
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 01 March 2023
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1097779
This article is part of the Research Topic Exploring the Interplay Between Clinical and Non-Clinical Outcomes for Children and Adults with Inflammatory Bowel Disease View all 6 articles
 Alex Krauthammer1,2*
Alex Krauthammer1,2* Ilana Weintraub1
Ilana Weintraub1 Ron Shaoul3
Ron Shaoul3 Raffi Lev-Tzion4
Raffi Lev-Tzion4 Efrat Broide5
Efrat Broide5 Michael Wilschanski6
Michael Wilschanski6 Aaron Lerner7
Aaron Lerner7 Baruch Yerushalmi8
Baruch Yerushalmi8 Dror S. Shouval9
Dror S. Shouval9 Hussein Shamaly10
Hussein Shamaly10 Yael Haberman-Ziv1,2
Yael Haberman-Ziv1,2 Batia Weiss1,2
Batia Weiss1,2
Objective and aim: Infantile-onset inflammatory bowel disease (IO-IBD), defined as IBD diagnosed at age 2 years or younger, tends to be more severe and refractory to conventional treatment than IBD diagnosed at a later age. However, data about IO-IBD and its long-term follow up are limited. We thus aimed to evaluate the presentation and long-term outcomes of patients with IO-IBD in a retrospective multicenter study.
Methods: Medical records of patients diagnosed with IO-IBD in eight medical centers during 2000–2017 with at least 1-year follow up were reviewed. Demographics and disease characteristics at diagnosis including age of onset, disease phenotype and location, surgeries, medical therapy, and comorbid conditions were recorded.
Results: Twenty-three patients with IO-IBD (16 males, 70%) were identified and followed for a median (range) of 51.2 (26.0–110.3) months. The mean ages at presentation and at the last follow up were 14 ± 9.8 and 101 ± 77 months, respectively. Six (26%) patients needed ileostomy already at the time of diagnosis and 20 (87%) were treated with corticosteroids. During long-term follow up, remission was achieved in 16 (73%) patients; of whom, 3 (14%) were without medications and 7 (32%) were in remission with the use of 5-aminosalicylic acid only. One patient needed hemicolectomy and one developed a severe EBV related infection.
Conclusion: The majority of patients with IO-IBD achieved long-term remission, despite a severe disease presentation at diagnosis. Surgery rate however is high, mainly during the first months from diagnosis.
Very early onset inflammatory bowel disease (VEO-IBD), defined as disease onset at age 6 years or younger, represents 6%–15% of pediatric IBD incidences (1, 2). Two subclassifications of VEO-IBD are neonatal IBD, defined as disease appearance in the first 28 days of life, and infantile-onset IBD (IO-IBD), defined when symptoms’ onset is at age 2 years or younger (3–6). Studies of VEO-IBD, have reported inconclusive disease course and natural history compared to IBD diagnosed at a later age (5–7). IO-IBD differs from IBD diagnosed at older ages by a predominant colonic involvement, an association with monogenic diseases (10%–20%), a high rate of positive family history of IBD, and poor response to therapy (2, 7–19). Prior studies identified that IO-IBD patients require more aggressive medical treatment and have higher rate of surgical intervention compared to later onset disease (2, 12, 13, 20). Data on long-term outcomes of IO-IBD are scarce (17, 21). Small-scale studies reported variable outcomes, ranging from high rates of surgery to complete remission during follow-up (21, 22). We aimed to report the long-term outcome of a cohort of patients with IO-IBD in a retrospective, multi-center study.
The databases of children with IBD, followed at eight medical centers in Israel from January 2000 to December 2017 were reviewed. Infants with formal diagnosis of IBD at age 2 years or younger, with a follow-up period of at least 12 months, were included. IBD was diagnosed according to standard criteria (23, 24). Demographic, clinical, laboratory, endoscopic, and histological details at diagnosis and during follow up were retrieved from the children's medical records. Those with evidence of infectious or allergic colitis, and known immune deficiencies or systemic diseases presenting prior to the gastrointestinal symptoms, were excluded.
