic arthritis patients previously exposed to biologics [abstract]. Arthritis Rheumatol. (2015) 67(suppl 10):1255–6. Retrieved March 23, 2021, from https://acrabstracts.org/abstract/efficacy-of-canakinumab-in-systemic-juvenile-idiopathic-arthritis-patients-previously-exposed-to-biologics/. doi: 10.1002/art.39448
11. Quartier P, Alexeeva E, Constantin T, Chasnyk V, Wulffraat N, Palmblad K, et al. Paediatric rheumatology international trials organisation and the pediatric rheumatology collaborative study group. Tapering canakinumab monotherapy in patients with systemic juvenile idiopathic arthritis in clinical remission: results from a phase IIIb/IV open-label, randomized study. Arthritis Rheumatol. (2021) 73(2):336–46. doi: 10.1002/art.41488
12. Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. (1997) 40(7):1202–9. doi: 10.1002/art.1780400703
13. Consolaro A, Negro G, Chiara Gallo M, Bracciolini G, Ferrari C, Schiappapietra B, et al. Defining criteria for disease activity states in nonsystemic juvenile idiopathic arthritis based on a three-variable juvenile arthritis disease activity score. Arthritis Care Res (Hoboken). (2014) 66(11):1703–9. doi: 10.1002/acr.22393
14. Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N. Childhood arthritis rheumatology research alliance; pediatric rheumatology collaborative study group; paediatric rheumatology international trials organisation. American college of rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). (2011) 63(7):929–36. doi: 10.1002/acr.20497
15. Ruperto N, Ravelli A, Pistorio A, Malattia C, Cavuto S, Gado-West L, et al. Cross-cultural adaptation and psychometric evaluation of the childhood health assessment questionnaire (CHAQ) and the child health questionnaire (CHQ) in 32 countries. Review of the general methodology. Clin Exp Rheumatol. (2001) 19(4 Suppl 23):S1–9. PMID: 11510308
16. Langan SM, Schmidt SA, Wing K, Ehrenstein V, Nicholls SG, Filion KB, et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). Br Med J. (2018) 363:k3532. doi: 10.1136/bmj.k3532
17. Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Lovell DJ; PRINTO; PRCSG. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. (2012) 367(25):2396–406. doi: 10.1056/NEJMoa1205099
18. Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. (2005) 201:1479–86. doi: 10.1084/jem.20050473
19. Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. (2000) 191(2):313–20. doi: 10.1084/jem.191.2.313
20. Ohshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Nomura S, et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci U S A. (1998) 95(14):8222–6. doi: 10.1073/pnas.95.14.8222
21. Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, Mori M, et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. (2005) 52(3):818–25. doi: 10.1002/art.20944
22. Oike T, Kanagawa H, Sato Y, Kobayashi T, Nkatsukasa H, Miyamoto K, et al. IL-6, IL-17 and Stat3 are required for auto-inflammatory syndrome development in mouse. Sci Rep. (2018) 8:15783. doi: 10.1038/s41598-018-34173-5
23. Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, et al. Igg1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci U S A. (1989) 86(19):7547–51. doi: 10.1073/pnas.86.19.7547
24. De Benedetti F, Rucci N, Del Fattore A, Peruzzi B, Paro R, Longo M, et al. Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. (2006) 54(11):3551–63. doi: 10.1002/art.22175
25. Fantuzzi G, Dinarello CA. The inflammatory response in interleukin-1 beta-deficient mice: comparison with other cytokine-related knock-out mice. J Leukoc Biol. (1996) 59(4):489–93. doi: 10.1002/jlb.59.4.489
26. Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, et al. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. (1994) 180(4):1243–50. doi: 10.1084/jem.180.4.1243
27. Fall N, Barnes M, Thornton S, Luyrink L, Olson J, Ilowite NT, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. (2007) 56(11):3793–804. doi: 10.1002/art
28. Shimamoto K, Ito T, Ozaki Y, Amuro H, Tanaka A, Nishizawa T, et al. Serum interleukin 6 before and after therapy with tocilizumab is a principal biomarker in patients with rheumatoid arthritis. J Rheumatol. (2013) 40(7):1074–81. doi: 10.3899/jrheum.121389
29. Uno K, Yoshizaki K, Iwahashi M, Yamana J, Yamana S, Tanigawa M, et al. Pretreatment prediction of individual rheumatoid arthritis Patients’ response to anti-cytokine therapy using Serum cytokine/chemokine/soluble receptor biomarkers. PloS one. (2015) 10(7):e0132055. doi: 10.1371/journal.pone.0132055
30. Diaz-Torne C, Ortiz MDA, Moya P, Hernandez MV, Reina D, Castellvi I, et al. The combination of IL-6 and its soluble receptor is associated with the response of rheumatoid arthritis patients to tocilizumab. Semin Arthritis Rheum. (2018) 47(6):757–64. doi: 10.1016/j.semarthrit.2017.10.022
31. Nishina N, Kikuchi J, Hashizume M, Yoshimoto K, Kameda H, Takeuchi T. Baseline levels of soluble interleukin-6 receptor predict clinical remission in patients with rheumatoid arthritis treated with tocilizumab: implications for molecular targeted therapy. Ann Rheum Dis. (2013) 73(5):945–7. doi: 10.1136/annrheumdis-2013-204137
