- 1Department of Otolaryngology, Children's Hospital of Chongqing Medical University, Chongqing, China
- 2Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing, China
- 3National Clinical Research Center for Child Health and Disorders, Chongqing, China
- 4China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Chongqing, China
- 5Department of Physical Examination, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Objective: The aim of this study was to describe a novel surgical technique of endoscopic percutaneous repair in pediatric patients with type 1, type 2 and type 3 laryngeal cleft (LC).
Methods: A retrospective study involving 12 patients with LC was performed at a tertiary pediatric hospital between February 2021 and June 2022. Endoscopic percutaneous repair was performed in all the patients. Information such as demographics, comorbidities, history of tracheostomy and the open approach for the repair, type of cleft and complications were analyzed.
Results: Twelve patients were diagnosed with LC. The median age of the patients at the time of surgery was 8.50 months (interquartile range, 49.50 months). Seven patients had tracheomalacia, four patients had subglottic stenosis, three patients had laryngomalacia. No surgical complications occurred in the 10 patients who underwent the primary procedure. For two patients who underwent a secondary procedure, endoscopic percutaneous repair failed again to heal the cleft. During the follow-up period after surgery, none of the patients had stridor, recurrent pneumonia, feeding difficulties, or dyspnea. Follow-up modified barium swallow postoperatively demonstrated no aspiration in 10 patients. Only the 2 patients with a secondary procedure had intermittent cough while taking large gulps of water. The cure rate of endoscopic percutaneous repairer was 83.3% (95% confidence interval: 73.9%–92.8%).
Conclusion: Endoscopic percutaneous repair should be considered as an alternative to the open transcervical approach and the traditional endoscopic approach for type 1, type 2 and type 3 LC.
Introduction
Laryngeal clefts (LC) are rare congenital airway anomalies that were charactered by a craniocaudal fissure in the separation between the laryngotracheal airway and pharyngoesophageal tract. LC were first reported by Richter in 1792 (1). Symptoms include aspiration, recurrent pneumonia and feeding difficulties. The Benjamin and Inglis classification is the most widely used system to describe LCs as follows: type 1, interarytenoid defect; type 2, partial defect in the cricoid cartilage; type 3, complete defect of the cricoid cartilage with or without cervical tracheal involvement; and type 4, extending into the thoracic trachea (2). For type 1, type 2 and most type 3 LC, the endoscopic repair is the preferred option (3–6). However, repairing using traditional endoscopic approaches, especially for type 3 LC, is difficult. For some type 3 LC, the open transcervical approach for the repair is generally recommended (7, 8). But the anterior midline incision of the larynx and trachea may affect the stability of the airway structure (4, 5). To address this issue, we were inspired by the endoscopic percutaneous suture lateralization for neonatal bilateral vocal folds described by Montague et al. (9), in which one end of the suture was pierced percutaneously through the airway by a 22-gauge needle and then removed from the skin using another tractive suture. Similarly, we hypothesized that if one end of the suture was pierced percutaneously through the airway and the esophagus and then pulled out by another tractive suture, “simple suture procedures of LC” could be achieved. Herein, we describe a novel technique for endoscopic percutaneous repair of type 1, Type 2 and type 3 LC.
Materials and methods
The present study retrospectively reviewed the medical records of patients who underwent endoscopic percutaneous repair of LC at the Children's Hospital of Chongqing Medical University between February 2021 and June 2022. Their data were analyzed from February 2022 to July 2022. Information on age, sex, gestational age, comorbidities, history of tracheostomy, open approach for repair, type of cleft, main symptoms, and complications was collected. The extent of the cleft was examined during the operation. The Institutional Review Board of Chongqing Medical University approved the study protocol. The study was complied with the Helsinki Declaration of 1975 (revised in 2008).
All the patients underwent endoscopic percutaneous repair of LC. Surgical repair was performed under general intravenous anesthesia using propofol and spontaneous respiration. The vocal cords are also anesthetized with topical lidocaine (2 mg/kg). All operations were performed under a Benjamin-Holinger (Storz) laryngoscope and 4 mm 0° telescope.
