- 1Department of Pediatrics and Child Health Nursing, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 2School of Public Health, University of Technology Sydney, Sydney, NSW, Australia
- 3Social and Population Health Unit, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 4Department of Surgical Nursing, School of Nursing, College of Medicine and Health Sciences, and Specialized Hospital, University of Gondar, Gondar, Ethiopia
- 5Department of Pediatrics and Child Health Nursing, School of Nursing, College of Medicine and Health Sciences, Specialized Hospital, University of Gondar, Gondar, Ethiopia
Introduction: Globally, opportunistic infections are the leading causes of morbidity and mortality among HIV-infected children, contributing to more than 90% of HIV-related deaths. In 2014, Ethiopia launched and began to implement a “test and treat” strategy aiming to reduce the burden of opportunistic infections. Despite this intervention, opportunistic infections continue to be a serious public health issue, with limited evidence available on their overall incidence among HIV-infected children in the study area.
Objective: The study aimed to assess the incidence of opportunistic infections and to identify predictors of their occurrence among HIV-infected children receiving antiretroviral therapy at Amhara Regional State Comprehensive Specialized Hospitals in 2022.
Methods: A multicenter, institution-based retrospective follow-up study was conducted among 472 HIV-infected children receiving antiretroviral therapy at Amhara Regional State Comprehensive Specialized Hospitals from May 17 to June 15, 2022. Children receiving antiretroviral therapy were selected using a simple random sampling technique. Data were collected using national antiretroviral intake and follow-up forms via the KoBo Toolbox. STATA 16 was used for data analyses, and the Kaplan–Meier method was used to estimate probabilities of opportunistic infection-free survival. Both bi-variable and multivariable Cox proportional hazard models were employed to identify significant predictors. A P-value <0.05 was taken to indicate statistical significance.
Results: Medical records from a total of 452 children (representing a completeness rate of 95.8%) were included and analyzed in the study. The overall incidence of opportunistic infections among children receiving ART was 8.64 per 100 person-years of observation. The predictors of elevated incidence of opportunistic infections were: a CD4 cell count below a specified threshold [AHR: 2.34 (95% CI: 1.45, 3.76)]; co-morbidity of anemia [AHR: 1.68 (95% CI: 1.06, 2.67)]; ever having exhibited only fair or poor adherence to ART drugs [AHR: 2.31 (95% CI: 1.47, 3.63)]; never having taken tuberculosis-preventive therapy [AHR: 1.95 (95% CI: 1.27, 2.99)]; and not having initiated antiretroviral therapy within 7 days of HIV diagnosis [AHR: 1.82 (95% CI: 1.12, 2.96)].
Conclusion: In this study, the incidence of opportunistic infections was high. Early initiation antiretroviral therapy has direct effect on boosting the immunity, suppressing viral replications and increases the CD4 count, so that the occurrence of opportunistic infection will reduce the incidence of OIs.
Background
Opportunistic infections (OIs) occur more frequently and with higher severity among individuals with low immunity, such as people living with human immunodeficiency virus (PLHIV) (1, 2). Immunosuppression is the hallmark of HIV infection; this predisposes PLHIV to OIs and contributes greatly to HIV-related morbidity and mortality in HIV-infected children across the globe (3). Opportunistic infections mainly affect the neurological, gastrointestinal, respiratory, and integumentary systems (4, 5).
As per the 2020 statistical report on global HIV and acquired immune deficiency syndrome (AIDS), among 37.7 million PLHIV, 1.7 million were children (6); and, according to the 2019 report, nearly 95,000 HIV-infected children died, mainly due to OIs (7).
The incidence of HIV-related OIs remains high in developed and resource-limited countries, as illustrated by the incidence by person-year observation (PYO) in the United States of America (4.99), Latin America (1.1 and 23.5), Brazil (2.63), and Ethiopia (5.53 and 9.7) (8–13). Globally, OIs are the leading cause of HIV-associated morbidity and mortality, accounting for more than 90% of all HIV-related fatalities (5, 10, 14). In developed countries, the life expectancy of newly HIV-infected children receiving antiretroviral therapy (ART) has reached the lifespan of the general population (15), in contrast, sub-Saharan African children have shorter life expectancies due to the high burden of OIs (16, 17). Among all HIV-related OIs, pneumonia and tuberculosis (TB) are the leading causes of hospital admissions for HIV-infected children receiving ART in Europe and East China, and across low- and middle-income countries (4, 8, 12, 14, 18).
Existing studies conducted in the USA (5, 19), Brazil (20), India (21), Nigeria (22), Uganda (23), South Africa (24), Tanzania (25), and Ethiopia (8, 12, 26–29) have identified baseline cluster of differentiation 4 (CD4) cell counts, poor and fair ART adherence, not taking cotrimoxazole preventive therapy (CPT), TB preventive therapy (TPT), and co-morbidity of malnutrition as contributing factors for the occurrence of OIs, even in the ART era.
In developing countries, the increased rate of OIs among HIV-infected children is related to inadequate medical care; challenges in ART adherence; delays in the provision of effective HIV treatment (5); less advanced technology for early diagnosis, prevention, and management of OIs; and lack of prophylaxis (26). Unless OIs are diagnosed and treated early, they markedly affect the treatment outcomes of PLHIV, which leads to poor quality of life, hastening of disease progression, and increasing medical costs, creating the potential risk of treatment failure and impairing the patient's response to ART regimens (30, 31).
In response to this problem, Ethiopia has implemented the Health Sector Transformation Plan II (HSTP-II), with a target of ensuring that 95% of HIV patients are receiving ART in order to achieve effective viral suppression, which in turn should result in a reduction of the incidence of OIs by the year 2025 (32).
Similarly, the Federal Ministry of Health in Ethiopia sets strategies to reduce the occurrence of OIs through reduction of HIV exposure, preventive therapies, immunization (4), and the use of highly active antiretroviral therapy (HAART). Many initiatives have already been adopted to reduce the incidence of OIs based on the suggested solutions. However, OIs remain the main factor in morbidity and mortality of children with HIV. In the meantime, Ethiopia has also adopted a “test and treat” strategy that has been in operation since June 10, 2014, targeting initiation of ART for all children who test positive for HIV, regardless of their CD4 cell count or WHO clinical stage; the aim of this initiative is to reduce HIV-related mortality and morbidity due to OIs (33, 34).
Despite the implementation of the test and treat approach, there is limited evidence on the overall incidence of OIs at Amhara Regional State comprehensive specialized hospitals. In addition, previous studies have not considered rapid initiation of ART as an independent predictor of incidence of OIs.
This study was conducted in support of the Ethiopian HSTP-II goal of reducing HIV/AIDS and its complications by the end of 2025, as well as the targets of the Sustainable Development Goals (32). The results will help health professionals and clinicians by increasing existing knowledge, enabling prioritization of identified predictors, and identifying further measures for prevention and management of OIs. The study will provide input to program planners and decision-makers at various levels in the domain of HIV/AIDS care and support, and will also provide input for the implementation of regional and national programs. The findings will additionally serve as baseline data for further research.
Against this background, this study aimed to assess the incidence of opportunistic infections and the predictors of OI incidence among HIV-infected children receiving ART at Amhara Regional State comprehensive specialized hospitals, Ethiopia, in 2022.
Methods and materials
Study design, period, and setting
A multicenter institution-based retrospective follow-up study was conducted between May 17 and June 15, 2022. The study was conducted across seven Amhara Regional State comprehensive specialized hospitals (University of Gondar, Felege-Hiwot, Debre-Markos, Dessie, Woldia, Debre Birhan, and Debre Tabor).
The Amhara Region is one of the 11 regional states of Ethiopia and spans the northwestern, northeastern, and north-central parts of Ethiopia, with an estimated area of 159,173.66 square kilometers. It is administratively organized into 12 zones, three city administrations, and 183 woredas. According to the January 2022 Ethiopian Demographic and Health Survey report, the total population of the region is estimated at 30,848,988 (35). According to the annual performance report of the Amhara National Regional Health Bureau, the region has 858 health centers, 3,560 health posts, and 81 hospitals. These hospitals also have specialized units for ART care and related services (36).
Since 2005, the Amhara Regional State comprehensive specialized hospitals (CSHs) have been providing free ART services as part of the national AIDS control and prevention program. Seven of these hospitals were included in the study (the Tibebe-Ghion comprehensive specialized hospital was excluded due to an inadequate study population), and across these hospitals, 928 HIV-infected children were newly enrolled to receive ART from June 10, 2014, to February 28, 2022.
