- 1Department of Pediatric, Ningbo Yinzhou No. 2 Hospital, Ningbo, China
- 2The Public Health College, Zhejiang Chinese Medical University, Hangzhou, China
- 3The First Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 4State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, Zhejiang University of Technology, Hangzhou, China
- 5The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 6The Medical Imaging College, Hangzhou Medical College, Hangzhou, China
Background: The kangaroo-mother care method (KMC) is a skin-to-skin contact-centered care approach with numerous benefits for neonates, but its impact on the treatment of jaundiced neonates is unknown. This study aimed to investigate the efficacy of KMC combined with neonatal phototherapy (NNPT) in treating neonates with non-pathological jaundice.
Methods: Relevant articles were searched in PubMed, Embase, Web of Science, and Cochrane Library databases from database establishment to April 2022. The outcomes included, without limitation, serum bilirubin levels, and duration of phototherapy.
Results: This meta-analysis included five studies (4 randomized controlled trials and 1 observational study) involving four hundred eighty-two neonates with non-pathological jaundice. The results showed that the group receiving KMC combined with NNPT had lower serum bilirubin at 72 h after intervention [weighted mean difference (WMD) = −1.51, p = 0.03], shorter duration of phototherapy [standard mean difference (SMD) = −1.45, p < 0.001] and shorter duration of hospitalization (SMD = −1.32, p = 0.002) compared to NNPT group. There was no difference in peak bilirubin in both groups of neonates (WMD = −0.12, p = 0.62).
Conclusions: KMC combined with NNPT helped to treat non-pathological jaundice in newborns compared to NNPT alone.
Introduction
Unconjugated hyperbilirubinemia often occurs in neonates. Jaundice symptoms are often physiological or caused by breastfeeding (1). It affects 85% of neonates (2), and while the prognosis is favorable for most of them, pathological jaundice, bilirubin encephalopathy, hearing loss, and seizures may develop without appropriate monitoring or treatment (3). It is a disease burden in countries of all income levels and often arouses physicians' concerns and parents’ anxiety (4).
Neonatal phototherapy (NNPT), exchange transfusion, and pharmacotherapy are applied to treat neonatal jaundice (5). Since the 1950s, NNPT has been the preferred treatment for neonatal jaundice, as it can reduce serum indirect bilirubin levels, and prevent acute and chronic encephalopathy (6–8). However, some studies showed that NNPT could cause short-term side effects such as heat and water-electrolyte imbalance, bronze baby syndrome, the influence on the retina of the eye (7, 9, 10), and long-term side effects such as tumors (7, 11, 12) and allergic diseases (7, 10).
Kangaroo-mother care method (KMC) was first reported in a Columbia hospital in 1984. KMC entails skin-to-skin care of the neonate in a kangaroo position at or shortly after birth (with the naked infant in a prone position on the mother's exposed chest and abdomen), as well as breastfeeding and close follow-up, as appropriate (13, 14). Studies showed that KMC facilitated a reduction in neonatal illness and the occurrence of nosocomial infections, decreasing mortality in low-birth-weight infants, and lessening the duration of hospital stays and medical costs (13, 15). It has also been reported that jaundiced newborns performing KMC favored a shorter duration of phototherapy (16, 17). To date, no relevant meta-analysis has been found in the database. Therefore, the present study aimed to investigate the efficacy of KMC combined with NNPT in treating neonatal non-pathological jaundice.
Method
Search strategy
This meta-analysis was directed by recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (18). Two researchers systematically retrieved electronic databases (PubMed, Embase, Web of Science, and Cochrane Library databases) using prearranged terms from database inception to April 2022 and primarily searched for articles related to KMC intervention for neonatal jaundice. The “PICOS” principles (Participant, Intervention, Comparison, Outcome, Study design) were used throughout the meta-analysis. The entire formula used for searching was as follows: (“Infant, Newborn” [Mesh] OR “Infants, Newborn” OR “Newborn Infant” OR “Newborn Infants” OR “Newborns” OR “Newborn” OR “Neonate” OR “Neonates” OR “Infant” [Mesh] OR “Infants”) AND (“Jaundice” [Mesh] OR “Icterus” OR “Hyperbilirubinemias” OR “Bilirubinemia” OR “Bilirubinemias” OR “Hyperbilirubinemia” [Mesh]) AND (“Kangaroo-Mother Care Method” [Mesh] OR “Care Method, Kangaroo-Mother” OR “Care Methods, Kangaroo-Mother” OR “Kangaroo Mother Care Method” OR “Kangaroo-Mother Care Methods” OR “Method, Kangaroo-Mother Care” OR “Methods, Kangaroo-Mother Care” OR “Kangaroo Mother Care” OR “Care, Kangaroo Mother” OR “Kangaroo-Mother Care” OR “Care, Kangaroo-Mother” OR “Skin-to-skin contact” OR “KMC” OR “SSC”). In addition, we reviewed the reference lists of the retrieved articles to identify more relevant studies. The Prospero registration number for this meta-analysis was CRD42022344508.
Inclusion and exclusion criteria
The studies included met the following criteria: (1) the original research was a randomized controlled trial (RCT) or observational study. (2) The study population was non-pathologically jaundiced newborns. (3) The intervention group used KMC based on NNPT. (4) Control group had NNPT without KMC. (5) Articles published in English.
The exclusion criteria were as follows: (1) relevant or detailed data could not be extracted, or the article could not be integrated with data from other articles. (2) The study was still in the protocol stage or was ongoing, or the full text was unavailable. (3) The study was from the same group of participants. The latest or most complete research was included as the article is continuously updated.