The primary outcome was clinical remission at the last visit. Secondary outcomes included the need for surgery and steroid dependency. Disease activity and clinical remission were evaluated by using the weighted Pediatric Crohn's Disease Activity Index for patients with a CD-like phenotype and the Pediatric Ulcerative Colitis Activity Index for patients with UC and IBD unclassified (IBD-U) (25, 26). Growth failure was defined as a difference of height Z-score >1 between the pre-illness and the last available height Z-score (27). Anemia was defined as a hemoglobin level <11 gr/dl, thrombocytosis as platelets >450,000, hypoalbuminemia as serum albumin <3.5 gr/dl, and C-reactive protein (CRP) elevation as >5 mg/L.
The study was approved by the institutional review board of each institution.
The data are presented as percentages for descriptive variables; and as medians and interquartile (lower and upper) ranges (IQRs), or as means and standard deviations (SDs) for continuous variables, as appropriate. We did not perform statistical analysis between subgroups (by disease location), because of the small sample size. The data were analyzed by a biostatistician using IBM SPSS statistics version 24 (Armonk, NY: IBM Corp).
During the study period, 23 patients with IO-IBD, of whom 16 were males (70%), met the inclusion criteria. Two patients had neonatal onset IBD both diagnosed at age 1 month. The mean age at diagnosis was 14.0 ± 9.8 months; 10 (44%) patients were classified as having UC and 13 (56%) as CD (Table 1). The median duration of follow-up was 51.2 (interquartile range [IQR] 26–110) months (Table 1). The most common presenting symptoms were diarrhea (23, 100%) and hematochezia (20, 87%). Extraintestinal manifestations were present in 10 (44%). Perianal involvement was present in 7 (30%) patients, of them 2 (9%) had perianal fistulas and 5 (22%) had deep fissures. The most prevalent laboratory abnormalities at presentation were iron deficiency anemia in 16/20 (80%), thrombocytosis in 13/19 (60%), hypoalbuminemia in 10/19 (53%), and elevated CRP in 14/17 (83%) (Table 2). Whole-exome sequencing (WES), performed in 10 patients, revealed two monogenic diseases: one with an IL-10 receptor mutation and one with a CARMIL2 mutation.
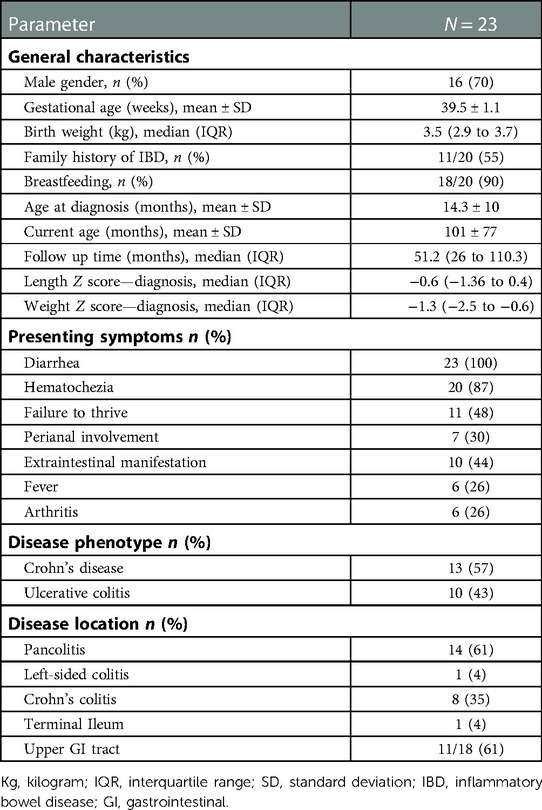
Table 1. Demographic and clinical characteristics of children with infantile-onset inflammatory bowel disease.

Table 2. Laboratory data at presentation and initial treatment in children with infantile-onset inflammatory bowel disease.
