
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 03 March 2023
Sec. Pediatric Neurology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1104001
 Yeping Wang1,2
Yeping Wang1,2 Xiaoyan Ren1,3
Xiaoyan Ren1,3 Yu Shen1,3
Yu Shen1,3 Yi Hua1
Yi Hua1 Lu Xu1
Lu Xu1 Weiran Zhang1
Weiran Zhang1 Guoxia Sheng1
Guoxia Sheng1 Peifang Jiang1
Peifang Jiang1 Zhefeng Yuan1
Zhefeng Yuan1 Liu Liu1*†,‡
Liu Liu1*†,‡ Feng Gao1*†,‡
Feng Gao1*†,‡
Objective: To explore the clinical characteristics of pediatric anti-gamma-aminobutyric acid-B receptor (GABABR) encephalitis to enhance the understanding and improve the diagnostic and therapeutic strategies for this disease.
Methods: We report a rare case of a female pediatric patient with anti-GABABR encephalitis who was treated at the Children's Hospital of Zhejiang University School of Medicine. Literature search was performed to explore the clinical characteristics of pediatric anti-GABABR encephalitis.
Results: The patient exhibited recurrent epileptic seizure, status epilepticus, and psychiatric symptoms at the age of 11 years and 10 months. Anti-GABABR antibodies were positive in cerebrospinal fluid and serum. Brain magnetic resonance imaging (MRI) exhibited abnormal signals in the left hippocampus. Symptoms and abnormality of brain MRI were improved after administration of immunosuppressants, anti-seizure and antipsychotic drugs. Two of pediatric anti-GABABR encephalitis with clinical data were identified through literature search. Analysis of these three cases suggested that the pediatric patients primarily experienced limbic encephalitis, with no tumor incidence. A favorable immunotherapy response was demonstrated with a superior prognosis in all the cases.
Conclusions: We reported a pediatric anti-GABABR encephalitis case with early age of onset. Promt autoimmune antibody testing and tumor screening, as well as immunomodulatory treatment immediately after a definitive diagnosis are warranted to improve prognosis.
Autoimmune encephalitis (AE) broadly refers to a type of encephalitis that is mediated by autoimmune mechanisms, particularly by antineuronal antibodies such as those against neuronal cell surface receptors or synaptic proteins, and intracellular targets (1, 2). With the advancement of experimental antibody detection techniques and expansion of the neuronal antibody spectrum, an increasing number of antibody-related AE cases have been diagnosed in recent years.
GABA is a vital inhibitory neurotransmitter in the central nervous system that plays a critical role in controlling neuronal excitability by binding to specific receptors. GABAA, GABAB, and GABAC receptors are the three classes of GABA receptors. Among these receptors, GABAAR and GABAcR are ligand-gated chloride channels, whereas GABABR is a G-protein coupled receptor. However, GABABR plays an essential role in neurotransmission and maintenance of synaptic stability (3). It is widely distributed in the brain and spinal cord, with the highest concentrations in the hippocampus, thalamus, and cerebellum. Therefore, it is closely associated with learning, memory, and cognitive functions (4).
Anti-gamma-aminobutyric acid-B receptor (GABABR) encephalitis is a relatively rare type of AE. Additionally, most anti-GABABR encephalitis cases have been reported in adults, and the disease has been rarely observed in children. Here we elaborate on the clinical manifestations of a case of pediatric anti-GABABR encephalitis and analyze the clinical characteristics of the disease through literature review.