Population selection and participation
The source population was all HIV-infected children aged <15 years and on ART follow-up in CSHs; the study population consisted of those newly enrolled for ART between June 10, 2014, and February 28, 2022. Newly HIV-infected children who had started receiving ART within this period were included in the study. Records with incomplete baseline information (CD4 count, hemoglobin level, weight, and height), unknown date of ART initiation, or unknown outcome status were excluded from the study.
Sample size determination; sampling techniques and procedures
In order to determine the required sample size in relation to the first specific objective, we used a single-population proportion formula, considering an estimated incidence of opportunistic infections among HIV-infected children of 24.9%, which was taken from a study conducted at Debre Markos CSH 24.9% (12); this calculation yielded a sample size of 287.
For the second objective, the sample size was calculated on the basis of common significant predictor variables using Cox proportional hazard models, implemented in STATA version 16 (Table 1). This calculation yielded a sample size of 472 for the second objective, which was taken as the final required sample size for this study.
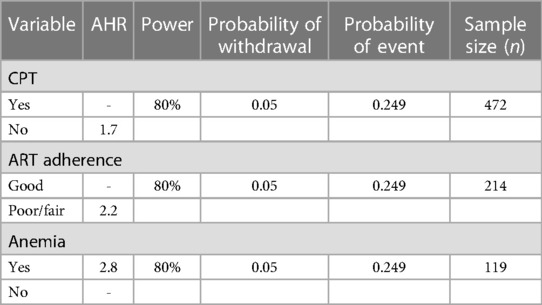
Table 1. Determination of estimated sample size required based on predictors of incidence of OIs among HIV-infected children receiving ART, using cox-model in STATA version 16.
Samples were drawn proportionally from each of the seven CSHs, and records were selected using simple random techniques (Figure 1).
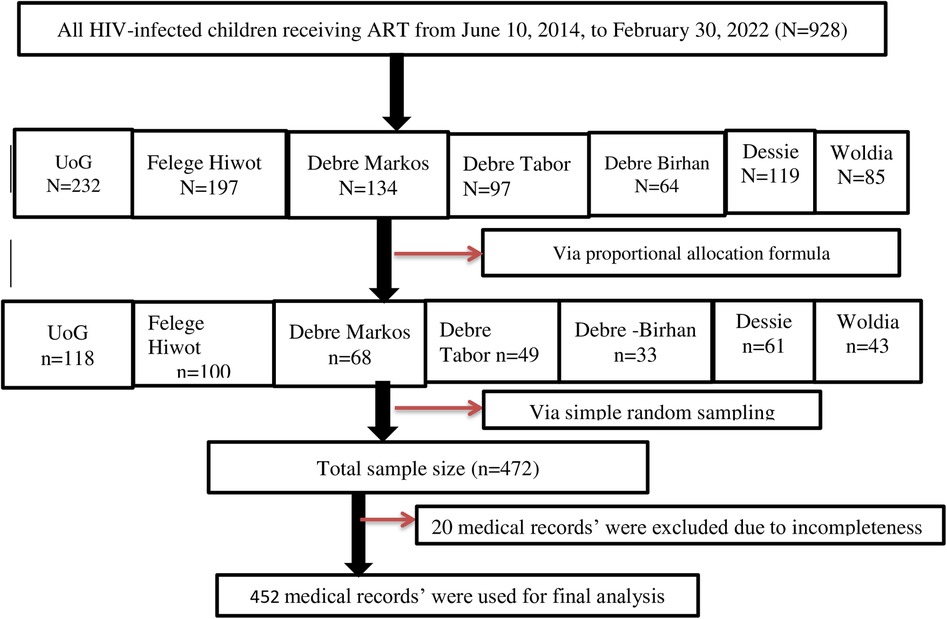
Figure 1. Schematic diagram of the sampling procedure for assessment of the incidence and predictors of OIs among HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals in Ethiopia in 2022.
Study variables and their measurements
The outcome variable was incidence of opportunistic infections among HIV-infected children receiving ART. The independent variables were: (1) sociodemographic characteristics (age, sex, residence, current HIV status of the parents, educational status of the caregiver, and marital status of the caregiver); (2) baseline clinical, nutritional, and laboratory characteristics (CD4 count, hemoglobin level, anthropometric indices, HIV disclosure status, functional and developmental status); (3) ART and medication-related characteristics: baseline ART regimen, presence of regimen change, level of ART adherence, receipt of TPT, receipt of CPT, ART side effects, and initiation of ART within seven days of diagnosis (4).
Opportunistic infections were considered for any type of infections with the clinical signs and symptoms of opportunistic disease occurring during the follow-up period (34). An OI event was deemed to have occurred when an HIV-infected child developed any OI after ART initiation during the follow-up period.
Survival was deemed to have occurred if the participant had no occurrence of any OI during the follow-up period.
Censored: Records of HIV-infected children (whether still living or not) were treated as censored if the child was transferred out to another health institution, was lost to follow-up, or was still on active ART follow-up but no evidence for any OI occurrence by the end of the study.
Time to develop OI was the time from ART initiation to the occurrence of an OI event during the follow-up period.
Stunting was recorded if the child had a height-for-age (HFA) or length-for-age (LFA) Z-score below −2 SD (34).
Wasting was recorded if weight-for-height (WFH) Z-score was less than −2 SD for children under 5 years old, or if body mass index (BMI)-for-age Z-score was less than −2 SD for children over 5 years old (34).
Level of adherence to ART was recorded as “good” in cases of compliance equal to or greater than 95% (or ≤ 3 missed doses per month); “fair” in cases of 85%–94% compliance (or between 4 and 8 missed doses per month); or “poor” in cases of less than 85% compliance (or ≥9 missed doses per month), as documented by ART health personnel in all cases (4).
Rapid initiation of ART was recorded in cases of initiation of ART care and support on the same day that HIV was confirmed or within 7 days (37).
Anemia (low hemoglobin level) was defined as a hemoglobin level of less than 10 mg/dl (12).
CD4 count or percentage (%) below the threshold was recorded based on thresholds specified by age category, as follows: CD4 cell count <1,500/mm3 or below 25% for children aged <12 months; CD4 cell count <750/mm3 or below 20% for children aged 12–35 months; CD4 cell count <350/mm3 or below 15% for children aged 36–59 months; and CD4 cell count <200/mm3 or below 15% for children aged age ≥60 months (34).
Incidence of OIs was calculated by dividing all newly occurring OI events by the total number of months of follow-up time across all patients.
Data collection tool, procedure, and quality control
The data extraction tool was pretested on 5% of the sample size (22) 2 weeks before the actual data collection period at UoG CSH. Additionally, a 1-day practical training session on how to review ART follow-up and medical records, data collection methods, and the objective of the study was provided for the data collectors and supervisors. Data were collected using the KoBo Toolbox, which was prepared with relevant restrictions by trained nurses working in the selected hospitals. In addition to the data collector, supervisors and the principal investigator carefully monitored the entire data collection process and daily submission reports.
Data were collected from ART intake and follow-up forms and from children's charts using a data extraction tool adopted from the 2021 Ethiopian ART guidelines (34). Data were collected using smartphones by seven nurses with bachelor's degrees and three supervisors who were familiar with the ART follow-up process and had received basic ART training. The occurrence of OIs was ascertained during data extraction by reviewing health professionals’ reports on the children's charts.
Data processing and analyses
The collected data were exported to STATA version 16 (MP) statistical software for management and analysis. The WHO anthro and anthroPlus software packages were used to generate anthropometric indices. Descriptive statistics (means, frequencies, and percentages) were computed and are presented in the tables and figures. In addition, a log-rank test was used to compare OI-free survival time for different levels of each categorical predictor variable. Multi-collinearity was tested by calculating the Variance Inflation Factor (VIF = 1.21) as a measure of the associations between predictor variables.
The assumptions of the Cox proportional hazard test were checked using Schoenfeld residuals and via a log-log plot all of the covariates (graphical check). The data fulfilled the assumptions, and the overall global model satisfied the proportional hazard assumption (global test, P = 0.9539).
The Cox–Snell residual test was used to check the goodness of fit. A residual is a difference between an observed data point and a predicted or fitted value; as the graph in Figure 2 indicates, the Cox regression model fit with the Cox–Snell residual and the predicted hazard at 45°.
At this time, all assumptions were fulfilled. Shared frailty (theta) was estimated to be significantly different from zero (theta: 4.00e-19; 95% CI: 0; Prob ≥ chibar2 = 0.5); this shows that the distribution of unmeasured variables did not differ between Comprehensive Specialized Hospitals. A multivariable Cox proportional hazards model was used to identify predictors. A 95% confidence interval (CI) for adjusted hazard ratio (AHR) was estimated to determine the strength of associations. A P-value ≤0.05 was taken to indicate statistical significance for associations between predictors and incidence of OIs.