Indicator measurement
KMC may encompass a range of mother-to-infant interventions, primarily referring to skin-to-skin contact between mother and infant (14). NNPT is a method of treating neonatal jaundice by irradiating the skin to lower serum bilirubin levels. During NNPT, the baby is naked except for the eyes and genitals.
Data extraction and quality evaluation
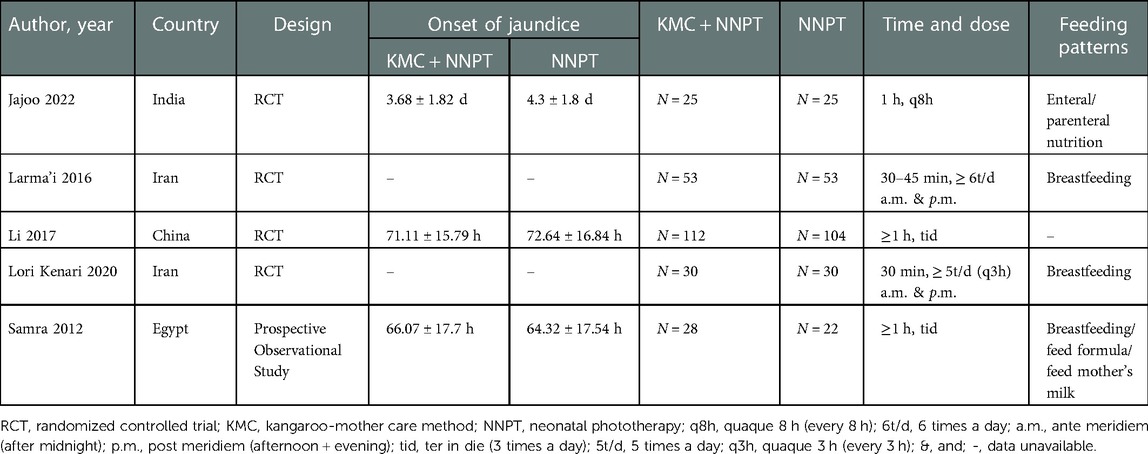
Two independent researchers extracted data from articles that met the inclusion criteria using a predesigned data extraction form and assessed study quality. When disagreements arose, a consensus was completed with a third researcher. The following information was extracted: authors, year of publication, country, study design, the onset of jaundice, the sample size of intervention and control groups, KMC usage frequency, feeding patterns, gestational age, weight, outcome indicators, and inclusion and exclusion criteria for neonatal recruitment in studies.
The methodological quality of RCT was assessed using the Cochrane Collaboration Risk of Bias Assessment Tool (19), categorized as low risk, high risk, or unclear risk. The quality of observational studies was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) with a maximum score of 9, and articles with high study quality scored 6–9 (20). The evaluation results were displayed in the form of graphs or charts.
Statistical analysis
The extracted data were analyzed by Review Manager 5.3 analysis software. Continuous variables were evaluated by weighted mean difference (WMD) or standardized mean difference (SMD) and 95% confidence interval (CI). WMD truly reflected the trial effect in original units, and SMD was suitable for pooled analysis of data with different units or large differences in means. A value of p < 0.05 was statistically significant. The X2 test was primarily used to detect the heterogeneity in the study, which was quantified using the I2 statistic (21). As I2 ≥ 50%, heterogeneity was considered statistically significant, and a random-effects model was used; otherwise, a fixed-effects model was used (22, 23). Funnel plots were used to test for publication bias or other biases (24, 25). When the number of included original studies was more than ten (26), the stability of the results could be tested by excluding literature on a case-by-case basis for sensitivity analysis.
Results
Literature retrieval
A total of 140 relevant articles were retrieved from electronic databases, and one relevant article was included in the reference list after review. After removing duplicate research papers, there were 86 articles left, and 76 were excluded by reading the titles and abstracts; the remaining ten articles were obtained in full and reviewed. Five of these articles were excluded for the following reasons: articles were published in a language other than English (n = 1). The study population was not non-pathologically jaundiced neonates (n = 2). Relevant data were unavailable for the article (n = 2). Finally, the rest five articles were included in this meta-analysis (16, 17, 27–29). A detailed PRISMA flowchart of the detailed search process and reasons for exclusion is displayed in Figure 1.
Study characteristics and quality assessment
The characteristics of all articles included in the meta-analysis are listed in Table 1. Five articles were selected, including four RCTs (17, 27–29) and one prospective observational study (16), conducted in different countries: two in Iran (27, 29), one in India (17), one in China (28), and one study in Egypt (16). The analysis included four hundred eighty-two neonates with non-pathological jaundice, with two hundred forty-eight in the intervention group and two hundred thirty-four in the control group. The intervention was KMC combined with NNPT, and the control measure was NNPT. Information on the recruitment criteria for neonates of original studies is presented in Supplementary Table S1.
This meta-analysis assessed the quality of all five included studies, and the quality of the four RCTs was appraised using the Cochrane Collaboration Risk of Bias Assessment Tool. Three studies were assessed as an unclear risk due to insufficient information on the generated sequences in random sequence generation, and the remaining one was evaluated as low risk. Regarding allocation concealment, three studies were categorized as unclear risk and one as high risk. About implementation blinding, three studies were judged as high risk and one as low risk. Four studies were rated as low risk concerning incomplete outcome data, selective outcome reporting, and free of other biases. NOS was used to rate the quality of one observational study with a score of seven. Detailed quality assessment results are presented in Supplementary Table S2.