Patient 1 was a 16-year-old girl, who experienced 2–3 episodes of seizure per day in sleep, with the onset age of 11 years and 10 months. The seizure was manifested as secondarily generalised tonic–clonic seizures that lasted 2–3 min before self-resolution. The patient experienced one episode of status epilepticus (SE) lasting 2 h. No abnormalities were observed in the medical, personal, or family history and upon physical examination. CSF test exhibited a white blood cell count of 30 × 106/L, comprising mainly monocytes (90%). No abnormalities were observed in CSF biochemistry, smear, and culture. Enteroviruses (hepatitis E virus, enterovirus type 71, coxsackievirus A16), Epstein–Barr virus, herpes simplex virus, and oligoclonal bands were negative in blood and CSF. Blood biochemistry, genetic metabolic profile, tumor markers, antinuclear antibodies, and erythrocyte sedimentation rate were normal. Video-electroencephalography (VEEG) indicated slowing of the basic rhythm in background activity during waking periods. Persistent polymorphic δ and θ rhythms were observed in the left temporo-occipital region during wakefulness and sleep (Figure 1A). The B-scan ultrasonography of the thyroid, liver, gallbladder, kidneys, retroperitoneum, and uterus; chest computed tomography (CT); electrocardiogram; and head magnetic resonance imaging (MRI) were normal. The patient experienced recurrent seizure incidence despite being symptomatically treated with acyclovir, mannitol, and levetiracetam (12.5 mg/kg·d). On day 23 after disease onset, brain MRI exhibited hyperintensities on T2 weighted image (T2WI) and fluid-attenuated inversion recovery (FLAIR) in the left medial temporal hippocampus (Figures 1B,C). Anti-GABABR antibodies were positive in both CSF (1:3.2) and serum (1:32). However, antibodies against NMDAR, AMPAR, GABAAR, LGI1, and CASPR2 and paraneoplastic antibodies were not observed in the CSF or serum. The patient was then administered levetiracetam (25 mg/kg·d) combined with carbamazepine (10 mg/kg·d) to control epileptic seizures, followed by administration of methylprednisolone (10 mg/kg × 3d). Although the epileptic events reduced, symptoms such as disorganised speech, easily frightening, agitation, and auditory hallucinations appeared. Further treatment with methylprednisolone (18.75 mg/kg × 3d) combined with intravenous immunoglobulin (IVIG; 20 g × 3d) and sertraline (50 mg qn) was administered. The patient scored 3 on the mRS when the symptoms were most severe. Following treatment, the epileptic seizures ceased one month after onset, psychiatric symptoms disappeared, and the mRS score was 0. Re-examination of brain MRI indicated a slight improvement in abnormal hippocampal signals, which were significantly improved three years after disease onset (Figures 1D,E).
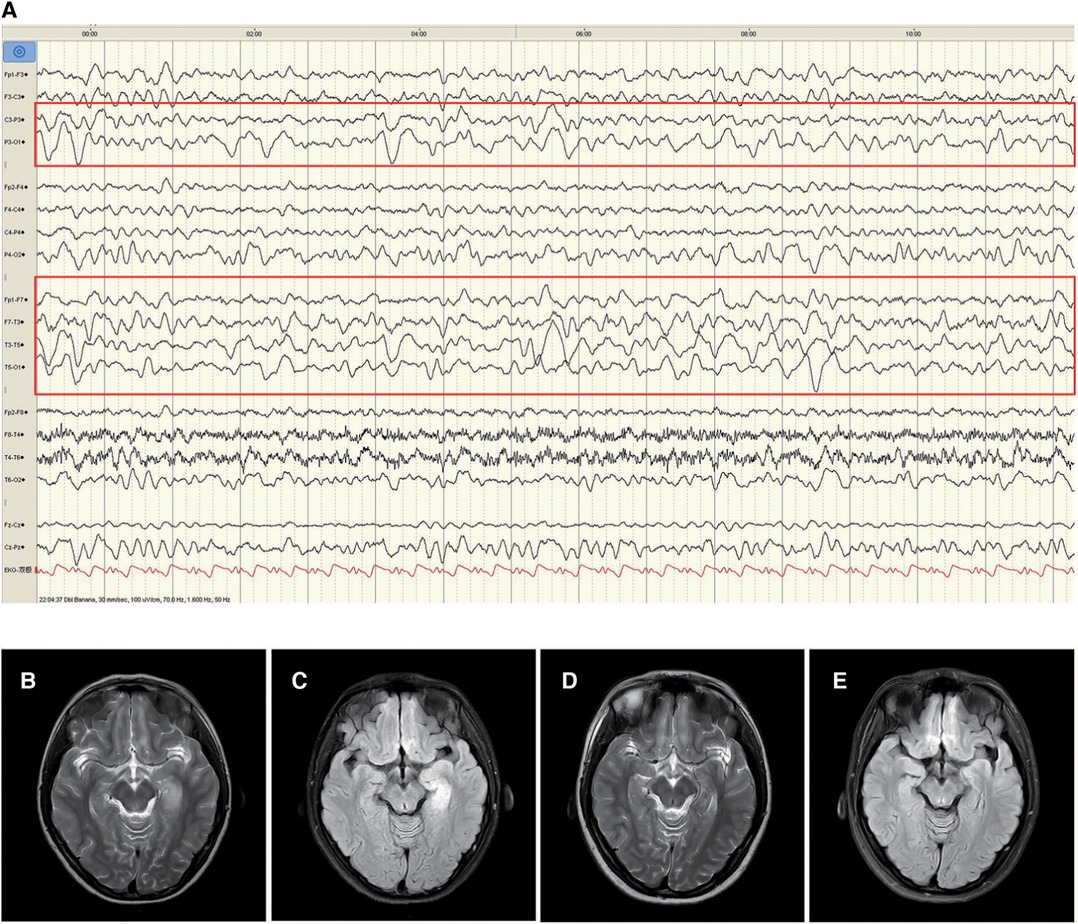
Figure 1. Electroencephalogram (EEG) and changes in brain magnetic resonance imaging (MRI) of patient 1. The left temporo-occipital polymorphic δ and θ activities were observed in the EEG of the patient in the waking phase at disease onset (A, red rectangle). MRI T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities in the hippocampal gyrus in the left medial temporal lobe (B and C). Improvement of abnormal signal in MRI T2/FLAIR (D and E) three years after treatment.