Ethical considerations
Ethical clearance was obtained from the Institutional Review Board (IRB) of the University of Gondar on behalf of the Ethical Review Committee of the School of Nursing with ref. no. SN/1228/2014 on 03/05/2022 (G.C) and with ref. no. SN/1228/2014. A written permission letter was obtained from Amhara Public Health Institute for each hospital (ref. no. አሕጤኢ/ዋ/ዳ/03/1438). Finally, before the data were collected, a letter of permission was obtained from each of the respective CSH administrators to collect data from each ART clinic. At the time of data extraction, personal identifiers (names and contact numbers) were removed. The data were kept strictly confidential and used only for research purposes.
Results
Socio-demographic characteristics of HIV-infected children receiving ART
A total of 452 HIV-infected children receiving ART participated in the study; their medical records were complete in 95.8% of cases. The mean (±SD) age of participating children was 7.43 (±4.16) years. Twenty-nine percent of children were in the <5 years age group; 58.2% of the children were boys, and more than two-thirds (70.6%) were urban residents. In terms of parental characteristics, 54.2% were married, 33.85% had no formal education, and 70% were living (Table 2).
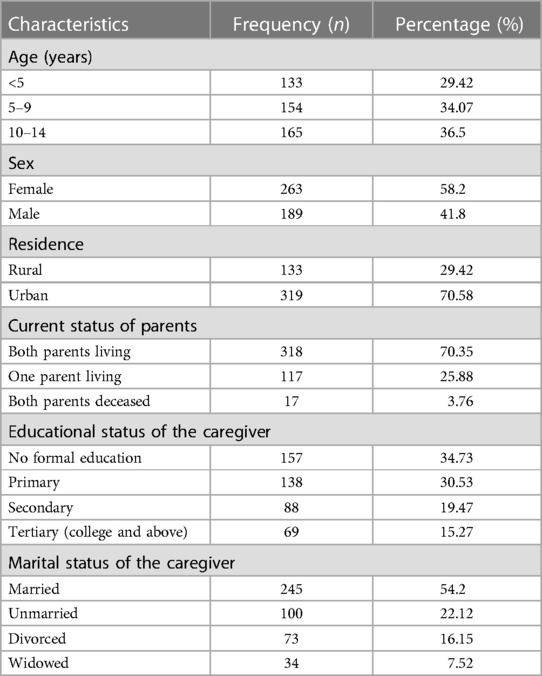
Table 2. Socio-demographic characteristics of HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022 (n = 452).
Baseline clinical, nutritional, and laboratory characteristics
The majority of children (78.54%) had CD4 cell counts above the threshold; in terms of functional status, 72.87% were working; and 71.11% had appropriate motor developmental status. In terms of nutritional status, approximately 16.37% were anemic; wasting and stunting were present in 30.75% and 48.89% of children, respectively (Table 3).
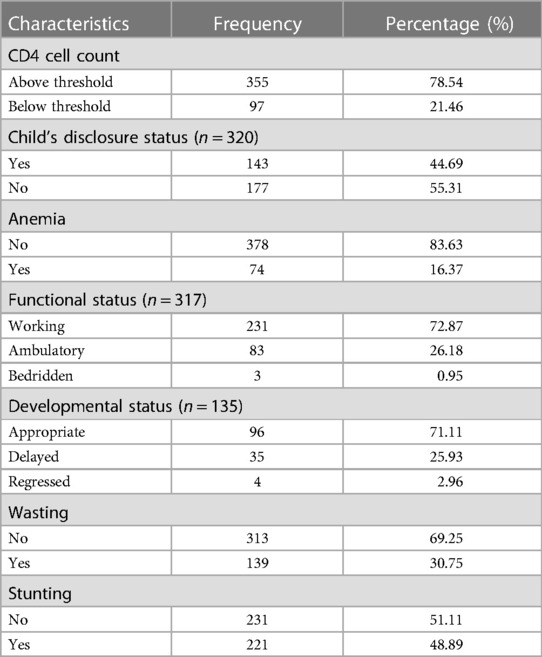
Table 3. Baseline clinical, nutritional, and laboratory test characteristics of HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022.
ART and other medication-related characteristics
The proportion of children exhibiting good adherence to ART and receiving TPT and CPT during the follow-up period were 70.58%, 64.38%, and 82.74%, respectively. In addition, only 37.83% of participants had initiated ART within 7 days after admission (Table 4).
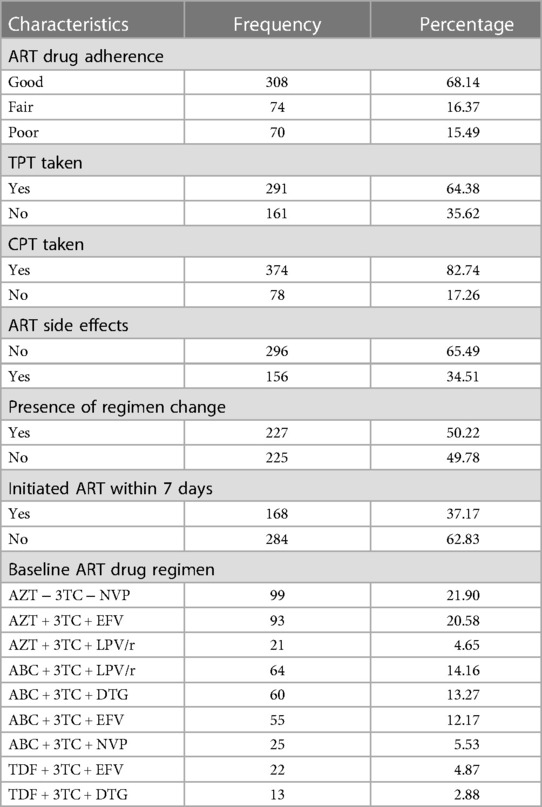
Table 4. ART and medication-related characteristics of HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022 (n = 452).
Incidence of opportunistic infections among HIV-infected children receiving ART
Across all 452 HIV-infected children receiving ART, follow-up periods ranged from 1 to 92 months, with a mean observation period of 36.8 months. The total observation time was 16,667.00 person-months (1,388.92 PYO).
New cases of opportunistic infection were observed in 120 participants (26.55%, 95% CI: 22.67, 30.88) within the follow-up period, and 73.45% were censored.
The overall incidence of OIs among HIV-infected children receiving ART across the seven Amhara Regional State CSHs was 8.64 (95% CI: 7.22, 10.33) per 100 PYO. The incidences of OIs in Woldia, University of Gondar, and Felege Hiwot CSHs were 12.86, 10.43, and 5.46 per 100 PYO, respectively (Table 5).
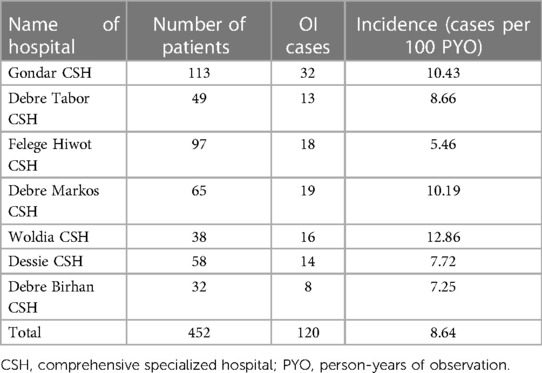
Table 5. Incidence of OIs by hospital among HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022 (n = 452).
The mean survival time was 67.57 months (95% CI: 64.02, 71.12). During the follow-up period, pneumonia was the most commonly observed OI, affecting nearly 28.33% of children, followed by tuberculosis (26.7%), diarrheal disease (10.9%), herpes zoster (8.33%), and acute upper respiratory tract infections (7.5%) (Figure 3).