All patients had colonic inflammation at diagnosis, of whom, 14 (61%) had pancolitis. Terminal ileum (TI) involvement was observed in 1 (4%) patient who was classified as CD. Esophagogastroduodenoscopy (EGD) was completed in 18 (78%) patients, of them, 11 (61%) had signs of macroscopic inflammation and histologically, chronic inflammation was absent in 8/18 (44%) patients. Classification of the 13 patients with CD included 11 with inflammatory disease (B1), one with stricturing disease (B2) and 2 with fistulizing disease (B3). Granulomas were found in intestinal biopsies of 4/13 (31%) of the CD patients.
The initial medical therapy after diagnosis was a combination of corticosteroids with 5-aminosalicylic acid (5-ASA) or an immunomodulatory agent [6-mercaptopurine (6-MP) or azathioprine (AZA)] in 20/23 (87%) patients. Monotherapy with infliximab, 5-ASA, or AZA was initiated in one patient each (Table 3). Following induction, an additional six patients needed escalation to biological therapy and 10/20 (50%) were steroid dependent. Information on nutritional therapy at presentation was available for 16 patients, 8 of whom were treated with total parenteral nutrition (TPN). A response to biological treatment was observed in 4/7 (57%) patients. However, in three of them treatment was discontinued due to allergic reactions (Table 3). Diverting ileostomy was performed in 6 (26%) patients, three with a CD phenotype (one with IL-10 receptor mutation) due to intractable inflammation and steroid dependency, and three with a UC phenotype (one with CARMIL 2 mutation, due to perforation during colonoscopy).
Information regarding the clinical state and treatment at the end of the follow up was available for 22 patients. The median follow-up time was 51.2 (26–110.3) months and the mean patients’ age at the last follow-up was 101 ± 77 months. Height Z-score and weight Z-score, both at diagnosis and at the end of follow up, were available in 8 and 12 patients, respectively. Thus, at diagnosis, mean height and weight Z-score of those patients were (−0.82 ± 1.69) and (−1.77 ± 1.92), as compared to (−0.89 ± 0.98) and (−0.89 ± 1.17) at the end of follow up, (p = 0.46 and p = 0.1), respectively. Growth failure was observed in 1/8 (12.5%) patients with available height measurements at diagnosis and end of follow up. Both height Z-score and weight Z-score at diagnosis and end of follow-up were available in 7 patients. Three patients (2 with CD, 1 with UC), had both stunting (Z-core ≤ 2) and wasting (Z-score ≤ 2) at the end of follow-up. The mean final height Z-score of all patients with available data was −0.8 ± 1.1. In general, the weight Z-score showed a tendency for improvement, when final weight Z-score was compared to weight Z-score at diagnosis: −0.7 ± 1.2 vs. −1.5 ± 1.7, p < 0.06.
Long-term remission, [median 51.2 (26–110.3) months], was achieved in 16/22 (73%) patients, of these, one underwent colectomy and ileostomy at diagnosis and one underwent ileostomy and hemicolectomy due to perforation during bowel dilation later in the course of disease. At last follow-up, three patients (aged 1.3, 8.2, and 12.0 years, respectively, Table 3), were in clinical remission without medical treatment, two of them (#12 and #16) with no surgeries and one (#2), after ileostomy and total colectomy very early in the course of the disease. Another seven patients were in remission with 5-ASA treatment only (3 CD, 4 UC). Monotherapy with infliximab, methotrexate, or AZA/6MP was administered to one patient each, combination therapy with infliximab and AZA/6MP to one patient, and 5-ASA with AZA/6MP to one patient. Three patients (14%) remained steroid dependent. Overall, in 11/22 (50%) patients, the treatment was de-escalated or stopped during follow-up; and in 4/22 (18%), the treatment was escalated to biological agents or immunomodulators.