First, needle and suture were prepared. A single strand of 4-0 or 5-0 Medtronic absorbable suture (suture #1) was placed in a 22-gauge indwelling needle (BD). The tip of the suture #1 was slightly exposed to the bevel of the needle (Figure 1A). A single strand of 5-0 Prolene suture (suture #2) was placed in a 22-gauge indwelling needle. Through the bevel of the needle, suture #2 was folded back into two strands, and a loop was formed at the front end (Figure 1B).
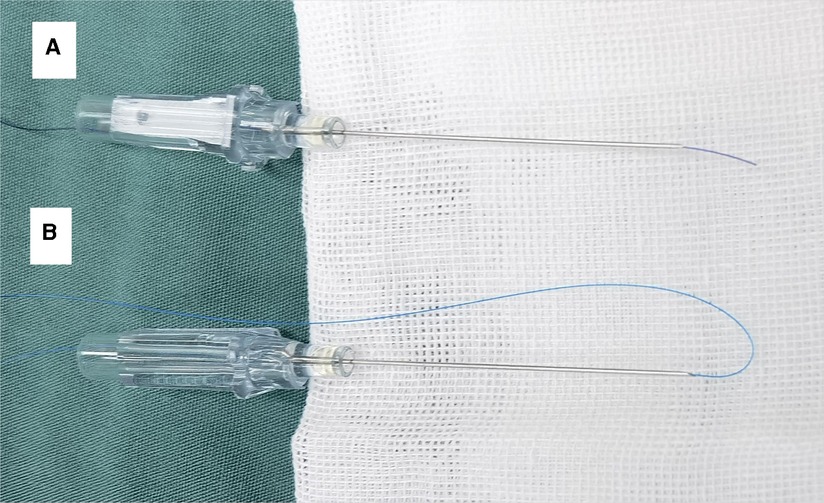
Figure 1. Needle and suture were prepared: (A) suture #1 (4-0 medtronic absorbable suture) was placed in a 22-gauge indwelling needle, and the tip of the suture #1 was slightly exposed to the bevel of the needle; (B) suture #2 (5–0 prolene suture) was placed in a 22-gauge indwelling needle, and a loop was formed at the front end.
Second, fresh wounds at the free edge of the laryngeal fissure mucosa were created. The edge and apex of the cleft were then denuded by needle-tip electrocautery (Storz) (10). Subsequently, a normal saline cotton ball was used to wipe the wound surface to make fresh wound (Figure 2).
Third, cleft was closed percutaneously. A 22-gauge needle with suture #1 was placed percutaneously within about 3 mm to the left of the midline of the neck. After entering the airway, the needle tip then penetrated the mucosal at the left edge of the cleft (approximately 0.5 mm from the needle insertion point to the free edge of cleft) and entered the esophagus (Figure 3A). The single-strand suture #1 was then drawn into the esophagus with laryngeal forceps, and the 22-gauge needle was withdrawn from the esophagus and airway (Figure 3B). Similarly, another 22-gauge needle with suture #2 was placed percutaneously in the esophagus from the right side (Figure 3C). The loop of suture #2 was drawn into the esophagus, and the 22-gauge needle was withdrawn (Figure 3D). The end of suture #1 was passed though the loop of suture #2 (Figure 3E). The loop of suture #2 was then drawn into the airway with the end of suture #1 (Figure 3F). After suture #2 is removed, both ends of suture #1 in the airway are pulled out of the barrel of the Benjamin-Holinger laryngoscope (Figures 3G–H). When tying the knots, a knot pusher (Storz) was routinely used. The knot was tied to the side of the airway (Figure 3I). The cleft was closed, from the caudal to the cranial part, with a single layer. Approximately 1–6 sutures were used depending on the extent of the cleft.