Serum bilirubin at 72 h post-intervention
Serum bilirubin levels after 72 h in participants after KMC combined with NNPT and NNPT alone were provided by two studies (27, 29), with eighty-three neonates in each group. Neonates receiving KMC combined with NNPT had lower serum bilirubin at 72 h post-intervention than controls (WMD = −1.51, 95%CI: (−2.85)–(−0.16), p = 0.03), (Figure 2). It indicated that KMC combined with NNPT contributed to a decrease in serum bilirubin 72 h after the intervention compared to the control group.
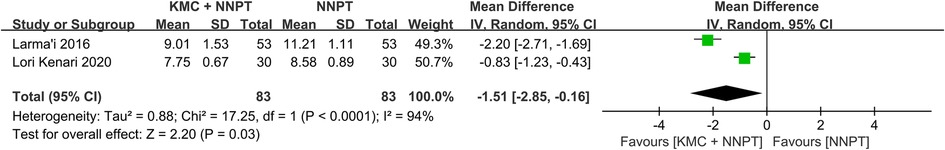
Figure 2. Forest plot comparing KMC + NNPT with NNPT alone on serum bilirubin at 72 h after intervention in neonates with non-pathological jaundice.
Peak bilirubin
The effect of KMC combined with NNPT vs. NNPT alone on peak bilirubin in neonates with non-pathological jaundice was reported in two studies (16, 28). It was found that in the intervention group (one hundred forty) and in the control group (one hundred twenty-six) showed homogeneity between studies with no statistical difference between both groups (WMD = −0.12, 95%CI: (−0.58)–0.34, p = 0.62, I2 = 0%) (Figure 3).
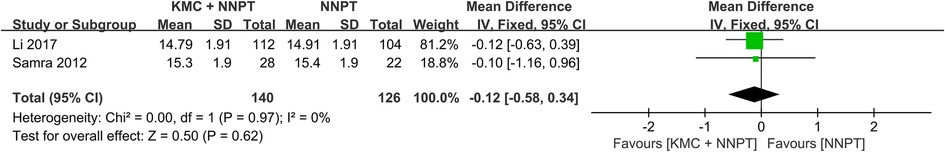
Figure 3. Forest plot comparing KMC + NNPT with NNPT alone on peak bilirubin in neonates with non-pathological jaundice.
Duration of phototherapy
The duration of phototherapy in neonates with non-pathological jaundice in the KMC combined with NNPT group (two hundred forty-eight) and in the control group (two hundred thirty-four) was reported in five studies (16, 17, 27–29). It was found that neonates who received KMC combined with NNPT had a shorter duration of phototherapy than the NNPT alone group. However, there was relatively large heterogeneity between the included studies (SMD = −1.45, 95%CI: (−1.92)–(−0.98), p < 0.001, I2 = 78%) (Figure 4). It showed that KMC combined with NNPT helped reduce phototherapy duration.
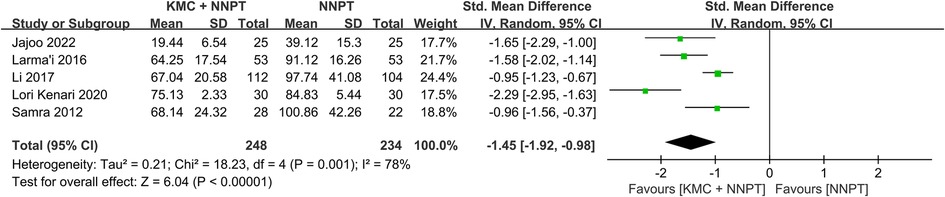
Figure 4. Forest plot comparing KMC + NNPT with NNPT alone on the duration of phototherapy in neonates with non-pathological jaundice.
Duration of hospitalization
According to statistics from three studies (one hundred eight neonates in each group) (17, 27, 29), who received KMC combined with NNPT spent less time in the hospital than control (SMD = −1.32, 95%CI: (−2.17)–(−0.46), p = 0.002) (Figure 5). It demonstrated that the intervention facilitated a shorter duration of hospitalization for neonates with non-pathological jaundice.
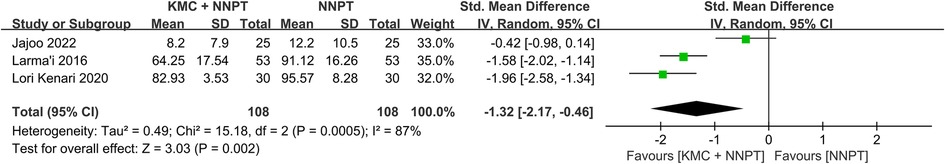
Figure 5. Forest plot comparing KMC + NNPT with NNPT alone on the duration of hospitalization in neonates with non-pathological jaundice.
Publication bias
The presence of publication bias was tested by funnel plot. Compared with the control group, the funnel plot of KMC combined with NNPT on the duration of phototherapy showed a roughly symmetrical inverted funnel shape indicating no apparent publication bias (Supplementary Figure S1).
Discussion
Jaundice may occur in neonates for the following reasons, (1) high number and short life span of red blood cells in neonates (30). (2) Low levels of bindable proteins in hepatocytes (31). (3) Low catalytic activity of uridine diphosphate glucuronosyltransferase (UDPGT) (31). (4) Higher β-glucuronidase in the neonatal intestine increases the hydrolysis of bound bilirubin (32). (5) Neonatal bilirubin is less stable and easily hydrolyzed in the intestine and transported back to the liver and circulatory system via enterohepatic circulation (31).