A literature search was conducted with the keywords ‘GABABR' and ‘encephalitis’ in databases such as PubMed and CNKI until December 2022. Only the articles written in English or Chinese were included. Anti-GABABR encephalitis cases of pediatric patients (aged less than 18 years) with complete or partial clinical data were identified, and the clinical characteristics of all these pediatric patients were summarized. And the patients with only anti-GABABR-positive and not secondary to any infections were included in analysis.
The database search yielded seven pediatric anti-GABABR encephalitis cases (5–10). In 2014, Kruer et al. (8) reported a case of anti-GABABR encephalitis in a 3-year-old male patient. The patient exhibited severe clinical phenotypes and rapid progression manifesting as confusion, lethargy, opsoclonus, dystonic tongue movements, ataxia, and chorea at onset, followed by complex partial seizures and SE during later phases. Laboratory tests indicated positive anti-GABABR antibodies in serum and CSF (1:32 and 1:3.2, respectively). A white blood cell count of 154/µl and a protein level of 59 mg/dl were observed in the initial CSF analysis. EEG exhibited generalised slow waves and epileptiform discharge. Brain MRI exhibited multifocal T2/FLAIR hyperintensities in the brainstem and cerebellum with involvement of the basal ganglia and hippocampus. Seizures were difficult to control even after treatment with various antiseizure medications (ASMs), intravenous immunogloblin (IVIG), and methylprednisolone. The patient died of sepsis four weeks later. The case of the same patient was reported by Petit-Pedrol et al. (11) in 2014; the patient tested positive for anti-GABAAR antibodies in CSF (1:320) and serum (+, titer was not reported) in addition to anti-GABABR antibodies. However, the clinical features of this patient were highly consistent with anti-GABAAR encephalitis. Additionally, Liu et al. (9) reported a case of an 11-year-old male patient who experienced AE 24 days after having Japanese encephalitis, and the main clinical manifestations were fever, headache, irritability, and aggressive behaviour. Anti-GABABR antibodies were positive in CSF and negative in serum. EEG and brain MRI findings were unknown. The patient displayed some mood problems even after immunotherapy. Neither of these two patients were recruited for analysis and discussion in consideration since they did not met the inclusion criteria. Another cohort study described a case of pediatric anti-GABABR encephalitis in a 16-year-old patient, although no clinical data of the patient were reported (7). Kang et al. reported 103 antibody-positive AE patients, which including two patients with anti-GABABR antibodies (10). These two patients were presented with epilepsy and behavioral symptoms. Abnormalities in EEG and brain MRI were also found. Regrettably, one of these two patients was positive for both anti-CASPR2 and anti-GABABR antibodies, while the detailed clinical information of these two cases was difficult to distinguish from the paper. Therefore, these cases were also excluded from the analysis.