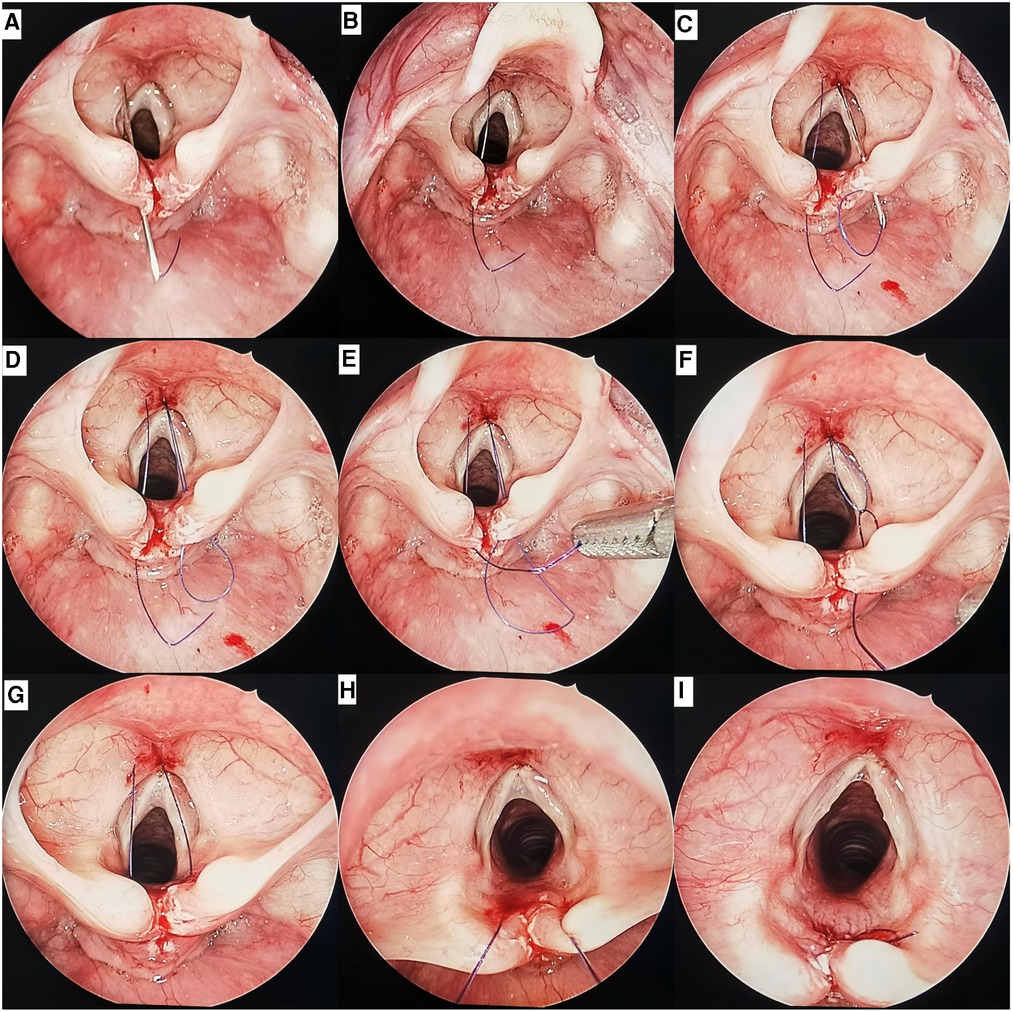
Figure 3. Endoscopic views of a type 1 cleft by endoscopic percutaneous repair: (A) the 22-gauge needle with suture #1 inserted into the airway percutaneously, then penetrated the mucosal at the left edge of the cleft and entered the esophagus. (B) The end of suture #1 was pulled into the esophagus, the 22-gauge needle was withdrawn. (C) The 22-gauge needle with suture #2 inserted into the airway percutaneously, then penetrated the mucosal at the right edge of the cleft and entered the esophagus. (D) The loop of suture #2 was pulled into the esophagus, the 22-gauge needle was withdrawn. (E) The end of suture #1 was passed though the loop of suture #2. (F) The end of suture #1 was pulled into the airway by the loop of suture #2. (G) Both ends of suture #1 were located in the airway. (H) The both ends of suture #1 was pulled out of the barrel. (I) The knot was tied on the side of the airway.