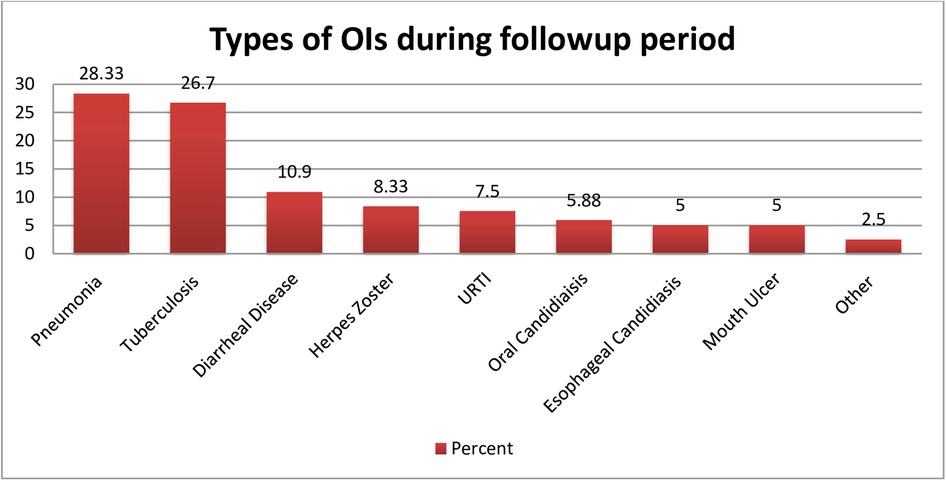
Figure 3. Common types of opportunistic infection observed among HIV-infected children receiving ART during the follow-up period at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022. Other = cryptococcal meningitis, Pneumocystis carinii pneumonia (PCP).
Comparison of incidence of OIs among levels of categorical variables
The incidence of opportunistic infections per 100 PYO was found to be higher in children with anemia (24.75), those with a CD4 cell count below the threshold (18.71), those who reported never taking CPT (16.82) or TPT (15.76), and those who ever exhibited fair or poor adherence to ART (18.94) (Table 6).
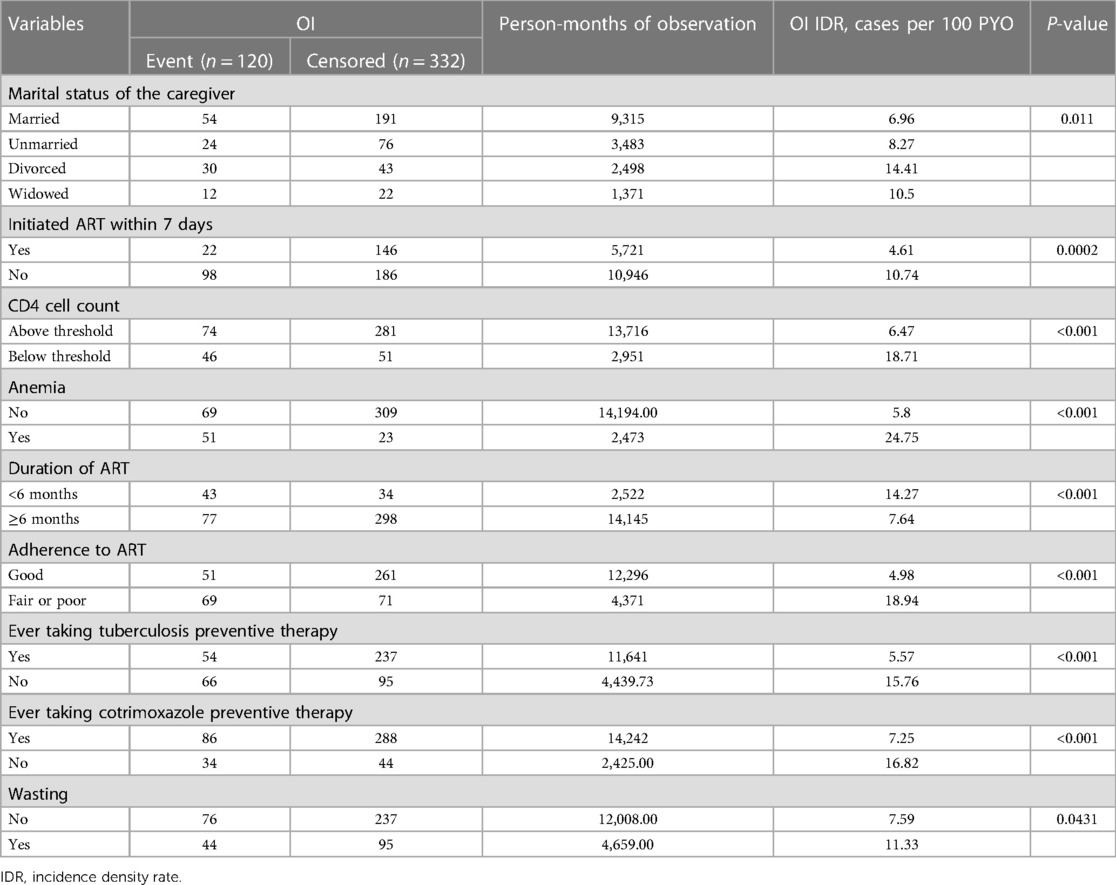
Table 6. Incidence of opportunistic infections (cases per 100 PYO) among HIV-infected children receiving ART in Amhara regional state comprehensive specialized hospitals, 2022 (n = 452), stratified by variables investigated.
The incidences of OIs within follow-up periods of less than 6 months, between 6 and 12 months, between 12 and 36 months, between 36 and 72 months, and between 72 and 92 months were 14.27, 7.51, 7.08, 9.22, and 1.97 per 100 PYO, respectively. In this study, the incidence of OIs in the first 6 months of follow-up was high, at 14.27 per 100 PYO.
Kaplan–Meier failure function or probability of OI
The probability of an OI occurring during the entire follow-up period was 0.424 (95% CI: 0.358, 0.497) by the end of follow-up; 0.064 (95% CI: 0.045, 0.0.091) after 6 months; 0.098 (95% CI: 0.0.074, 0.131) after 1 year; 0.218 (95% CI: 0.179, 0.264) after 3 years; 0.398 (95% CI: 0.339, 0.464) after 5 years; and 0.424 (95% CI: 0.358, 0.497) after 7 years (Figure 4).
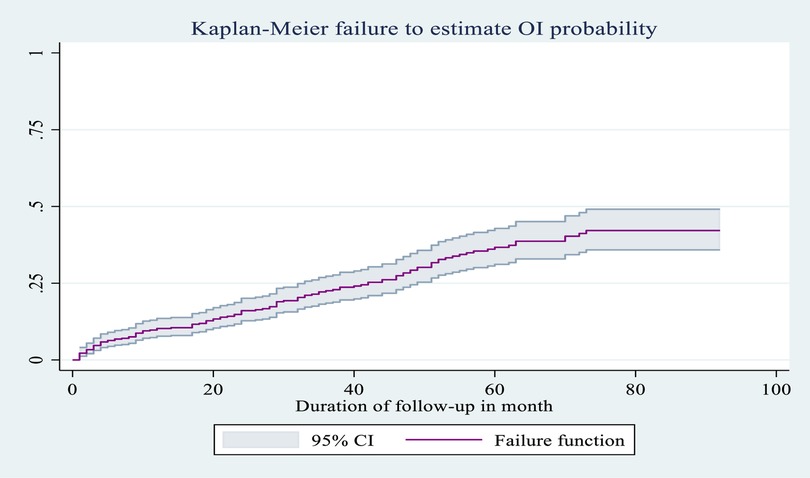
Figure 4. Overall Kaplan–Meier failure as an estimate of the probability of occurrence of OI (with 95% confidence interval) among HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022.
Comparison of probability of OI-free survival among levels of categorical variables
Children who ever exhibited poor or fair adherence to ART during the follow-up period had a lower probability of OI-free survival compared to those who exhibited good adherence to ART (log-rank test, χ2 = 66.91, P < 0.0001). Comparisons of OI-free survival probability among levels of this and other categorical variables are shown in Figures 5–8.

Figure 5. Kaplan–Meier estimates of probability of OI-free survival by ART adherence level among HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022.
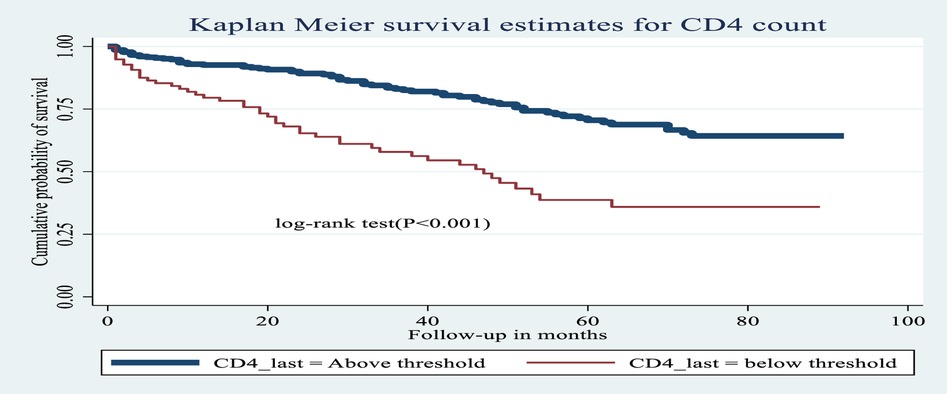
Figure 6. Kaplan–Meier estimates of probability of OI-free survival by CD4 cell count (above or below threshold) among HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022.