Jeffery et al. (6) and Höftberger et al. (5) each reported a case of a patient aged ≤18 years having anti-GABABR encephalitis in their cohort studies, with partial descriptions of clinical data. These two patients were labelled Patient 2 and Patient 3, respectively. Both patients were aged 16 years at disease onset. Patient 2 presented with seizures, delirium, and psychosis, with a serum anti-GABABR antibody titer of 1:1920; the symptoms were completely resolved after treatment with steroids and plasma exchange (PLEX). Patient 3 presented with typical limbic encephalitis (LE) (details unknown), with a positive anti-GABABR antibody in serum; the symptoms were completely resolved after treatment with steroids, IVIG, and PLEX. Tumor was not observed in these two patients. The clinical characteristics of the three analyzed pediatric cases with anti-GABABR encephalitis are summarized in Table 1. The mean age of disease onset was 14.6 years (11 years 10 months–16 years). All the three patients exhibited typical clinical symptoms of LE, tested negative in tumor screening, and displayed a good prognosis after immunotherapy.
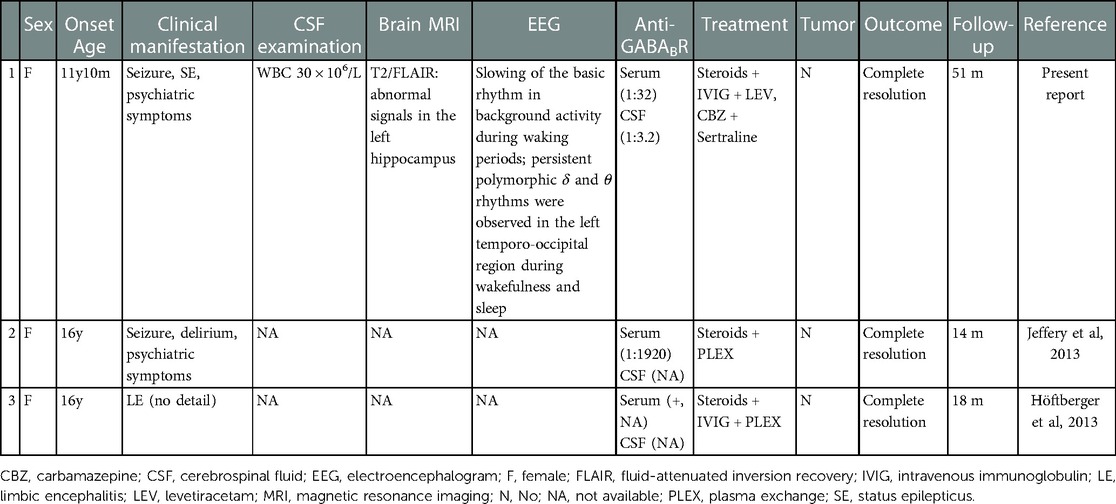
Table 1. Clinical characteristics of three idiopathic pediatric patients with anti-GABABR encephalitis.
This case study reports a pediatric anti-GABABR encephalitis case with the earliest onset age to date. The patient exhibited severe epileptic seizure, SE, psychiatric problems, behavioral abnormalities, elevated CSF cell counts, left hippocampal hyperintensities (as indicated on the T2WI and FLAIR MRI), and positive anti-GABABR in serum and CSF. Immunotherapy was found to be an effective strategy. In addition, only seven pediatric anti-GABABR encephalitis cases were identified through literature search, of which two cases with only anti-GABABR-positive and not secondary to any infections, and their clinical characteristics were analyzed.