After the operation, three patients with type 3 LC without tracheotomy were monitored in the pediatric intensive care unit for 3 days. The remaining patients return directly to the general ward postoperatively. Antibiotics were intravenously administered for 7 days. Systemic glucocorticoids (dexamethasone 0.5 mg/kg) were administered for 3 days. The patients were fed using a gastric tube immediately postoperatively and were switched to oral feeding 4 weeks later. Proton pump inhibitors were routinely administered for at least 4 weeks. All the patients underwent flexible fiberoptic laryngoscopy to assess the surgical incision 1 weeks postoperatively. Modified barium swallow (MBS) was performed to evaluate postoperative swallow function 3 months postoperatively. Descriptive statistics were used to analyze the overall group. All data are presented as medians and interquartile ranges (IQRs) for non-normally distributed continuous data. Categorical data are presented as percentages.
Results
A total of 12 patients (6 male and 6 female) who had LC were included in the present study (Table 1). The main symptoms were difficulties during feeding in 7 patients, recurrent pneumonia in 3 patients, stridor in 4 patients and dyspnea in 3 patients with type 3 LC. Congenital anomalies were identified in 6 patients (50%); 4 patients had subglottic stenosis; 3 patients had VACTERL (vertebral, anal, cardiac, tracheal, esophageal, renal, and limb anomalies) association; and 1 patient had congenital tracheal stenoses. According to the Myer-Cotton classification system, the 4 patients with subglottic stenosis were all grade I (0%–50% obstruction of subglottic airway) (11). The associated comorbidities were observed in 10 patients (83%): 7 patients had tracheomalacia, 3 patients had laryngomalacia, 2 patients had pharyngomalacia, 2 patients had bronchiectasis and atelectasis, and 2 patients had gastroesophageal reflux disease. According to the Benjamin–Inglis classification system (2), 5 patients had type 1 LC, 2 patients had type 2 LC, 5 patients had type 3 LC, and none of the patients had type 4 LC. In 3 patients with type 3 LC, the cleft extended through the cricoid cartilage and ended above the first tracheal ring. In 2 patients with type 3 LC, the cleft passed to the first tracheal ring and ended above the second tracheal ring (Table 2). The median age of the patients at the time of surgery was 8.50 months (IQR, 49.50 months). Of the 12 patients treated using the endoscopic percutaneous approach, 10 patients (83%) were treated with a primary procedure and 2 patients (17%) with a secondary procedure after a previous surgery using an open transcervical approach with tracheostomy. The median operative time of endoscopic percutaneous repair in the series was 89.00 min (IQR, 51.25 min). No surgical complications occurred in any of the patients who underwent a primary procedure. For 2 patients who underwent a secondary procedure, endoscopic percutaneous repair failed again to heal the cleft. The cleft was repaired using the second open transcervical approach. The tracheostomy tube was successfully removed 3 months postoperatively. During the follow-up period, none of the patients had stridor, recurrent pneumonia, feeding difficulties, or dyspnea postoperatively. All the patients had aspiration of nectar-thick liquids, as documented by MBS before the repair. Follow-up MBS postoperatively indicated no aspiration in 10 patients. Only the 2 patients who underwent a secondary procedure had intermittent cough while taking in large gulps of water. The cure rate of endoscopic percutaneous repair was 83.3% (95% confidence interval: 73.9%-92.8%).
Discussion
The present study demonstrated that endoscopic percutaneous repair of the LC is a feasible technique. During the surgery, the cleft was successfully closed in all patients, and no surgical complications occurred. Apart from two patients who required open transcervical repair, none of the other patients had secondary dehiscence.