Initially, NNPT was used to treat unconjugated hyperbilirubinemia to reduce the use of blood exchange therapy. The mechanism of NNPT action lies in the conversion of naturally toxic nonpolar Z, Z-bilirubin into polar and water-soluble photoisomerization products as well as structural isomers (30, 33). The irreversibly formed structural isomer lumirubin (30) has greater solubility and a short half-life in plasma (34). It is readily excreted from urine and bile (30). It was considered the main mechanism (34). In addition, a small fraction of bilirubin is converted into products excreted through urine by degradation (35) or photooxidation (30, 36).
NNPT using visible light to treat neonatal unconjugated hyperbilirubinemia is often considered an inexpensive, simple, most used, and relatively safe treatment option (30, 33, 37). However, there are some short- or long-term side effects of NNPT (33), such as damage to the erythrocyte membrane and bronchial and pulmonary dysplasia (38). As Bergman (39) pointed out, “skin-to-skin contact is the natural “habitat’ for human infants.” When this contact is hindered, the newborn will cry. Most notably, the use of NNPT alone separates the mother from the infant, whereas the combination of skin-to-skin contact care meets the emotional needs of the mother and infant (40) and reduces neonatal stress response (41). For decades, KMC has been regarded as a resultful, noninvasive, simple, and cost-effective care method, particularly when used in low-resource settings in developing countries (42–45). KMC has multiple benefits for neonates in the short or long term, such as promoting breastfeeding (45, 46), stabilizing neonatal physiology (47), and regulating microbiota (48).
The mechanisms by which KMC is effective in jaundiced newborns include the direct effect of the intervention on the newborn, the feedback effect of maternal benefit, and other benefits to the newborn. Firstly, babies who receive KMC develop specific behavioral patterns, such as “crawling” to their mother's nipples (49, 50), and the mother's lactation reflex is enhanced, resulting in increased milk production (49). Consequently, KMC contributed to breastfeeding (13, 51–53). Breastfeeding reduced the enterohepatic circulation of bilirubin and lowered serum bilirubin levels via the three pathways described below. (1) Breastfeeding increased neonatal feeding, providing adequate nutrition and calories to babies (54), allowing them to gain body weight (49, 54), improving intestinal motility, and accelerating meconium excretion (55, 56). Meconium excretion was associated with unconjugated bilirubin levels (54) and bilirubin enterohepatic circulation (55, 56). Additionally, increased caloric intake helped reduce the release of fatty acids that interfered with the formation of conjugated bilirubin (57) or favored UDPGT production (58), lowering serum bilirubin concentration. (2) Breastfeeding decreased supplemental formula, and therefore water intake was reduced (55, 59). Therefore, the enterohepatic circulation of bilirubin was decreased, and jaundice was also improved. (3) Breastfeeding led to earlier maturation of bilirubin-binding enzymes in the liver of babies (16, 60). Apart from breastfeeding reduced enterohepatic circulation of serum bilirubin through various pathways, neonates receiving KMC lay prone on the mother's chest and abdomen, and the vibrations generated by the contact accelerated intestinal excretion, which contributed to bilirubin elimination from the body via the digestive pathway (16, 27). Thus, serum bilirubin levels were reduced. The decrease in serum bilirubin at 72 h after KMC intervention could be explained by the decrease in serum bilirubin levels.
Simultaneously, the benefits of KMC for the mother are fed back to the newborn, assisting the neonate in reducing the degree of jaundice and resulting in a virtuous circle. This study would describe three parts: the mother's emotional, vagal, and hormonal regulation. (1) KMC shortened mother-infant separation time due to NNPT, improving the mother's mood (61), promoting breastfeeding (62), and helping the neonate recover from the jaundice state. (2) Frequent touching of the newborn by mother receiving KMC stimulated the vagus nerve of the newborn (63) and promoted gastric motility, which helps to lower serum bilirubin (64), thereby shortening the length of hospital stay. (3) KMC regulated neonatal-related hormone levels synergistically by regulating the mother's endocrine system. On the one hand, salivary cortisol is a stress hormone released by the hypothalamic-pituitary-adrenal axis (65, 66). KMC reduced salivary cortisol levels in neonates (51, 67), improving sleep-wake cycles (51). On the other hand, KMC synergistically promoted the synthesis of oxytocin in the hypothalamus of mothers and infants (67). It is important in reducing maternal stress anxiety and neonatal stress response, enabling positive maternal-infant interactions, and promoting breastfeeding (68–70). Apart from this, increased oxytocin release raised the temperature at the mother's breast, promoting a synergistic regulation of the mother's body temperature and the heat requirements of the newborn, which facilitated the maintenance of body temperature stability (51, 71).
Finally, other effects of KMC on the baby can also impact the recovery of jaundiced newborns. (1) KMC alleviated neonatal pain (72, 73). (2) KMC reduced the amount of energy consumed by newborns due to crying (51, 74), so more energy was available for increased bowel movements, weight gain, and health recovery. All of this contributed to lower neonatal serum bilirubin levels and shortened the duration of phototherapy and hospital stay.