Five out of six patients, who underwent surgery at the time of diagnosis, were with active disease despite the surgical procedure [of them one with IL-10 receptor mutation (patient #13), one with CARMIL 2 mutation (patient #4) and one patient in whom later in the course of the disease ileitis was demonstrated on MRE (patient #5)]. These five patients were treated with TPN, steroids, cyclosporine or anti-tumor necrosis factor (TNF)-α medications. At the end of follow up, the patient with CARMIL 2 mutation was treated with Budesonide gel for upper GI inflammation and was waiting for initiation of Sirolimus. The patient with IL-10 receptor mutation had an episode of severe sepsis after a single dose of Etanercept given for his arthritis, and at the end of follow up was without regular treatment except antibiotics and topical treatment for skin manifestations. His parents refused bone marrow transplant. Four patients (Table 3) were followed for 10 years or longer; of them, three were in remission and one was steroid dependent after surgery at diagnosis.
Severe long-term complications included bowel perforation during endoscopic follow up in one patient, and an Epstein-Barr virus infection in one patient (#10), in which lymphoma was suspected and eventually ruled out after the patient was lost to follow-up in our center.
The main findings of the current study are the variability in the long-term outcomes of children with IO-IBD. We report long-term remission in 73% of the patients, despite a severe inflammatory course in infancy; however, steroid dependency was common and 26% required surgical intervention.
All patients in our cohort had colonic disease, classified as UC or CD, and none were classified as having IBD-U. Other studies reported various sub-classifications of disease in IO-IBD. A retrospective study of 1,370 children with early-onset IBD in the U.S. reported similar proportions of CD, UC, and indeterminate colitis (about 33% at each subgroup) in children under age 2 years (18). In contrast, a study of 62 children who presented with IBD before age 2 years reported IBD-U in 71% (22). Notably, one-third of that cohort had monogenic diseases. In a case series of six Asian patients, 3 had CD, 2 UC, and 1 IBD-U (21). Other studies reported IBD-U in 10%–28% of children with VEO-IBD (<6 years) (16, 28), though data were not presented separately for IO-IBD. A recent population-based study from Israel classified children with VEO and IO-IBD as CD or UC only, with no IBD-U classification (29). This is due to the lack of inclusion of IBD-U code by the Israeli Health Maintenance Organizations. The differences in the distribution of UC, CD, and IBD-U between studies may also be due to differences between the populations, including the proportions of patients with monogenic disease, and differences in the rate of WES testing. In addition, we might have overlooked some patients who might have fallen under classification of IBD-U, because only 78% of our patients had EGD performed and none had small bowel imaging.
Lack of chronic inflammation on biopsy, observed in 44% of our patients on EGD, might be explained by the early performance of endoscopic evaluation due to severe disease at presentation.
We found causative mutations in 2/10 (20%) patients in whom WES was performed. As genetic testing was not available and affordable during the first years of the study period, not all the patients were evaluated. Similar studies reported various rates of mutation detection in IO-IBD. In a study from China, 16/54 (30%) patients underwent genetic evaluation; 9/54 (17%) had monogenic diseases (12). Among 62 children with IO-IBD in the UK, causative mutations were detected in 31% (22). However, a number of studies of VEO-IBD that included patients with IO-IBD did not report any genetic analyses (13, 16, 18, 22). A family history of IBD was present in 55% of our patients. This is similar to reports of 18%–44% in a number of western studies (18, 22), but contrasts to the lack of family history in studies from Asia (12, 21).
The clinical presentation of our group is in line with previous studies; bloody diarrhea was the most common symptom, and anemia hypoalbuminemia and elevated CRP were the most common laboratory findings. Fecal calprotectin was not consistently available at diagnosis as in other studies of the same age group (22).