Endoscopic percutaneous repair has clear advantages over open transcervical surgery. Firstly, anterior incision of the larynx and trachea was avoided, thus reducing the potential risk of laryngotracheal frame instability (4, 5, 12). Secondly, the endoscopic percutaneous approach may avoid postoperative tracheal intubation or tracheostomy (4, 13, 14). Lastly, some of the risks associated with the open transcervical approach, including recurrent laryngeal nerve injury, neck infection, and pharyngeal fistula, are rare (5).
In our experience, the deepest part of the type 3 LC has insufficient space, and repair of this part using the traditional endoscopic approach is very difficult. To ensure operability of the procedure, a smaller suture needle, such as a 6-0 Vicryl suture on a P-1 needle, was selected (4, 5). The smaller suture needle is likely to be completely submerged into the tissue, and the needle cannot be released. Moreover, the needle holder cannot hold the suture needle firmly, which may easily cause it to fall off. Therefore, less tissue is sutured at the edge of the cleft, which may increase the risk of secondary dehiscence after traditional endoscopic repair. Endoscopic percutaneous repair can solve the aforementioned problems with the traditional endoscopic approach. The 22-gauge indwelling needle can be freely moved in and out of the larynx and trachea, ensuring that as much tissue as possible is sutured to both sides of the cleft. This increases the strength of the wound-healing scar tissue. Therefore, endoscopic percutaneous repair for type 3 LC has better advantages over the traditional endoscopic approach.
The endoscopic percutaneous approach depends on good endoscopic exposure. The lateral edges and distal end of the cleft must be visible and accessible for needle placement. For patients with the Pierre Robin syndrome, Treacher Collins syndrome, or Down syndrome, endoscopic exposure may be difficult (6, 15, 16), and an open approach needs to be considered.
In our series two of the five children with type 3 LC underwent three separate repairs before closure was successful. The initial management of the two children was through tracheotomy and transcervical repair. The mucosa at the lateral edges of the cleft was thin and brittle, which may interfere with healing after endoscopic percutaneous approach. The cleft was successfully repaired by second transcervical approach with costal cartilage grafting. In the second transcervical operation, the esophageal mucosa at the edge of the cleft was sufficiently dissociated to ensure that the thick and healthy mucosal tissue was sutured. Koltai et al. have reported that one child had two previous transcervical repairs of the type 2 LC that left the interarytenoid tissues thin, friable, and covered with granulations (17). Therefore, endoscopic percutaneous repair should be carefully considered for children with type 3 LC who have undergone repeated operations.
In our study, tracheomalacia, subglottic stenosis, and laryngomalacia were the most common airway comorbidities and anomalies. Seven of 12 patients (58%) had tracheomalacia. These tracheomalacia cases were mild and did not result in severe dyspnea. Our results demonstrated that the mild tracheomalacia did not have a significant effect on the repair of LC. For severe tracheomalacia, which results in severe dyspnea, tracheotomy may be required to create a safe airway before the repair of LC (5). A very strong correlation between LC and subglottic stenosis was identified. A previous study has reported that 26% of patients with LC have subglottic stenosis (18). Four of 12 patients (33%) in our series had subglottic stenosis, which were all grade I. Our results demonstrated that the grade I subglottic stenosis did not affect the success rate of LC repair in our series of patients. For the patients with LC and severe subglottic stenosis (grade II-IV), endoscopic percutaneous repair is not recommended. First, the airway is bound to become narrow after LC is closed (4). Second, endoscopic airway surgery will inevitably lead to varying degrees of mucosal edema (4). One millimeter of annular edema at the subglottic level reduces the cross-sectional area by nearly 60% (19). All of the abovementioned factors can lead to postoperative airway stenosis and long-term tube placement in patients with LC and severe subglottic stenosis. Therefore, we recommend open surgery with costal cartilage grafting for patients with LC and severe subglottic stenosis (20–22). Laryngomalacia is the common associated anomaly in LC (23). Three patients (25%) in our series had laryngomalacia. The upper glottic airway narrowed after LC closure. The aryepiglottic folds were divided to expand the airway during LC repair (24).