Fifteen cases of adult anti-GABABR encephalitis were first reported by Lancaster et al. in 2010 (12). This disease is a GABABR antibody-mediated AE that often involves the limbic system and exhibits the median onset age of 50–65 years on adults (Table 2, Supplementary data S1). The disease exhibits a male predisposition (60%–82%) in adults (13), with seizures as the first symptom followed by incidences of confusion and memory deficits, abnormal behavior, hallucinations, language disorder, sleep disorder, and cerebellar ataxia in a few cases (14–16). Epileptic seizures in patients with anti-GABABR encephalitis are predominantly present as generalized tonic-clonie seizure (17–19). SE is common and displays a high propensity of progressing into refractory epilepsy when multiple ASMs are ineffective. The three pediatric cases summarized in this study presented with LE with epileptic seizures. SE occurred only in Patient 1, although its incidence in other two patients reported in literature is unknown due to lack of information. These clinical characteristics are similar to those reported in studies on adult anti-GABABR encephalitis (Table 2).
Slow-wave activity was observed in the EEG of Patient 1 in this study with no information on the other two included cases. EEG abnormalities comprising predominantly slow waves, followed by epileptiform discharge (18–21) were detected in more than 80% of adult anti-GABABR encephalitis cases (22, 23). Si et al. indicated that the distribution range of the slow-wave activity reflects the severity of anti-GABABR encephalitis (24). In addition, changes in brain imaging can also facilitate the diagnosis and treatment of anti-GABABR encephalitis. Signal anomalies in the unilateral or bilateral medial temporal lobe were observed in T2/FLAIR-weighted images of approximately 45% of patients with anti-GABABR encephalitis (2), and some patients developed hippocampal sclerosis with disease progression (25). Furthermore, limbic system involvement was associated with a poor prognosis (26). In Patient 1 in this study, abnormalities were seen in T2/FLAIR-weighted images in the left hippocampus in early phase of the disease and were significantly reduced after treatment. Although the specificity of imaging manifestations is limited in the acute phase of anti-GABABR encephalitis, long-term brain MRI follow-up can help clinicians in understanding the underlying pathological and physiological processes, thereby providing a theoretical basis for diagnosis and treatment.
Research suggests that other neuronal antibodies, including those against VGCC, AMPAR, and GABAAR and classic paraneoplastic antibodies (Hu, Ri, amphiphysin, SOX-1), could be detected in approximately 7%–40% of patients with GABABR encephalitis (5, 19, 25), leading to variation and overlap of clinical syndromes. Although the Patient 1 was tested to rule out antibodies against NMDAR, AMPAR, GABAAR, LGI1, and CASPR2, a comprehensive screening against the entire set of known AE antigens was not performed, which is one of the limitations of this study. In the other aspect, tumor incidence was identified in the anti-GABABR encephalitis population, as most of the patients were middle-aged or elderly people. Studies have revealed that 50%–60% of patients with anti-GABABR encephalitis develop tumor. Small cell lung carcinoma has been the mostly commonly observed tumor, followed by thymic carcinoma, bladder cancer, and breast cancer (5, 27, 28). Furthermore, tumor progression is the most common cause of death in anti-GABABR encephalitis (21). However, this type of AE is highly responsive to immunotherapy. No tumor was found in the three cases of pediatric anti-GABABR encephalitis summarized in this study. Nevertheless, considering the correlation between the disease and tumor, susceptibility to tumor in pediatric patients with anti-GABABR encephalitis cannot be ruled out. Tumor was identified after encephalitis in the majority of previous cohort studies. Therefore, long-term follow-up is needed for pediatric patients with anti-GABABR encephalitis, despite negative results observed in the initial tumor screening. Graus et al. updated the diagnostic criteria for PNS, which recommend repeated tumor screening every 4–6 months for 2 years for patients with high-risk phenotypes (e.g., encephalomyelitis, LE, opsoclonus-myoclonus) and high-risk antibodies (e.g., Hu, CV2, SOX1, Yo), if no tumor is found in the initial examination (29).