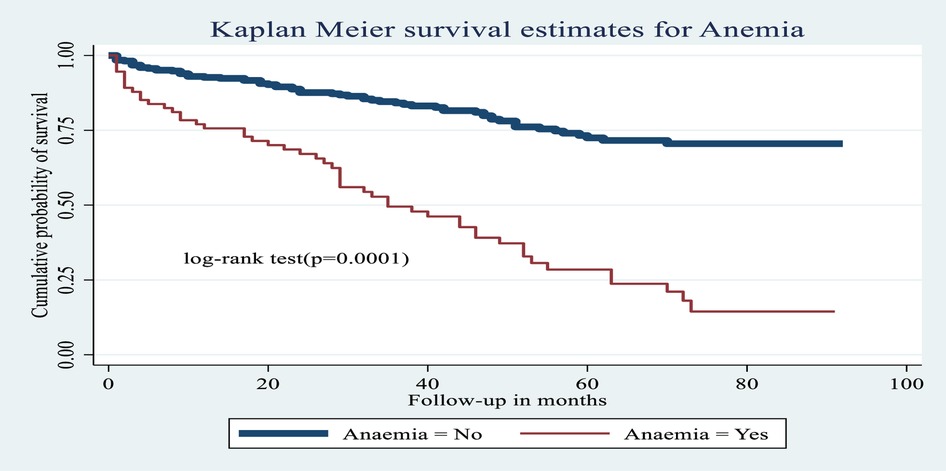
Figure 7. Kaplan–Meier estimates of probability of OI-free survival with and without anemia among HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022.
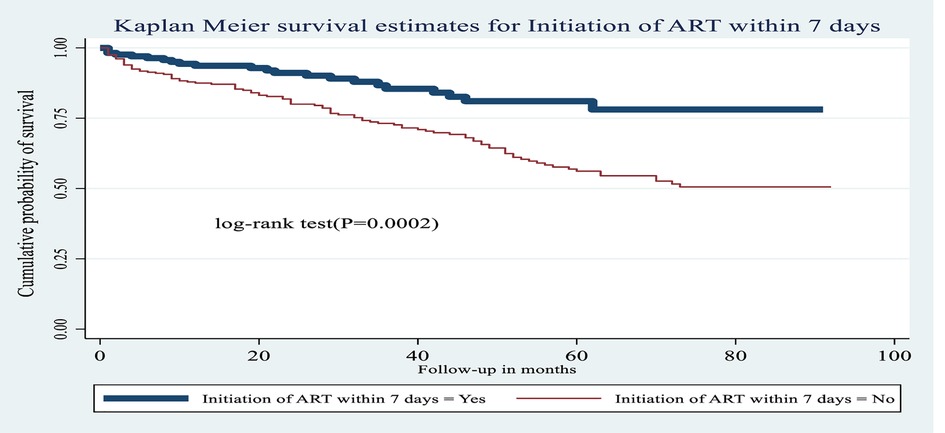
Figure 8. Kaplan–Meier estimates of probability of OI-free survival by ART initiation time among HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022.
Predictors of incidence of OIs among HIV-infected children receiving ART at Amhara regional state CSHs
In the multivariable Cox regression analysis, only CD4 cell count, anemia status, ART adherence level, ever taking TPT, and initiation of ART within 7 days of admission showed statistically significant associations with incidence of OIs among HIV-infected children receiving ART.
CD4 cell count showed a statistical association with incidence of OIs: specifically, the hazard of developing OIs among children presenting with a CD4 cell count below the threshold was 2.34 times greater [AHR: 2.34 (95% CI: 1.45, 3.76)] than among children with a CD4 count above the threshold.
The hazard of developing OIs among children with anemia was 1.68 times greater [AHR: 1.68 (95% CI: 1.06, 2.67)] than among non-anemic children.
Adherence to ART was also found to be statistically significant factor for incidence of OIs: specifically, HIV-infected children receiving ART who exhibited fair or poor ART adherence were 2.31 times more likely [AHR: 2.31 (95% CI: 1.47, 3.63)] to develop OIs than those children who exhibited good ART adherence.
The risk of developing OIs among HIV-infected children receiving ART who never took TPT was 1.95 times greater [AHR: 1.95 (95% CI: 1.27, 2.99)] than that of those who did take TPT during the follow-up period.
Lastly, the risk of developing OIs among HIV-infected children who did not initiate ART within 7 days of admission was 1.82 times greater [AHR: 1.82 (95% CI: 1.12, 2.96)] than among those who had initiated ART within 7 days, or immediately after diagnosis. Predictors of the incidence of OIs among HIV-infected children receiving ART are listed in Table 7.
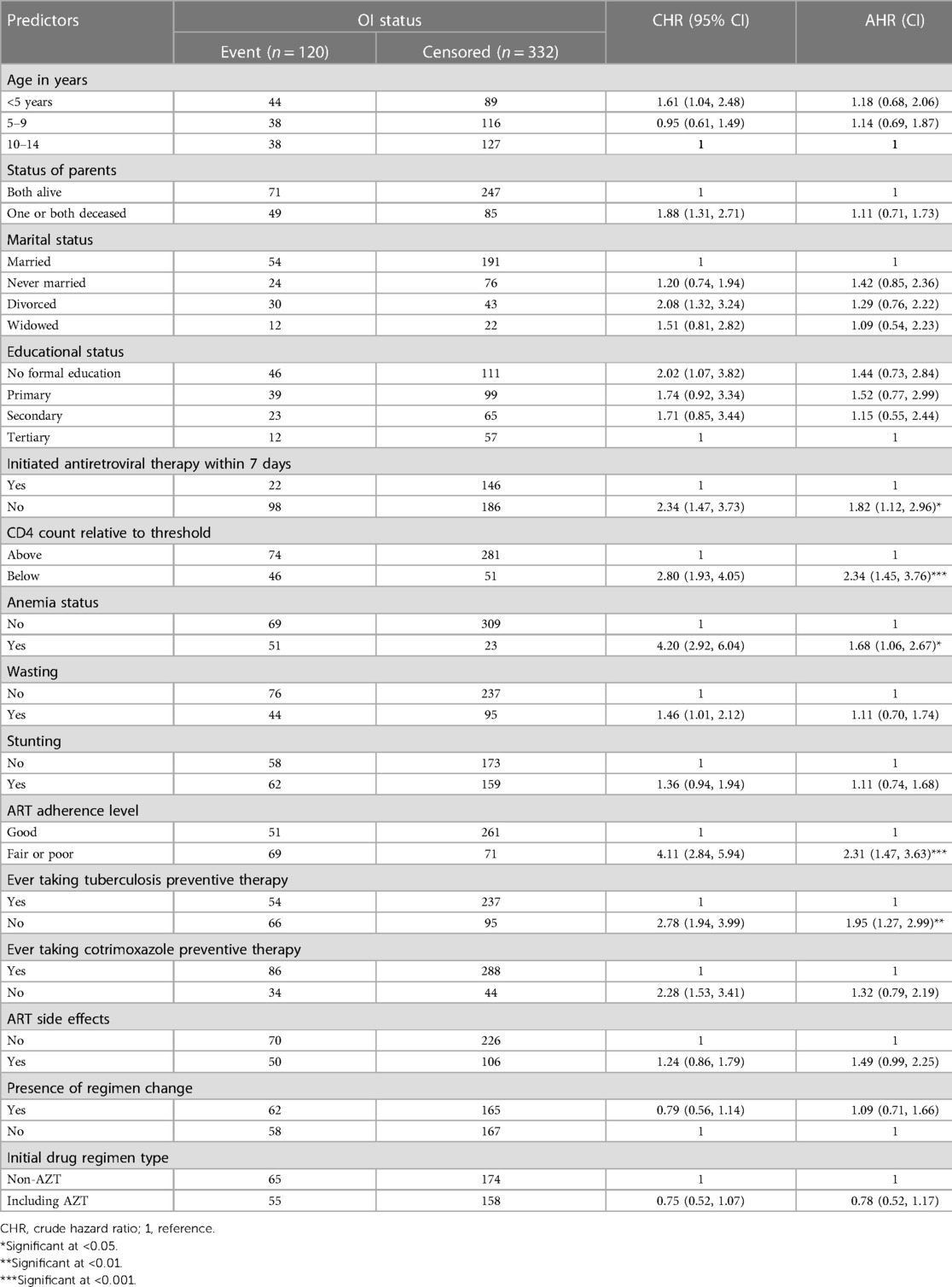
Table 7. Bi-variable and multivariable Cox proportional hazard analysis of predictors of incidence of OIs among HIV-infected children receiving ART at Amhara regional state comprehensive specialized hospitals, Ethiopia, 2022 (n = 452).