In conclusion, endoscopic percutaneous repair with the open transcervical approach significantly reduces perioperative and postoperative morbidity. Endoscopic percutaneous repair has better maneuverability and firmer sutures for type 3 LC than the traditional endoscopic approach. Sufficient exposure and a healthy mucosal structure at the lateral edges of the cleft are the major factors that should be considered when determining whether a patient is a candidate for endoscopic percutaneous repair. For the patients with LC and severe subglottic stenosis (grade II-IV), we recommend open repair rather than endoscopic percutaneous repair. Endoscopic percutaneous repair may be a suitable technique for the treatment of type 1, type 2, and type 3 LC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by The institutional review board of the Children's Hospital of Chongqing Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
XT: study design, data acquisition, analysis and interpretation, manuscript drafting, fnal approval, and accountability for all aspects of the work; YY: data acquisition, manuscript revising, final approval and accountability for all aspects; ZZ: data analysis and interpretation, manuscript revising, final approval and accountability for all aspects of the work; RS: study design, data analysis and interpretation, manuscript drafting, final approval and accountability for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Program for Youth Innovation in Future Medicine, Chongqing Medical University (W0128).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Richter CF. Dissertation medico de infanticide in artis obstetriciae [Dissertation /master's thesis]. Leipzig (SN): University of Leipzig (1792).
2. Benjamin B, Inglis A. Minor congenital laryngeal clefts: diagnosis and classification. Ann Otol Rhinol Laryngol. (1989) 98:417–20. doi: 10.1177/000348948909800603
3. Timashpolsky A, Schild SD, Ballard DP, Leventer SP, Rosenfeld RM, Plum AW. Management of type 1 laryngeal clefts: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. (2021) 164:489–500. doi: 10.1177/0194599820947742
4. Adil E, Al Shemari H, Rahbar R. Endoscopic surgical repair of type 3 laryngeal clefts. JAMA Otolaryngol Head Neck Surg. (2014) 140:1051–5. doi: 10.1001/jamaoto.2014.2421
5. Sandu K, Monnier P. Endoscopic laryngotracheal cleft repair without tracheotomy or intubation. Laryngoscope. (2006) 116:630–4. doi: 10.1097/01.mlg.0000200794.78614.87
6. Garabedian EN, Pezzettigotta S, Leboulanger N, Harris R, Nevoux J, Denoyelle F, et al. Endoscopic surgical treatment of laryngotracheal clefts: indications and limitations. Arch Otolaryngol Head Neck Surg. (2010) 136:70–4. doi: 10.1001/archoto.2009.197
7. Kawaguchi AL, Donahoe PK, Ryan DP. Management and long-term follow-up of patients with types III and IV laryngotracheoesophageal clefts. J Pediatr Surg. (2005) 40:158–64.; discussion 164-5. doi: 10.1016/j.jpedsurg.2004.09.041
8. Kubba H, Gibson D, Bailey M, Hartley B. Techniques and outcomes of laryngeal cleft repair: an update to the great ormond street hospital series. Ann Otol Rhinol Laryngol. (2005) 114:309–13. doi: 10.1177/000348940511400410
9. Montague GL, Bly RA, Nadaraja GS, Conrad DE, Parikh SR, Chan DK. Endoscopic percutaneous suture lateralization for neonatal bilateral vocal fold immobility. Int J Pediatr Otorhinolaryngol. (2018) 108:120–4. doi: 10.1016/j.ijporl.2018.02.032