The cessation time of NNPT in the included studies was defined according to a decline in serum bilirubin to certain levels. The findings of this study revealed that the serum bilirubin level was lower in the KMC group after 72 h of intervention than in the control group. Therefore, the time required by NNPT to reduce the serum bilirubin levels to the corresponding threshold was also shorter in the KMC group, which might explain the shorter duration of phototherapy in the KMC group. Most included studies were neonates admitted for jaundice (16, 27, 29), and the main treatment objective was to improve jaundice and reduce serum bilirubin levels (75). In the KMC group, there was an advantage in the shortening of hospital stays due to lower serum bilirubin levels and less duration of phototherapy. Less time spent in the hospital helped to increase mother-infant interaction, reducing the financial burden on families and lower medical costs (76). Peak bilirubin is the highest value of total serum bilirubin (77). This study showed no difference in peak bilirubin between the two groups, and the mechanism needs further investigation.
Since the outbreak of coronavirus disease 2019 (COVID-19), the epidemic has spread rapidly among people in close contact (78), so methods of separating people from each other, such as wearing masks and drawing one-meter lines, have become popular. However, this posed a challenge to maternity (79), which might mean that mothers could not be in close contact with their newborns, adversely affecting both mother and baby. If COVID-19 is suspected or diagnosed, the World Health Organization recommends that mothers and infants could use skin-to-skin contact and other measures (80). However, there was no evidence of vertical transmission from a coronavirus-infected mother (81), and increased virus transmission was not observed in newborns receiving skin-to-skin care (82). As a result, the World Health Organization recommendations for neonatal skin care could be followed (80).
As far as we know, no previous meta-analyses have been conducted to evaluate the effect of KMC combined with NNPT on outcomes in jaundiced neonates. Only one systematic review (76) of qualitative analyses reported changes in bilirubin levels and phototherapy duration. We are the first meta-analysis comparing KMC combined with NNPT vs. NNPT alone for neonatal jaundice in strict accordance with PRISMA guidelines.
Although the present meta-analysis generated objective results, there were some limitations. The first was the limited number of studies that could be included, which prevented our study from determining the effect of KMC on other outcomes, such as skin bilirubin. Secondly, some studies reported that in breastfed newborns, proper feeding can alleviate jaundice (60). Feeding patterns was correlated with neonatal jaundice, but there are limited data related to feeding patterns. Therefore, subgroup analysis could not be done in this study to explore its effect on jaundice. In addition, the design of the included studies prevented us from exploring the role of KMC on different types of jaundice in newborns. Finally, the overall findings should be treated with caution because the small number of studies, as well as the different sample sizes, design methods, and confounding factors of different studies, made this study somewhat heterogeneous, resulting in a decrease in credibility inevitably.
In conclusion, KMC combined with NNPT was more effective than NNPT alone in treating non-pathological jaundice in newborns. KMC combined with NNPT reduced serum bilirubin at 72 h after intervention in jaundiced neonates and shortened the duration of phototherapy and hospitalization. However, there was no apparent benefit of the intervention in peak bilirubin. Additional large RCTs are required to provide more data to validate the findings of this study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
SPZ designed the research process. XH and MLC searched the database for corresponding articles. RRF and MLC extracted useful information from the articles above. WH and RRF used statistical software for analysis. XH, MLC and YJH drafted the meta-analysis. XH, SPZ and HJST polished this article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1098143/full#supplementary-material.
Abbreviations
KMC, kangaroo-mother care method; NNPT, neonatal phototherapy; WMD, weighted mean difference; SMD, standardized mean difference; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; NOS, Newcastle-Ottawa Quality Assessment Scale; CI, confidence interval; UDPGT, uridine diphosphate glucuronosyltransferase; COVID-19, coronavirus disease 2019; q8h, quaque 8 h (every 8 h); 6t/d, 6 times a day; a.m., ante meridiem (after midnight); p.m., post meridiem (afternoon + evening); tid, ter in die (3 times a day); 5t/d, 5 times a day; q3h, quaque 3 h (every 3 h); &, and; GA, gestational age; wk, weeks; -, data unavailable.
References
1. Abbey P, Kandasamy D, Naranje P. Neonatal jaundice. Indian J Pediatr. (2019) 86(9):830–41. doi: 10.1007/s12098-019-02856-0
2. Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage–mechanisms and management approaches. N Engl J Med. (2013) 369(21):2021–30. doi: 10.1056/NEJMra1308124
4. Olusanya BO, Kaplan M, Hansen TWR. Neonatal hyperbilirubinaemia: a global perspective. Lancet Child Adolesc Health. (2018) 2(8):610–20. doi: 10.1016/S2352-4642(18)30139-1
5. Mishra S, Agarwal R, Deorari AK, Paul VK. Jaundice in the newborns. Indian J Pediatr. (2008) 75(2):157–63. doi: 10.1007/s12098-008-0024-7