Discussion
In this study, we planned to assess the incidence of OIs and predictors of their occurrence among HIV-infected children receiving ART in Amhara Regional State CSHs in 2022; we found that the overall incidence of OIs among HIV-infected children receiving ART within the study area was 8.64 (95% CI: 7.22, 10.33) per 100 PYO. This finding was consistent with that of a study conducted in Debre Markos CSH, Ethiopia (9.7 per 100 PYO) (8). However, this was a higher rate than observed in a study conducted in Northwest Ethiopia at the UoG and Debre Tabor CSHs (5.53 per 100 PYO) (12). These discrepancies in findings resulted from outcome ascertainment, in that our study considered all types of OIs, while the aforementioned studies focused only on advanced OIs. ART on the same day of HIV status confirmation without intensive counseling and adequate preparation may lead to poor adherence in the early phase of ART intuition which increase the incidence of OIs (37, 38).
Additionally, the incidence of OIs observed in this study was higher than that of studies conducted in the United States of America (4.99 per 100 PYO) (39), Latin America (1.1 per 100 PYO) (13), and Brazil (2.63 per 100 PYO) (14). This difference is due to developed countries and middle-income countries providing better healthcare services and facilities to prevent the occurrence of OIs in comparison to resource-limited countries like Ethiopia. Additionally, dissimilarities could be due to low levels of education (in this study, two-thirds of the participants’ caregivers had no formal education or a primary education only), poverty, the burden of communicable diseases due to poor hygiene and sanitation, overcrowded living conditions, and malnutrition, which are common problems in developing countries (5) that could further exacerbate immunosuppression and co-morbidity with other diseases.
In contrast to this study, another study conducted in Latin America found a higher incidence of OIs among HIV-infected children (23.5 per PYO) (11). This discrepancy results from the nature of the study designs: the Latin America study was a prospective cohort study in which data were gathered using unbiased techniques [observation and clinical evaluation: physical examinations, medical history, and advanced laboratory investigations (viral load test)], as well as regular follow-ups that could increase the detection of new OIs. Another possible reason for the discrepancy could be the timing of the study period, given the recent accessibility of new ART drugs with reduced side effects, such dolutegravir. In the current study, 34.51% of the participants reported experiencing side effects from ART (11, 34).
Many predictors showed statistically significant associations with the incidence of OIs among HIV-infected children receiving ART. In this regard, children who presented with a CD4 cell count below the threshold were 2.34 times more likely to develop OIs [AHR: 2.34 (95% CI: 1.45, 3.76)] than those children with a CD4 cell count above the threshold. The finding is supported by studies conducted in Latin America (11), India (21), the USA (19), and Northern Ethiopia (40), and at Debre Markos CSH (8). The reason is that CD4 cells play an important role in mounting an immune response to infections. These cells release cytokines that lead to the activation of antigen-presenting cells, phagocytic cells, natural killer cells, and cytotoxic T cells. A decrease in CD4 cell count thus leads to a decline in both humoral and cell-mediated immunity (41). This results in the depletion of CD4 cells and immunodeficiency, and the likelihood of OIs increases (21, 42).
The risk of developing OIs was 1.68 times greater among children with anemia [AHR: 1.68 (95% CI: 1.06, 2.67)] as compared to non-anemic children. This finding is supported by various studies conducted at Debre Tabor and University of Gondar CSHs (12), in the Metekel zone (43), and in Addis Ababa (44), Northern Ethiopia (40), Adama (29), Nigeria (45), Uganda (23), Tanzania (25) and South Africa (24, 46). The occurrence of anemia at the start of a chronic disease is caused by baseline pro-inflammatory cytokine upregulation. Evidence suggests that the dysregulated iron axis in HIV-related anemia may provide highly favorable intracellular macrophage conditions for microorganisms like TB bacilli, and subsequently results in the development of OIs (24, 47). Another clinical reason could be that CD4 cell count is significantly associated with the likelihood of anemia; this is explained by an increased viral burden, which may cause anemia through increased cytokine-mediated myelosuppression and a higher burden of OIs (48). Anemia in turn exerts an effect on O2 intake capacity, which leads to a synergistic effect with OIs, worsening the prognosis of the disease process and potentially ending in death (48).
HIV-infected children receiving ART who exhibited fair or poor ART adherence were 2.31 times more likely [AHR: 2.31 (95% CI: 1.47, 3.63)] to develop OIs as compared to those children who exhibited good ART adherence. This result is consistent with studies conducted at Debre Markos CSH (8), at Debre Tabor and University of Gondar (12), at Bahir Dar Public Hospitals (49), in Brazil (22), and in India (50). A possible reason that poor or fair adherence to a regime of HIV medications directly increases viral replication to its maximum, compromising health and quality of life; thus, the risk of developing OIs is maximized, and the risk of drug resistance is increased (34, 37, 38).
The risk of developing OIs among children who never took TPT was 1.95 times higher [AHR: 1.95 (95% CI: 1.27, 2.99)] than among those who took TPT during the follow-up period. This finding is comparable with findings reported in Nigeria (51), at UoG (52), in Northwest Ethiopia Gondar (53), in Adama (29), in Addis Ababa (54), at SNNP Region Hospitals (55), and at Arba Minch Hospital (56). This implies that TPT plays a role in decreasing mycobacterium load and reducing the progression of latent bacilli to active TB (57).
The risk of developing OIs among HIV-infected children receiving ART was 1.82 times higher among those who had not initiated ART within 7 days of admission or enrolment [AHR: 1.82 (95% CI: 1.12, 2.96)] than among those who had initiated ART within this time frame. Recently, the WHO has strongly recommended initiating ART on the same day that HIV is confirmed, or within 7 days, after ensuring the patient's willingness and readiness to start ART immediately (37). If children do not start ART quickly, the incidence of OIs is likely to be increased. Early ART commencement improves drug uptake, HIV care retention, and viral suppression (58). By 2025, 95% of patients undergoing ART will have achieved viral suppression, which will play a role in lowering the incidence of OIs. This might be achieved through rapid ART commencement (59).
Despite the interesting research topic and strong methodological considerations throughout the course of our study, the retrospective nature of data collection obliged us to rely on existing and previously recorded information on the type of OIs that occurred during follow-up, which could mean that important variables were missed. Likewise, the use of subjective measures (given the limited capacity to establish definitive diagnoses of OIs) means that asymptomatic OIs could surely have been missed, in turn resulting in an underestimate for the study's outcome of interest. Additionally, considering the use of children's charts as data sources, non-registered variables that could have influenced the occurrence of OIs (income, caregiver's occupational status, family size, and viral load) were not investigated.
Conclusion
In this study, a high incidence of OIs was observed during a specified follow-up period among HIV-infected children receiving ART at Amhara Regional State CSHs. Having a CD4 cell count below the threshold, ever exhibiting only fair or poor adherence to ART, co-morbidity of anemia, ever taking TPT, and not having initiated ART within 7 days were significant predictors of an increased incidence of OIs among this group.
Recommendation
Caregivers are recommended to bring their children for early screening, and to engage with follow-up care to the greatest extent possible. CSHs should closely monitor children who present with a baseline CD4 cell count below the threshold, or with a low hemoglobin level or anemia, and should place more emphasis on working with the caregivers of HIV-infected children in order to achieve good ART adherence during the follow-up period. Ideally, ART should be initiated immediately or within 7 days of the HIV diagnosis being confirmed. Further prospective cohort studies should be conducted, incorporating important predictors of OIs like income status, occupational status of the caregiver, family size, and viral load; further qualitative research on ART drug adherence level should also be conducted.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
GM developed the research idea, designed the study, and was involved in proposal writing, training and supervision of the data collectors, analysis and interpretation the results, and preparation of the manuscript. BB, WK, AA, ME, and AW participated in critical revision of the proposal, study design, analysis and interpretation of the results, and writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Financial support was received from University of Gondar. The funding institution had no role in the preparation of the manuscript or in the decision to publish.