10. Fracchia MS, Diercks G, Yamasaki A, Hersh C, Hardy S, Hartnick M, et al. Assessment of the feeding swallowing impact survey as a quality of life measure in children with laryngeal cleft before and after repair. Int J Pediatr Otorhinolaryngol. (2017) 99:73–7. doi: 10.1016/j.ijporl.2017.05.016
11. Myer CM 3rd, O'Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol. (1994) 103:319–23. doi: 10.1177/000348949410300410
12. Cotton RT, Schreiber JT. Management of laryngotracheoesophageal cleft. Ann Otol Rhinol Laryngol. (1981) 90:401–5. doi: 10.1177/000348948109000424
13. DuBois JJ, Pokorny WJ, Harberg FJ, Smith RJ. Current management of laryngeal and laryngotracheoesophageal clefts. J Pediatr Surg. (1990) 25:855–60. doi: 10.1016/0022-3468(90)90191-B
14. Myer CM 3rd, Cotton RT, Holmes DK, Jackson RK. Laryngeal and laryngotracheoesophageal clefts: role of early surgical repair. Ann Otol Rhinol Laryngol. (1990) 99:98–104. doi: 10.1177/000348949009900203
15. Iguchi H, Sasano N, So M, Hirate H, Sasano H, Katsuya H. Orotracheal intubation with an AirWay scope in a patient with treacher collins syndrome. J Anesth. (2008) 22:186–8. doi: 10.1007/s00540-007-0598-7
16. Nakazawa K, Ikeda D, Ishikawa S, Makita K. A case of difficult airway due to lingual tonsillar hypertrophy in a patient with down's syndrome. Anesth Analg. (2003) 97:704–5. doi: 10.1213/01.ANE.0000074347.64382.A4
17. Koltai PJ, Morgan D, Evans JN. Endoscopic repair of supraglottic laryngeal clefts. Arch Otolaryngol Head Neck Surg. (1991) 117:273–8. doi: 10.1001/archotol.1991.01870150041004
18. de Alarcón A, Osborn AJ, Tabangin ME, Cohen AP, Hart CK, Cotton RT, et al. Laryngotracheal cleft repair in children with Complex airway anomalies. JAMA Otolaryngol Head Neck Surg. (2015) 141:828–33. doi: 10.1001/jamaoto.2015.1419
19. Holinger LD. Evaluation of stridor and wheezing. In: Holinger LD, Lusk RP, Green CG, editors. Pediatric laryngology and bronchoesophagology. Philadelphia, PA: Lippincott-Raven Press (1997). p. 42.
20. Garabedian EN, Ducroz V, Roger G, Denoyelle F. Posterior laryngeal clefts: preliminary report of a new surgical procedure using tibial periosteum as an interposition graft. Laryngoscope. (1998) 108:899–902. doi: 10.1097/00005537-199806000-00020
21. Younis RT, Lazar RH, Astor F. Posterior cartilage graft in single-stage laryngotracheal reconstruction. Otolaryngol Head Neck Surg. (2003) 129:168–75. doi: 10.1016/S0194-5998(03)00604-1
22. Balakrishnan K, Cheng E, de Alarcon A, Sidell DR, Hart CK, Rutter MJ. Outcomes and resource utilization of endoscopic mass-closure technique for laryngeal clefts. Otolaryngol Head Neck Surg. (2015) 153:119–23. doi: 10.1177/0194599815576718
23. van der Doef HP, Yntema JB, van den Hoogen FJ, Marres HA. Clinical aspects of type 1 posterior laryngeal clefts: literature review and a report of 31 patients. Laryngoscope. (2007) 117:859–63. doi: 10.1097/MLG.0b013e318033c2e9
Keywords: laryngeal cleft, aspiration, dyspnea, endoscopic, repair
Citation: Tang X, Yang Y, Zhang Z and Sun R (2023) Endoscopic percutaneous repair of laryngeal cleft. Front. Pediatr. 11:1113894. doi: 10.3389/fped.2023.1113894
Received: 2 December 2022; Accepted: 8 February 2023;
Published: 23 February 2023.
Edited by:
Chao Chen, Fudan University, ChinaReviewed by:
David C. Van Der Zee, University Medical Center Utrecht, NetherlandsPatrick Stafler, Schneider Children's Medical Center, Israel
© 2023 Tang, Yang, Zhang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Sun ZW50MjAwMkAxNjMuY29t
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
 XinYe Tang1,2,3,4
XinYe Tang1,2,3,4 Rong Sun
Rong Sun