6. Woodgate P, Jardine LA. Neonatal jaundice: phototherapy. BMJ Clin Evid. (2015) 2015:0319.25998618
7. Faulhaber FRS, Procianoy RS, Silveira RC. Side effects of phototherapy on neonates. Am J Perinatol. (2019) 36(3):252–7. doi: 10.1055/s-0038-1667379
8. Okwundu CI, Okoromah CA, Shah PS. Cochrane review: prophylactic phototherapy for preventing jaundice in preterm or low birth weight infants. Evid Bsed Child Health. (2013) 8(1):204–49. doi: 10.1002/ebch.1898
9. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. (2004) 114(1):297–316. doi: 10.1542/peds.114.1.297
10. Xiong T, Qu Y, Cambier S, Mu D. The side effects of phototherapy for neonatal jaundice: what do we know? What should we do? Eur J Pediatr. (2011) 170(10):1247–55. doi: 10.1007/s00431-011-1454-1
11. Newman TB, Wickremasinghe AC, Walsh EM, Grimes BA, McCulloch CE, Kuzniewicz MW. Retrospective cohort study of phototherapy and childhood cancer in northern California. Pediatrics. (2016) 137(6):e20151354. doi: 10.1542/peds.2015-1354
12. Wickremasinghe AC, Kuzniewicz MW, Grimes BA, McCulloch CE, Newman TB. Neonatal phototherapy and infantile cancer. Pediatrics. (2016) 137(6):e20151353. doi: 10.1542/peds.2015-1353
13. Boundy EO, Dastjerdi R, Spiegelman D, Fawzi WW, Missmer SA, Lieberman E, et al. Kangaroo mother care and neonatal outcomes: a meta-analysis. Pediatrics. (2016) 137(1):e20152238. doi: 10.1542/peds.2015-2238
14. Chan GJ, Valsangkar B, Kajeepeta S, Boundy EO, Wall S. What is kangaroo mother care? Systematic review of the literature. J Glob Health. (2016) 6(1):010701. doi: 10.7189/jogh.06.010701
15. Lawn JE, Mwansa-Kambafwile J, Barros FC, Horta BL, Cousens S. “Kangaroo mother care” to prevent neonatal deaths due to pre-term birth complications. Int J Epidemiol. (2011) 40(2):525–8. doi: 10.1093/ije/dyq172
16. Samra NM, El Taweel A, Cadwell K. The effect of kangaroo mother care on the duration of phototherapy of infants re-admitted for neonatal jaundice. J Mater Fetal Neonatal Med. (2012) 25(8):1354–7. doi: 10.3109/14767058.2011.634459
17. Jajoo M, Dhingra D, Chandil A, Jain R. Effect of kangaroo mother care on duration of phototherapy on neonatal jaundice: a randomized controlled trial. Indian J Pediatr. (2022) 89(5):507–9. doi: 10.1007/s12098-021-04013-y
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
19. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
20. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
22. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. (1959) 22(4):719–48. doi: 10.1093/jnci/22.4.719
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
24. van Aert RCM, Wicherts JM, van Assen M. Publication bias examined in meta-analyses from psychology and medicine: a meta-meta-analysis. PLoS One. (2019) 14(4):e0215052. doi: 10.1371/journal.pone.0215052
25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
26. Hong J, He Y, Fu R, Si Y, Xu B, Xu J, et al. The relationship between night shift work and breast cancer incidence: a systematic review and meta-analysis of observational studies. Open Med. (2022) 17(1):712–31. doi: 10.1515/med-2022-0470
27. Rasouli Larmani N, Ahmadpourkacho M, Zahed Pasha Y, Hajiahmadi M, Mazloomi A. The effect of kangaroo mother care on the duration of phototherapy in term infants with hyperbilirubinemia. J Babol Univ Med Sci. (2016) 18(6):15–20. doi: 10.22088/JBUMS.18.6.15
28. Li X, Zhang Y, Li W. Kangaroo mother care could significantly reduce the duration of phototherapy for babies with jaundice. Int J Clin Exp Med. (2017) 10(1):1690–5.
29. Kenari RL, Aziznejadroshan P, Haghshenas Mojaveri M, Hajian-Tilaki K. Comparing the effect of kangaroo mother care and field massage on serum bilirubin level of term neonates with hyperbilirubinemia under phototherapy in the neonatal ward. Caspian J Intern Med. (2020) 11(1):34–40. doi: 10.22088/cjim.11.1.34
30. Stokowski LA. Fundamentals of phototherapy for neonatal jaundice. Adv Neonatal Care. (2011) 11(5 Suppl):S10–S21. doi: 10.1097/ANC.0b013e31822ee62c
31. Hansen TW. Recent advances in the pharmacotherapy for hyperbilirubinaemia in the neonate. Expert Opin Pharmacother. (2003) 4(11):1939–48. doi: 10.1517/14656566.4.11.1939
32. Agarwal R, Deorari AK. Unconjugated hyperbilirubinemia in newborns: current perspective. Indian Pediatr. (2002) 39(1):30–42.11805351
33. Ebbesen F, Hansen TWR, Maisels MJ. Update on phototherapy in jaundiced neonates. Curr Pediatr Rev. (2017) 13(3):176–80. doi: 10.2174/1573396313666170718150056
34. Ennever JF, Costarino AT, Polin RA, Speck WT. Rapid clearance of a structural isomer of bilirubin during phototherapy. J Clin Invest. (1987) 79(6):1674–8. doi: 10.1172/JCI113006
35. McDonagh AF. Phototherapy: from ancient Egypt to the new millennium. J Perinatol. (2001) 21(Suppl 1):S7–s12. doi: 10.1038/sj.jp.7210625
36. Allen D. Neonatal jaundice. Nurs Child Young People. (2016) 28(6):11. doi: 10.7748/ncyp.28.6.11.s15
37. Mreihil K, Benth J, Stensvold HJ, Nakstad B, Hansen TWR. Phototherapy is commonly used for neonatal jaundice but greater control is needed to avoid toxicity in the most vulnerable infants. Acta Paediatr. (2018) 107(4):611–9. doi: 10.1111/apa.14141
38. Vreman HJ, Wong RJ, Stevenson DK. Phototherapy: current methods and future directions. Semin Perinatol. (2004) 28(5):326–33. doi: 10.1053/j.semperi.2004.09.003
40. Arzani A, Zahedpasha Y, Ahmadpour-Kacho M, Khafri S, Aziznejad P. Kangaroo care effect on self-esteem in the mothers of low birth weight infants. J Babol Univ Med Sci. (2012) 366:52–8.