Treatment for AEs mainly includes immunomodulation therapy, treatment of primary tumor, and symptomatic treatment. Encephalitis associated with antibodies against cell-surface proteins are mediated primarily by humoral immunity which is respond good to immunosuppression. Furthermore, if a tumor is identified, early tumor treatment is particularly crucial for a good outcome. Studies have suggested that most patients with anti-GABABR encephalitis demonstrate complete or partial recovery of neurological functions after receiving immunotherapy (5, 15, 16, 30, 31). Moreover, immunotherapy delay is an independent predictor for epilepsy (32). Therefore, immunotherapy should be administered promptly after definitive diagnosis. Currently, the recommended first-line immunotherapies include treatment with steroid, IVIG, and PLEX, whereas second-line immunosuppressants such as rituximab and cyclophosphamide are generally used to treat refractory cases. All the three pediatric patients reported in this study received immunotherapy, did not develop tumor, and had a good outcome.
Given the low incidence of anti-GABABR encephalitis and the rarer incidence of the disease in pediatric patients, there were three pediatric cases with only anti-GABABR encephalitis could be analysed until now. Among of these cases, we reported a case with an early onset age of 11 years and 10 months. The phenotype of pediatric anti-GABABR encephalitis is similar to that in adults, in whom the disease presents primarily as LE; however, tumors are rare in pediatric cases. With timely immunotherapy administration, the prognosis of pediatric patients may become superior to that of adult patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics Committee of the Children’s Hospital of Zhejiang University School of Medicine. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
YW: designed and conceptualized the study, interpreted and analyzed the data, and drafted and revised the manuscript for intellectual content. XR, YS, and YH: played a major role in the acquisition of data and revised the manuscript for intellectual content. LY, WZ, and GS: revised the manuscript for intellectual content. LL and FG: designed and conceptualized the study, interpreted, and analyzed the data, and revised the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Key Research and Development Programme of Zhejiang Province (Grant No. 2020C03038), National Natural Science Foundation of China (Grant No. 82001232), Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ19H090002); Key social development projects of Jinhua science and technology plan project, Grant NO. 2022-3-128; Research and cultivation fund project of Jinhua Maternity and Child Health Care Hospital, Grant NO. HFB2021-2-03.
The authors are deeply grateful to the family who participated in this research. This work was supported by the Key Research and Development Programme of Zhejiang Province (Grant No. 2020C03038), National Natural Science Foundation of China (Grant No. 82001232), and Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ19H090002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1104001/full#supplementary-material.
1. Lancaster E, Dalmau J. Neuronal autoantigens–pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. (2012) 8(7):380–90. doi: 10.1038/nrneurol.2012.99
2. Dalmau J, Graus F. Antibody-Mediated encephalitis. N Engl J Med. (2018) 378(9):840–51. doi: 10.1056/NEJMra1708712