The most common induction treatment at presentation was corticosteroids, within the range of 80%–92% reported for other series of IO-IBD including recent study from 4 Israeli Health Maintenance Organizations (5, 24, 29). The treatment regimens included 5-ASA, AZA, 6-MP, infliximab, and nutritional support by TPN. Infliximab treatment failure was observed in all, but one of our patients during induction. This high failure rate corroborates other reports of VEO-IBD. One study reported a high rate of treatment discontinuation before week 14 (30), due to adverse effects and treatment failure. Another study reported a higher rate of treatment failure during the first year than in older children (31). Finally, recent data showed that up to 35% newly diagnosed IO-IBD patients at each year fail to respond to biologics (29). Data have not been published specifically on the response to anti-TNFα medications in IO-IBD. The lower response rate to anti-TNFα medications in younger children may reflect a higher predisposition to infections, different pharmacokinetics, or genetic background, leading to non-TNFα mediated inflammation (30, 32, 33). In addition, we did not use therapeutic drug monitoring during the study years, and a more intense treatment regimen may be needed to control inflammation in young patients.
Of our patients with available information regarding nutritional therapy, half needed TPN at presentation. This is similar to the proportion of 59% (10/17), in the Japanese cohort of VEO-IBD (15) but lower than 90% (9/10), reported among patients who were diagnosed with IBD during their first year of life (5). Notably, patients with monogenic disease were also described to have higher TPN requirements (34). In studies of older IBD patients at older ages (35), the TPN requirement for TPN was much lower. Taken together, TPN use at presentation appears to be inversely proportional to patient age at diagnosis. The young age, together with severe diarrhea and failure to thrive, and the low response rate to anti-TNFα medications, are possible causes of the high rates of TPN initiation in IO-IBD.
Surgery at an early stage after diagnosis was performed in 26% of our patients. This is within the range of 19%–50% reported by studies from Asia, England, France and Israel (5, 21, 22, 29). The need for early surgery reflects the severity of disease at presentation in this age group and was shown to have 1.4 times higher risk compared to toddler-onset group (29). Another possible explanation to high surgery rates at this age group is their biological treatment refractory “behavior” most probably due to suboptimal dosing of biological agents and unclear pharmacokinetics (30, 32, 33).
The long-term outcomes of the patients in our cohort were more favorable than previously described, despite the severe disease at presentation. Clinical remission was achieved in 73% of the patients after a median of 51 months. Notably, at the end of the follow up, about one-third of the patients were treated only with 5-ASA and 3 (14%) remained without any treatment, though one of them needed surgery early in the course of the disease. In other studies only 26% of children in the UK with IO-IBD did not require treatment escalation beyond first-line immunomodulator therapy (22), and 2 (20%) of 10 children diagnosed during their first year of life with IBD in France, stopped medications within 1 year and had long-term remission (5). The early surgery rate, 26% at the first hospitalization, was similar to that described in the literature for IO-IBD (7), and higher than in later-onset childhood IBD (36). Interestingly, after the initial surgeries, none of our patients required repeat surgery, except one patient who had a bowel perforation during colonoscopy. Similar to other studies of IO-IBD patients, one of our patients (#5) who was primarily diagnosed with UC and underwent total colectomy, developed ileitis during follow up, and most probably was wrongly diagnosed as UC at diagnosis (37, 38).
Despite presentation with severe disease at a very young age, none of our patients died. Mortality has rarely been reported in relation to IO-IBD. However, higher mortality rates, 7% and 19% were reported for IO-IBD cohorts with high proportions (17% and 44%) of monogenic diseases and immune deficiency (5, 39). The small size of our series precluded identifying factors that associate with such favorable outcomes as long-term remission and treatment de-escalation.
The variable outcomes between studies on IO-IBD and VEO-IBD may result from differences between the cohorts in the study periods, the availability of biologic treatment, the extent of genetic evaluations, and the proportions of patients with monogenic diseases.
Our study has limitations that are associated with its multicenter retrospective design. The genetic evaluation and treatment of VEO-IBD in general, and particularly of IO-IBD, have changed significantly over the past two decades. Secondly, our definition of remission was clinical, and did not include fecal calprotectin levels or consistent evaluation of endoscopic remission. In addition, small bowel imaging was not feasible in this age group at presentation. Nevertheless, our study contributes information on the long-term outcomes of children with IO-IBD as a distinct group, rather than as a part of VEO-IBD and shows similar results to data from a validated Israeli IBD Research Nucleus (29), thus providing validity to our results.