Acknowledgments
First, the authors would like to express their deepest gratitude to the University of Gondar, College of Medicine and Health Sciences, School of Nursing for providing me with this opportunity and financial support. In addition, we would like to express our special thanks to the data collectors and study supervisors. We would also like to acknowledge Amhara Public Health Institute for granting me permission to conduct the study at each hospital. Finally, we are grateful to the hospital head administrators, the ART clinic, and patient medical room staff members at each of the comprehensive specialized hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Global health sector strategy on HIV 2016-2021. Towards ending AIDS (2016). World Health Organization.
2. Center of Disease and control and prevention. AIDS and opportunistic infections in what are opportunistic infections? May 20,2021, center of Disease and control and preventions USA.
3. Boyd AT, Oboho I, Paulin H, Ali H, Godfrey C, Date A, et al. Addressing advanced HIV disease and mortality in global HIV programming. AIDS Res Ther. (2020) 17(1):40. doi: 10.1186/s12981-020-00296-x
4. Federal Minstry of Health. National guidlines for comprehensive HIV prevntion, care and treatment in Ethiopia, in prevention, screening, and management of common co infections FEBRUARY 2018, FMOH Addis Ababa.
5. Department of health and Human services in USA. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children, in recommendations from the national institutes of health, centers for disease control and prevention, HIV medicine association of the infectious diseases society of America, and pediatric infectious diseases society. January 24, 2022, America Acadamy of Pediatrics.
7. UNAIDS. Global HIV & AIDS statistics—2018 fact sheet. Global HIV and AIDs ststistics, World AIDS day 2019 fact sheet (2019). 1: p. 1–6.
8. Melkamu MW, Gebeyehu MT, Afenigus AD, Hibstie YT, Temesgen B, Petrucka P, et al. Incidence of common opportunistic infections among HIV-infected children on ART at Debre Markos referral hospital, northwest Ethiopia: a retrospective cohort study. BMC Infect Dis. (2020) 20(1):1–12. doi: 10.1186/s12879-020-4772-y
9. Prasitsuebsai W, Kariminia A, Puthanakit T, Lumbiganon P, Hansudewechakul R, Moy FS, et al. Impact of antiretroviral therapy on opportunistic infections of HIV-infected children in the therapeutic research, education and AIDS training Asia pediatric HIV observational database. Pediatr Infect Dis J. (2014) 33(7):747–52. doi: 10.1097/INF.0000000000000226
10. Candiani T, Pinto J, Cardoso CAA, Carvalho IR, Dias A, Carneiro M, et al. Impact of highly active antiretroviral therapy (HAART) on the incidence of opportunistic infections, hospitalizations and mortality among children and adolescents living with HIV/AIDS in Belo Horizonte, minas gerais state, Brazil. Cad Saude Publica. (2007) 23(Suppl 3):S414–23. doi: 10.1590/S0102-311X2007001500009
11. Alarcón JO, Freimanis-Hance L, Krauss M, Reyes MF, Cardoso CAA, Mussi-Pinhata MM, et al. Opportunistic and other infections in HIV-infected children in Latin America compared to a similar cohort in the United States. AIDS Res Hum Retroviruses. (2012) 28(3):282–8. doi: 10.1089/aid.2011.0057
12. Chanie ES, Bayih WA, Birhan BM, Belay DM, Asmare G, Tiruneh T, et al. Incidence of advanced opportunistic infection and its predictors among HIV infected children at debre tabor referral hospital and university of gondar compressive specialized hospitals, northwest Ethiopia, 2020: a multicenter retrospective follow-up study. Heliyon. (2021) 7(4):e06745. doi: 10.1016/j.heliyon.2021.e06745
13. Nesheim SR, Kapogiannis BG, Soe MM, Sullivan KM, Abrams E, Farley J, et al. Trends in opportunistic infections in the pre- and post-highly active antiretroviral therapy eras among HIV-infected children in the perinatal AIDS collaborative transmission study, 1986-2004. Pediatrics. (2007) 120(1):100–9. doi: 10.1542/peds.2006-2052
14. Haileamlak A, Hagos T, Abebe W, Abraham L, Asefa H, Teklu AM Predictors of hospitalization among children on ART in Ethiopia: a cohort study. Ethiop J Health Sci. (2017) 27(1):53–62. doi: 10.4314/ejhs.v27i1.6S
15. Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis. (2013) 26(1):17–25. doi: 10.1097/QCO.0b013e32835ba6b1
16. Samji H, Cescon A, Hogg R, Modur S, Althoff K, Buchacz K Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One. (2013) 8(12):e81355. doi: 10.1371/journal.pone.0081355
18. B-Lajoie M-R, Drouin O, Bartlett G, Nguyen Q, Low A, Gavriilidis G, et al. Incidence and prevalence of opportunistic and other infections and the impact of antiretroviral therapy among HIV-infected children in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. (2016) 62(12):1586–94. doi: 10.1093/cid/ciw139
19. Crowell TA, Lyall H, Malatinkova E, Bhagani S, Hsu D, Colby DJ, et al. Highlights from the 24(th) conference on retroviruses and opportunistic infections: 13-16 February 2017, Seattle, Washington, USA. J Virus Erad. (2017) 3(2):101–8. doi: 10.1016/S2055-6640(20)30291-0
20. Coelho L, Cardoso SW, Amancio RT, Moreira RI, Campos DP, Veloso VG, et al. Trends in AIDS-defining opportunistic illnesses incidence over 25 years in rio de janeiro, Brazil. PLoS One. (2014) 9(6):e98666. doi: 10.1371/journal.pone.0098666
21. Dhaka G, Sherwal B, Saxena S, Rai Y, Chandra J Current trends in opportunistic infections in children living with HIV/AIDS in a tertiary care hospital in northern India. Indian J Sex Transm Dis AIDS. (2017) 38(2):142–6. doi: 10.4103/2589-0557.216992
22. Iroezindu M. Disparities in the magnitude of human immunodeficiency virus-related opportunistic infections between high and low/middle-income countries: is highly active antiretroviral therapy changing the trend? Ann Med Health Sci Res. (2016) 6(1):4–18. doi: 10.4103/2141-9248.180234
23. Weissberg D, Mubiru F, Kambugu A, Fehr J, Kiragga A, von Braun A, et al. Ten years of antiretroviral therapy: incidences, patterns and risk factors of opportunistic infections in an urban Ugandan cohort. PloS One. (2018) 13(11):e0206796. doi: 10.1371/journal.pone.0206796
24. Brennan A, Bonawitz R, Schnippel K, Berhanu R, Maskew M, Long L, et al. Incident tuberculosis in HIV-positive children, adolescents and adults on antiretroviral therapy in South Africa. Int J Tuberc Lung Dis. (2016) 20(8):1040–5. doi: 10.5588/ijtld.15.0488
25. Li N, Manji KP, Spiegelman D, Muya A, Mwiru RS, Liu E, et al. Incident tuberculosis and risk factors among HIV-infected children in Tanzania. AIDS. (2013) 27(8):1273–81. doi: 10.1097/QAD.0b013e32835ecb24
26. Iacob SA, Iacob DG, Jugulete G. Improving the adherence to antiretroviral therapy, a difficult but essential task for a successful HIV treatment-clinical points of view and practical considerations. Front Pharmacol. (2017) 8:831. doi: 10.3389/fphar.2017.00831
27. Alebel A, Demant D, Petrucka P, Sibbritt D Effects of undernutrition on opportunistic infections among adults living with HIV on ART in northwest Ethiopia: using inverse-probability weighting. Plos One. (2022) 17(3):e0264843. doi: 10.1371/journal.pone.0264843
28. Alebel A, Engeda EH, Kelkay MM, Petrucka P, Kibret GD, Wagnew F, et al. Mortality rate among HIV-positive children on ART in northwest Ethiopia: a historical cohort study. BMC Public Health. (2020) 20(1):1–11. doi: 10.1186/s12889-020-09418-6
29. Beshir MT, Beyene AH, Tlaye KG, Demelew TM Incidence and predictors of tuberculosis among HIV-positive children at adama referral hospital and medical college, oromia, Ethiopia: a retrospective follow-up study. Epidemiol Health. (2019) 41:e2019028. doi: 10.4178/epih.e2019028
30. Sisay MM, Ayele TA, Gelaw YA, Tsegaye AT, Gelaye KA, Melak MF Incidence and risk factors of first-line antiretroviral treatment failure among human immunodeficiency virus-infected children in Amhara regional state, Ethiopia: a retrospective follow-up study. BMJ Open. (2018) 8(4):e019181. doi: 10.1136/bmjopen-2017-019181
31. Belay GM, Engeda EH, Ayele AD. Late antiretroviral therapy initiation and associated factors among children on antiretroviral therapy at university of gondar comprehensive specialized hospital, gondar, northwest Ethiopia: a cross-sectional study. BMC Res Notes. (2019) 12(1):1–7. doi: 10.1186/s13104-019-4279-z
33. FMOH. National guidlines for comprhensive HIV prevention, care and treatment in Ethiopia (2014). FMOH: Addis Ababa. p. 1–165.