3. Ulrich D, Bettler B. GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr Opin Neurobiol. (2007) 17(3):298–303. doi: 10.1016/j.conb.2007.04.001
4. Benarroch EE. GABAB Receptors: structure, functions, and clinical implications. Neurology. (2012) 78(8):578–84. doi: 10.1212/WNL.0b013e318247cd03
5. Hoftberger R, Titulaer MJ, Sabater L, Dome B, Rozsas A, Hegedus B, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. (2013) 81(17):1500–6. doi: 10.1212/WNL.0b013e3182a9585f
6. Jeffery OJ, Lennon VA, Pittock SJ, Gregory JK, Britton JW, McKeon A. GABAB Receptor autoantibody frequency in service serologic evaluation. Neurology. (2013) 81(10):882–7. doi: 10.1212/WNL.0b013e3182a35271
7. Hayden Z, Borocz K, Csizmadia Z, Balogh P, Kellermayer Z, Bodo K, et al. Single-center study of autoimmune encephalitis-related autoantibody testing in Hungary. Brain Behav. (2019) 9(12):e01454. doi: 10.1002/brb3.1454
8. Kruer MC, Hoeftberger R, Lim KY, Coryell JC, Svoboda MD, Woltjer RL, et al. Aggressive course in encephalitis with opsoclonus, ataxia, chorea, and seizures: the first pediatric case of gamma-aminobutyric acid type B receptor autoimmunity. JAMA Neurol. (2014) 71(5):620–3. doi: 10.1001/jamaneurol.2013.4786
9. Liu B, Liu J, Sun H, Xie M, Yang C, Pan Y, et al. Autoimmune encephalitis after Japanese encephalitis in children: a prospective study. J Neurol Sci. (2021) 424:117394. doi: 10.1016/j.jns.2021.117394
10. Kang Q, Liao H, Yang L, Fang H, Hu W, Wu L. Clinical characteristics and short-term prognosis of children with antibody-mediated autoimmune encephalitis: a single-center cohort study. Front Pediatr. (2022) 10:880693. doi: 10.3389/fped.2022.880693
11. Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. (2014) 13(3):276–86. doi: 10.1016/S1474-4422(13)70299-0
12. Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. (2010) 9(1):67–76. doi: 10.1016/S1474-4422(09)70324-2
13. Marinas JE, Matveychuk D, Dursun SM, Baker GB. Neuroimmunological antibody-mediated encephalitis and implications for diagnosis and therapy in neuropsychiatry. Acta Neuropsychiatr. (2020) 32(4):177–85. doi: 10.1017/neu.2019.50
14. McKay JH, Dimberg EL, Lopez Chiriboga AS. A systematic review of gamma-aminobutyric acid receptor type B autoimmunity. Neurol Neurochir Pol. (2019) 53(1):1–7. doi: 10.5603/PJNNS.a2018.0005
15. Wang Y, Yu Y, Hu Y, Li Y, Song F, Wang Y. Clinical and electroencephalographic features of the seizures in neuronal surface antibody-associated autoimmune encephalitis. Front Neurol. (2020) 11:280. doi: 10.3389/fneur.2020.00280
16. Cui J, Bu H, He J, Zhao Z, Han W, Gao R, et al. The gamma-aminobutyric acid-B receptor (GABAB) encephalitis: clinical manifestations and response to immunotherapy. Int J Neurosci. (2018) 128(7):627–33. doi: 10.1080/00207454.2017.1408618
17. Wu H, Wang Y, Wei K, Qiao S, Liu L, Zhang R, et al. Clinical characteristics and elevated ProGRP and positive oligoclonal bands of 13 Chinese cases with anti-GABABR encephalitis. Int J Dev Neurosci. (2021). doi: 10.1002/jdn.10121
18. Chen X, Liu F, Li JM, Xie XQ, Wang Q, Zhou D, et al. Encephalitis with antibodies against the GABAB receptor: seizures as the most common presentation at admission. Neurol Res. (2017) 39(11):973–80. doi: 10.1080/01616412.2017.1351062
19. van Coevorden-Hameete MH, de Bruijn M, de Graaff E, Bastiaansen D, Schreurs MWJ, Demmers JAA, et al. The expanded clinical spectrum of anti-GABABR encephalitis and added value of KCTD16 autoantibodies. Brain. (2019) 142(6):1631–43. doi: 10.1093/brain/awz094