41. Szucs KA, Rosenman MB. Family-centered, evidence-based phototherapy delivery. Pediatrics. (2013) 131(6):e1982–5. doi: 10.1542/peds.2012-3479
42. Vincent S. Skin-to-skin contact. Part two: the evidence. Pract Midwife. (2011) 14(6):44–6.21739738
43. Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. (2016) 2016(8):Cd002771. doi: 10.1002/14651858.CD002771.pub4
44. Goudarzvand L, Dabirian A, Nourian M, Jafarimanesh H, Ranjbaran M. Comparison of conventional phototherapy and phototherapy along with Kangaroo mother care on cutaneous bilirubin of neonates with physiological jaundice. J Mater Fetal Neonatal Med. (2019) 32(8):1280–4. doi: 10.1080/14767058.2017.1404567
45. Hubbard JM, Gattman KR. Parent-Infant skin-to-skin contact following birth: history, benefits, and challenges. Neonatal Netw. (2017) 36(2):89–97. doi: 10.1891/0730-0832.36.2.89
46. Chiou ST, Chen LC, Yeh H, Wu SR, Chien LY. Early skin-to-skin contact, rooming-in, and breastfeeding: a comparison of the 2004 and 2011 national surveys in Taiwan. Birth (Berkeley, Calif). (2014) 41(1):33–8. doi: 10.1111/birt.12090
47. Cho ES, Kim SJ, Kwon MS, Cho H, Kim EH, Jun EM, et al. The effects of kangaroo care in the neonatal intensive care unit on the physiological functions of preterm infants, maternal-infant attachment, and maternal stress. J Pediatr Nurs. (2016) 31(4):430–8. doi: 10.1016/j.pedn.2016.02.007
48. Filho F L, de Sousa SH, Freitas IJ, Lamy ZC, Simões VM, da Silva AA, et al. Effect of maternal skin-to-skin contact on decolonization of Methicillin-Oxacillin-Resistant Staphylococcus in neonatal intensive care units: a randomized controlled trial. BMC Pregnancy Childbirth. (2015) 15(63):63. doi: 10.1186/s12884-015-0496-1
49. Nirmala P, Rekha S, Washington M. Kangaroo mother care: effect and perception of mothers and health personnel. J Neonatal Nurs. (2006) 12(5):177–84. doi: 10.1016/j.jnn.2006.07.008
50. Messmer PR, Rodriguez S, Adams J, Wells-Gentry J, Washburn K, Zabaleta I, et al. Effect of kangaroo care on sleep time for neonates. Pediatr Nurs. (1997) 23(4):408–14.9282055
51. Moore ER, Bergman N, Anderson GC, Medley N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. (2016) 11(11):Cd003519. doi: 10.1002/14651858.CD003519.pub4
52. Karimi FZ, Miri HH, Khadivzadeh T, Maleki-Saghooni N. The effect of mother-infant skin-to-skin contact immediately after birth on exclusive breastfeeding: a systematic review and meta-analysis. J Turk Ger Gynecol Assoc. (2020) 21(1):46–56. doi: 10.4274/jtgga.galenos.2019.2018.0138
53. Feldman-Winter L, Kellams A, Peter-Wohl S, Taylor JS, Lee KG, Terrell MJ, et al. Evidence-based updates on the first week of exclusive breastfeeding among infants ≥35 weeks. Pediatrics. (2020) 145(4):e20183696. doi: 10.1542/peds.2018-3696
54. Buiter HD, Dijkstra SS, Oude Elferink RF, Bijster P, Woltil HA, Verkade HJ. Neonatal jaundice and stool production in breast- or formula-fed term infants. Eur J Pediatr. (2008) 167(5):501–7. doi: 10.1007/s00431-007-0533-9
55. Maisels MJ. Screening and early postnatal management strategies to prevent hazardous hyperbilirubinemia in newborns of 35 or more weeks of gestation. Semin Fetal Neonatal Med. (2010) 15(3):129–35. doi: 10.1016/j.siny.2009.10.004
56. Sievers E, Clausen U, Oldigs HD, Schaub J. Supplemental feeding in the first days of life—effects on the recipient infant. Ann Nutr Metab. (2002) 46(2):62–7. doi: 10.1159/000057642
58. Boskabadi H, Maamouri G, Bagheri S. Significant neonatal weight loss related to idiopathic neonatal hyperbilirubinemia. Int J Pediatr. (2014) 2(4.1):225–31. doi: 10.22038/ijp.2014.3168
59. Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Clinical report from the pilot USA kernicterus registry (1992 to 2004). J Perinatol. (2009) 29(Suppl 1):S25–S45. doi: 10.1038/jp.2008.211
60. Hassan B, Zakerihamidi M. The correlation between frequency and duration of breastfeeding and the severity of neonatal hyperbilirubinemia. J Mater Fetal Neonatal Med. (2018) 31(4):457–63. doi: 10.1080/14767058.2017.1287897
61. Flacking R, Lehtonen L, Thomson G, Axelin A, Ahlqvist S, Moran VH, et al. Closeness and separation in neonatal intensive care. Acta Paediatr. (2012) 101(10):1032–7. doi: 10.1111/j.1651-2227.2012.02787.x
62. Wouk K, Tucker C, Pence BW, Meltzer-Brody S, Zvara B, Grewen K, et al. Positive emotions during infant feeding and breastfeeding outcomes. J Hum Lact. (2020) 36(1):157–67. doi: 10.1177/0890334419845646
63. Field T, Diego M. Vagal activity, early growth and emotional development. Infant Behav Dev. (2008) 31(3):361–73. doi: 10.1016/j.infbeh.2007.12.008