34. FMOH. National comprehensive HIV prevention, care and treatment training for healthcare providers, M.O. health, editor. (2021) Ethiopian Federal Ministry of Health Addis Ababa. p. 1–502.
37. Le Thuy, World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy (2018).
38. Hibstie YT, Kibret GD, Talie A, Temesgen B, Melkamu MW, Alebel A Nearly one in every six HIV-infected children lost from ART follow-up at Debre Markos referral hospital, northwest Ethiopia: a 14-year retrospective follow-up study. PLoS One. (2020) 15(9):e0239013. doi: 10.1371/journal.pone.0239013
39. Ylitalo N, Brogly S, Hughes MD, Nachman S, Dankner W, Van Dyke R, et al. Risk factors for opportunistic illnesses in children with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Pediatr Adolesc Med. (2006) 160(8):778–87. doi: 10.1001/archpedi.160.8.778
40. Alemu YM, Andargie G, Gebeye E. High incidence of tuberculosis in the absence of isoniazid and cotrimoxazole preventive therapy in children living with HIV in northern Ethiopia: a retrospective follow-up study. PLoS One. (2016) 11(4):e0152941. doi: 10.1371/journal.pone.0152941
41. Ford N, Meintjes G, Vitoria M, Greene G, Chiller T The evolving role of CD4 cell counts in HIV care. Curr Opin HIV AIDS. (2017) 12(2):123–8. doi: 10.1097/COH.0000000000000348
42. Ravichandra RK, Bikash PB, Agarwalla S. Opportunistic infections in HIV infected children and its correlation with CD4 count. Int J Contemp Pediatr. (2017) 4(5):1743–7. doi: 10.18203/2349-3291.ijcp20173777
43. Kebede F, Tarekegn H, Molla M, Jara D, Abate A Incidence and predictors of pulmonary Tuberculosis among children who received antiretroviral therapy (ART), northwest Ethiopia: a multicenter historical cohorts study 2009–2019. J Trop Med. (2022) 2022:9925693. doi: 10.1155/2022/9925693
44. Alemu A, Yesuf A, Zerihun B, Getu M, Worku T, Bitew ZW Incidence and determinants of tuberculosis among HIV-positive individuals in Addis Ababa, Ethiopia: a retrospective cohort study. Int J Infect Dis. (2020) 95:59–66. doi: 10.1016/j.ijid.2020.02.053
45. Iroezindu M, Ofondu E, Hausler H, Van Wyk B Prevalence and risk factors for opportunistic infections in HIV patients receiving antiretroviral therapy in a resource-limited setting in Nigeria. J AIDs Clin Res. (2013) 3:2. doi: 10.4172/2155-6113.S3-002
46. Kerkhoff AD, Wood R, Cobelens FG, Gupta-Wright A, Bekker L-G, Lawn SD The predictive value of current haemoglobin levels for incident tuberculosis and/or mortality during long-term antiretroviral therapy in South Africa: a cohort study. BMC Med. (2015) 13(1):1–13. doi: 10.1186/s12916-015-0320-9
47. McDermid JM, Hennig BJ, van der Sande M, Hill AV, Whittle HC, Jaye A, et al. Host iron redistribution as a risk factor for incident tuberculosis in HIV infection: an 11-year retrospective cohort study. BMC Infect Dis. (2013) 13(1):48. doi: 10.1186/1471-2334-13-48
48. Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. AIDS. (1999) 13(8):943–50. doi: 10.1097/00002030-199905280-00010
49. Chekole B, Belachew A, Geddif A, Amsalu E, Tigabu A Survival status and predictors of mortality among HIV-positive children initiated antiretroviral therapy in bahir dar town public health facilities Amhara region, Ethiopia, 2020. SAGE Open Med. (2022) 10:20503121211069477. doi: 10.1177/20503121211069477
50. Isaakidis P, Paryani R, Khan S, Mansoor H, Manglani M, Valiyakath A, et al. Poor outcomes in a cohort of HIV-infected adolescents undergoing treatment for multidrug-resistant tuberculosis in mumbai, India. PLoS One. (2013) 8(7):e68869. doi: 10.1371/journal.pone.0068869
51. Chamla DD, Asadu C, Davies A, de Wagt A, Ilesanmi O, Adeyinka D, et al. Patching the gaps towards the 90–90–90 targets: outcomes of Nigerian children receiving antiretroviral treatment who are co-infected with tuberculosis. J Int AIDS Soc. (2015) 18:20251. doi: 10.7448/IAS.18.7.20251
52. Atalell KA. Survival and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at university of gondar comprehensive specialized hospital, northwest Ethiopia. A retrospective follow-up study. BMC Infect Dis. (2018) 13(5):e0197145. doi: 10.1371/journal.pone.0197145
53. Beshaw MA, Balcha SA. Effect of isoniazid prophylaxis therapy on the prevention of Tuberculosis incidence and associated factors among HIV infected individuals in northwest Ethiopia: retrospective cohort study. HIV AIDS. (2021) 13:617–29. doi: 10.2147/HIV.S301355
54. Semu M, Fenta TG, Medhin G, Assefa D Effectiveness of isoniazid preventative therapy in reducing incidence of active tuberculosis among people living with HIV/AIDS in public health facilities of Addis Ababa, Ethiopia: a historical cohort study. BMC Infect Dis. (2017) 17(1):1–8. doi: 10.1186/s12879-016-2109-7
55. Yirdaw KD, Jerene D, Gashu Z, Edginton M, Kumar AM, Letamo Y, et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PloS One. (2014) 9(8):e104557. doi: 10.1371/journal.pone.0104557
56. Abossie A, Yohanes T. Assessment of isoniazid preventive therapy in the reduction of tuberculosis among ART patients in arba minch hospital, Ethiopia. Ther Clin Risk Manag. (2017) 13:361. doi: 10.2147/TCRM.S127765
57. Ayele HT, Mourik MSv, Debray TP, Bonten MJ Isoniazid prophylactic therapy for the prevention of tuberculosis in HIV infected adults: a systematic review and meta-analysis of randomized trials. PloS One. (2015) 10(11):e0142290. doi: 10.1371/journal.pone.0142290
58. Ford N, Migone C, Calmy A, Kerschberger B, Kanters S, Nsanzimana S, et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS. (2018) 32(1):17. doi: 10.1097/QAD.0000000000001671
Keywords: antiretroviral therapy, HIV-infected, children, opportunistic infection, predictors, CD4
Citation: Mekonnen GB, Birhane BM, Engdaw MT, Kindie W, Ayele AD and Wondim A (2023) Predictors of a high incidence of opportunistic infections among HIV-infected children receiving antiretroviral therapy at Amhara regional state comprehensive specialized hospitals, Ethiopia: A multicenter institution-based retrospective follow-up study. Front. Pediatr. 11:1107321. doi: 10.3389/fped.2023.1107321
Received: 24 November 2022; Accepted: 30 March 2023;
Published: 2 May 2023.
Edited by:
Rakesh Kumar Pilania, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Yonas Bekele, National Institutes of Health (NIH), United StatesCarlos Daniel Grasa, University Hospital La Paz, Spain
© 2023 Mekonnen, Birhane, Engdaw, Kindie, Ayele and Wondim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gebrehiwot Berie Mekonnen Z2ViZXJlaHdvdDIwMDRAZ21haWwuY29t
Abbreviations AHR, adjusted hazard ratio; AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; CPT, cotrimoxazole preventive therapy; CD4, cluster of differentiation 4; CSHs, comprehensive specialized hospitals; HIV, human immunodeficiency virus; HSTP-II, health sector transformation plan II; OIs, opportunistic infections; PLHIV, people living with human immunodeficiency virus; PYO, person-year observations; SD, standard deviation; TB, tuberculosis; TPT, tuberculosis preventive therapy; UoG, University of Gondar; WHO, World Health Organization.
 Gebrehiwot Berie Mekonnen
Gebrehiwot Berie Mekonnen Binyam Minuye Birhane
Binyam Minuye Birhane Melaku Tadege Engdaw
Melaku Tadege Engdaw Wotetenesh Kindie4
Wotetenesh Kindie4 Amare Wondim
Amare Wondim