20. Qiao S, Zhang YX, Zhang BJ, Lu RY, Lai QL, Chen LH, et al. Clinical, imaging, and follow-up observations of patients with anti-GABAB receptor encephalitis. Int J Neurosci. (2017) 127(5):379–85. doi: 10.1080/00207454.2016.1176922
21. Lin J, Li C, Li A, Liu X, Wang R, Chen C, et al. Encephalitis with antibodies against the GABAB receptor: high mortality and risk factors. Front Neurol. (2019) 10:1030. doi: 10.3389/fneur.2019.01030
22. Yeshokumar AK, Coughlin A, Fastman J, Psaila K, Harmon M, Randell T, et al. Seizures in autoimmune encephalitis-A systematic review and quantitative synthesis. Epilepsia. (2021) 62(2):397–407. doi: 10.1111/epi.16807
23. Ghimire P, Khanal UP, Gajurel BP, Karn R, Rajbhandari R, Paudel S, et al. Anti-LGI1, anti-GABABR, and anti-CASPR2 encephalitides in Asia: a systematic review. Brain Behav. (2020) 10(10):e01793. doi: 10.1002/brb3.1793
24. Si Z, Wang A, Liu J, Zhang Z, Hu K. Typical clinical and imaging manifestations of encephalitis with anti-gamma-aminobutyric acid B receptor antibodies: clinical experience and a literature review. Neurol Sci. (2019) 40(4):769–77. doi: 10.1007/s10072-018-3679-5
25. Dogan Onugoren M, Deuretzbacher D, Haensch CA, Hagedorn HJ, Halve S, Isenmann S, et al. Limbic encephalitis due to GABAB and AMPA receptor antibodies: a case series. J Neurol Neurosurg Psychiatry. (2015) 86(9):965–72. doi: 10.1136/jnnp-2014-308814
26. Zhang X, Lang Y, Sun L, Zhang W, Lin W, Cui L. Clinical characteristics and prognostic analysis of anti-gamma-aminobutyric acid-B (GABA-B) receptor encephalitis in northeast China. BMC Neurol. (2020) 20(1):1. doi: 10.1186/s12883-019-1585-y
27. Gaspard N. Guilty by association: kCTD16 and GABABR antibodies in paraneoplastic limbic encephalitis. Epilepsy Curr. (2019) 19(6):372–5. doi: 10.1177/1535759719877891
28. Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. (2017) 97(2):839–87. doi: 10.1152/physrev.00010.2016
29. Graus F, Vogrig A, Muniz-Castrillo S, Antoine JG, Desestret V, Dubey D, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. (2021) 8(4). doi: 10.1212/NXI.0000000000001014
30. de Bruijn M, van Sonderen A, van Coevorden-Hameete MH, Bastiaansen AEM, Schreurs MWJ, Rouhl RPW, et al. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. Neurology. (2019) 92(19):e2185–e96. doi: 10.1212/WNL.0000000000007475
31. Kim TJ, Lee ST, Shin JW, Moon J, Lim JA, Byun JI, et al. Clinical manifestations and outcomes of the treatment of patients with GABAB encephalitis. J Neuroimmunol. (2014) 270(1–2):45–50. doi: 10.1016/j.jneuroim.2014.02.011
Keywords: anti-GABABR encephalitis, autoimmune encephalitis, pediatric, benign prognosis, immunotherapy
Citation: Wang Y, Ren X, Shen Y, Hua Y, Xu L, Zhang W, Sheng G, Jiang P, Yuan Z, Liu L and Gao F (2023) Case report: Pediatric anti-gamma aminobutyric acid-B receptor encephalitis with benign prognosis. Front. Pediatr. 11:1104001. doi: 10.3389/fped.2023.1104001
Received: 21 November 2022; Accepted: 15 February 2023;
Published: 3 March 2023.
Edited by:
Yi-Chia Wei, Chang Gung Memorial Hospital, TaiwanReviewed by:
Ruzica Kravljanac, The Institute for Health Protection of Mother and Child Serbia, Serbia© 2023 Wang, Ren, Shen, Hua, Xu, Zhang, Sheng, Jiang, Yuan, Liu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Gao ZXBpbGVwc3lAemp1LmVkdS5jbg== Liu Liu b3NpZXIwNjE1eHdAemp1LmVkdS5jbg==
†These authors have contributed equally to this work.
‡ORCID Liu Liu orcid.org/0000-0002-3641-5297 Feng Gao orcid.org/0000-0003-4907-7212
Specialty Section: This article was submitted to Pediatric Neurology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.