32. Jostock T, Müllberg J, Ozbek S, Atreya R, Blinn G, Voltz N, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. (2001) 268(1):160–7. doi: 10.1046/j.1432-1327.2001.01867.x
33. Mihara M, Kasutani K, Okazaki M, Nakamura A, Kawai S, Sugimoto M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. (2005) 5(12):1731–40. doi: 10.1016/j.intimp.2005.05.010
34. Doeleman MJH, van Maarseveen EM, Swart JF. Immunogenicity of biologic agents in juvenile idiopathic arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). (2019) 58(10):1839–49. doi: 10.1093/rheumatology/kez030
35. Sun H, Van LM, Floch D, Jiang X, Klein UR, Abrams K, et al. Pharmacokinetics and pharmacodynamics of canakinumab in patients with systemic juvenile idiopathic arthritis. J Clin Pharmacol. (2016) 56(12):1516–27. doi: 10.1002/jcph.754
36. Okano T, Inui K, Tada M, Sugioka Y, Mamoto K, Wakitani S, et al. Levels of interleukin-1 beta can predict response to tocilizumab therapy in rheumatoid arthritis: the PETITE (predictors of effectiveness of tocilizumab therapy) study. Rheumatol Int. (2016) 36(3):349–57. doi: 10.1007/s00296-015-3379-x
37. Gattorno M, Piccini A, Lasigliè D, Tassi S, Brisca G, Carta S, et al. The pattern of response to anti–interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. (2008) 58:1505–15. doi: 10.1002/art.23437
38. Brachat AH, Grom AA, Wulffraat N, Brunner I, Quartier P, Brik R, et al. Early changes in gene expression and inflammatory proteins in systemic juvenile idiopathic arthritis patients on canakinumab therapy. Arthritis Res Ther. (2017) 19:13. doi: 10.1186/s13075-016-1212-x
39. Klein A, Klotsche J, Hügle B, Minden K, Hospach A, Weller-Heinemann F, et al. Long-term surveillance of biologic therapies in systemic-onset juvenile idiopathic arthritis: data from the German BIKER registry. Rheumatology (Oxford). (2020) 59(9):2287–98. doi: 10.1093/rheumatology/kez577
40. Grom AA, Ilowite NT, Pascual V, Brunner HI, Martini A, Lovell D, et al. Rate and clinical presentation of macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis treated with canakinumab. Arthritis Rheumatol. (2016) 68(1):218–28. doi: 10.1002/art.39407
41. Schulert GS, Minoia F, Bohnsack J, Cron RQ, Hashad S, KonÉ-Paut I, et al. Effect of biologic therapy on clinical and laboratory features of macrophage activation syndrome associated with systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). (2018) 70(3):409–19. doi: 10.1002/acr.23277
42. Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. (2007) 34(5):1133–8. PMID: 17343315
43. Shimizu M, Nakagishi Y, Inoue N, Mizuta M, Ko G, Saikawa Y, et al. Interleukin-18 for predicting the development of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Clin Immunol. (2015) 160(2):277–81. doi: 10.1016/j.clim.2015.06.005
44. Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child. (2001) 85(5):421–6. doi: 10.1136/adc.85.5.421
45. Yokota S, Itoh Y, Morio T, Sumitomo N, Daimaru K, Minota S. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis under treatment with tocilizumab. J Rheumatol. (2015) 42(4):712–22. doi: 10.3899/jrheum.140288
46. Moussa T, Abdelhak M, Edens C. How do pediatric rheumatologists diagnose macrophage activation syndrome in systemic onset juvenile idiopathic arthritis? An examination of the CARRA registry [abstract]. Arthritis Rheumatol. (2020) 72(suppl 4):133–5. https://acrabstracts.org/abstract/how-do-pediatric-rheumatologists-diagnose-macrophage-activation-syndrome-in-systemic-onset-juvenile-idiopathic-arthritis-an-examination-of-the-carra-registry/ Accessed 24 March 2021. doi: 10.1002/art.41304
Keywords: systemic juvenile idiopathic arthritis, tocilizumab, canakinumab, second-line biologic, switching
Citation: Alexeeva E, Krekhova E, Dvoryakovskaya T, Isaeva K, Chomakhidze A, Chistyakova E, Lomakina O, Denisova R, Mamutova A, Fetisova A, Gautier M, Vankova D, Kriulin I and Saygitov R (2023) Efficacy and safety of canakinumab as a second line biologic after tocilizumab treatment failure in children with systemic juvenile idiopathic arthritis: A single-centre cohort study using routinely collected health data. Front. Pediatr. 11:1114207. doi: 10.3389/fped.2023.1114207
Received: 2 December 2022; Accepted: 24 January 2023;
Published: 22 February 2023.
Edited by:
Erkan Demirkaya, Western University, CanadaReviewed by:
Lianne Kearsley-Fleet, The University of Manchester, United KingdomKübra Öztürk, Istanbul Medeniyet University Göztepe Prof Dr Süleyman Yalçın City Hospital, Türkiye
© 2023 Alexeeva, Krekhova, Dvoryakovskaya, Isaeva, Chomakhidze, Chistyakova, Lomakina, Denisova, Mamutova, Fetisova, Gautier, Vankova, Kriulin and Saygitov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ekaterina Alexeeva YWxla2F0eWFAeWFuZGV4LnJ1
Specialty Section: This article was submitted to Pediatric Rheumatology, a section of the journal Frontiers in Pediatrics
 Ekaterina Alexeeva
Ekaterina Alexeeva Elizaveta Krekhova
Elizaveta Krekhova Tatyana Dvoryakovskaya1,2,3
Tatyana Dvoryakovskaya1,2,3