64. Lin CH, Yang HC, Cheng CS, Yen CE. Effects of infant massage on jaundiced neonates undergoing phototherapy. Ital J Pediatr. (2015) 41:94. doi: 10.1186/s13052-015-0202-y
65. McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. (2008) 583(2-3):174–85. doi: 10.1016/j.ejphar.2007.11.071
66. Handlin L, Jonas W, Petersson M, Ejdebäck M, Ransjö-Arvidson AB, Nissen E, et al. Effects of sucking and skin-to-skin contact on maternal ACTH and cortisol levels during the second day postpartum-influence of epidural analgesia and oxytocin in the perinatal period. Breastfeeding Med. (2009) 4(4):207–20. doi: 10.1089/bfm.2009.0001
67. Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin, cortisol, and triadic family interactions. Physiol Behav. (2010) 101(5):679–84. doi: 10.1016/j.physbeh.2010.08.008
68. Vittner D, McGrath J, Robinson J, Lawhon G, Cusson R, Eisenfeld L, et al. Increase in oxytocin from skin-to-skin contact enhances development of parent-infant relationship. Biol Res Nurs. (2018) 20(1):54–62. doi: 10.1177/1099800417735633
69. Uvänas-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: the role of the oxytocinergic system. Int J Behav Med. (2005) 12(2):59–65. doi: 10.1207/s15327558ijbm1202_3
70. Lee HJ, Macbeth AH, Pagani JH, Young WS 3rd. Oxytocin: the great facilitator of life. Prog Neurobiol. (2009) 88(2):127–51. doi: 10.1016/j.pneurobio.2009.04.001
71. Beiranvand S, Valizadeh F, Hosseinabadi R, Pournia Y. The effects of skin-to-skin contact on temperature and breastfeeding successfulness in full-term newborns after cesarean delivery. Int J Pediatr. (2014) 2014:846486. doi: 10.1155/2014/846486
72. Shukla VV, Bansal S, Nimbalkar A, Chapla A, Phatak A, Patel D, et al. Pain control interventions in preterm neonates: a randomized controlled trial. Indian Pediatr. (2018) 55(4):292–6. doi: 10.1007/s13312-018-1270-z
73. Okan F, Ozdil A, Bulbul A, Yapici Z, Nuhoglu A. Analgesic effects of skin-to-skin contact and breastfeeding in procedural pain in healthy term neonates. Ann Trop Paediatr. (2010) 30(2):119–28. doi: 10.1179/146532810X12703902516121
74. Kostandy R, Anderson GC, Good M. Skin-to-skin contact diminishes pain from hepatitis B vaccine injection in healthy full-term neonates. Neonatal Netw. (2013) 32(4):274–80. doi: 10.1891/0730-0832.32.4.274
76. Garg BD, Bansal A, Kabra NS. Role of kangaroo mother care in the management of neonatal hyperbilirubinemia in both term and preterm neonates: a systematic review. J Perinat Educ. (2020) 29(3):123–33. doi: 10.1891/J-PE-D-18-00043
77. Babu T A, Bhat BV, Joseph NM. Association between peak serum bilirubin and neurodevelopmental outcomes in term babies with hyperbilirubinemia. Indian J Pediatr. (2012) 79(2):202–6. doi: 10.1007/s12098-011-0501-2
78. Nussbaumer-Streit B, Mayr V, Dobrescu AI, Chapman A, Persad E, Klerings I, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. (2020) 9(9):Cd013574. doi: 10.1002/14651858.CD013574.pub2
79. Ionio C, Ciuffo G, Landoni M. Parent-infant skin-to-skin contact and stress regulation: a systematic review of the literature. Int J Environ Res Public Health. (2021) 18(9):4695. doi: 10.3390/ijerph18094695
80. Prince Sultan Military Medical City. New research highlights risks of separating newborns from mothers during COVID-19 pandemic. Keeping mothers and babies together could save more than 125 000 lives: WHO. Saudi Med J. (2021) 42(4):462–3.33795509
81. Pashaei Z, SeyedAlinaghi S, Qaderi K, Barzegary A, Karimi A, Mirghaderi SP, et al. Prenatal and neonatal complications of COVID-19: a systematic review. Health Sci Rep. (2022) 5(2):e510. doi: 10.1002/hsr2.510
Keywords: kangaroo-Mother care method, phototherapy, skin-to-skin contact, hyperbilirubinemia, infant, meta—analysis
Citation: Huang X, Chen M, Fu R, He W, He Y, Shentu H and Zhu S (2023) Efficacy of kangaroo mother care combined with neonatal phototherapy in newborns with non-pathological jaundice: A meta-analysis. Front. Pediatr. 11:1098143. doi: 10.3389/fped.2023.1098143
Received: 14 November 2022; Accepted: 6 January 2023;
Published: 25 January 2023.
Edited by:
Mary E. Sunday, Duke University, United StatesReviewed by:
Fabio Carmona, University of São Paulo, BrazilConcepcion De Alba-Romero, University Hospital October 12, Spain
© 2023 Huang, Chen, Fu, He, He, Shentu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suping Zhu a2FuZ3RoZXJAMTYzLmNvbQ==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Xiang Huang1
Xiang Huang1