In conclusion, though the evaluation and treatment of children with IO-IBD pose substantial challenges, a large proportion of children have favorable long-term prognosis, even following severe disease course at presentation. Prospective studies of this population are needed to identify predictors for favorable outcomes, to identify those with monogenic diseases, and to evaluate responses to biological treatment using therapeutic drug monitoring.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The study was approved by the institutional review board of each institution. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
AK conceptualized and designed the study, and drafted the initial manuscript. IW, RS, RL-T, EB, MW, AL, BY, DS, HS, and YH-Z carried out the initial analyses, and reviewed and revised the manuscript. BW designed the data collection instruments, coordinated and supervised the data collection, and critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
We would like to thank the teams of physicians, coordinators, and research staff at the participating sites, who helped conduct this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer JO declared a shared consortium [Very Early Onset Inflammatory Bowel Disease Consortium] with the author DS to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. (2004) 126(6):1504–17. doi: 10.1053/j.gastro.2004.01.063
2. Benchimol EI, Bernstein CN, Bitton A, Carroll MW, Singh H, Otley AR, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol. (2017) 112(7):1120–34. doi: 10.1038/ajg.2017.97
3. Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. (2014) 147(5):990–1007.e3. doi: 10.1053/j.gastro.2014.07.023
4. Shim JO. Recent advance in very early onset inflammatory bowel disease. Pediatr Gastroenterol Hepatol Nutr. (2019) 22(1):41–9. doi: 10.5223/pghn.2019.22.1.41
5. Ruemmele FM, El Khoury MG, Talbotec C, Maurage C, Mougenot JF, Schmitz J, et al. Characteristics of inflammatory bowel disease with onset during the first year of life. J Pediatr Gastroenterol Nutr. (2006) 43(5):603–9. doi: 10.1097/01.mpg.0000237938.12674.e3
6. Benchimol EI, Mack DR, Nguyen GC, Snapper SB, Li W, Mojaverian N, et al. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology. (2014) 147(4):803–13. doi: 10.1053/j.gastro.2014.06.023
7. Kelsen JR, Sullivan KE, Rabizadeh S, Singh N, Snapper S, Elkadri A, et al. North American society for pediatric gastroenterology, hepatology, and nutrition position paper on the evaluation and management for patients with very early-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2020) 70(3):389–403. doi: 10.1097/MPG.0000000000002567
8. de Ridder L, Weersma RK, Dijkstra G, van der Steege G, Benninga MA, Nolte IM, et al. Genetic susceptibility has a more important role in pediatric-onset crohn’s disease than in adult onset crohn’s disease. Inflamm Bowel Dis. (2007) 13(9):1083–92. doi: 10.1002/ibd.20171
9. Begue B, Verdier J, Rieux-Laucat F, Goulet O, Morali A, Canioni D, et al. Defective IL10 signaling defining a subgroup of patients with inflammatory bowel disease. Am J Gastroenterol. (2011) 106(8):1544–55. doi: 10.1038/ajg.2011.112
10. Sprockett D, Fukami T, Relman DA. Role of priority effects in the early-life assembly of the gut microbiota. Nat Rev Gastroenterol Hepatol. (2018) 15(4):197–205. doi: 10.1038/nrgastro.2017.173
11. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. (2004) 118(2):229–41. doi: 10.1016/j.cell.2004.07.002
12. Fang YH, Luo YY, Yu JD, Lou JG, Chen J. Phenotypic and genotypic characterization of inflammatory bowel disease in children under six years of age in China. World J Gastroenterol. (2018) 24(9):1035–45. doi: 10.3748/wjg.v24.i9.1035
13. Dhaliwal J, Walters TD, Mack DR, Huynh HQ, Jacobson K, Otley AR, et al. Phenotypic variation in paediatric IBD by age: a multi-center prospective inception cohort study of the Canadian children IBD network. J Crohns Colitis. (2019) 14(4):445–54. doi: 10.1093/ecco-jcc/jjz106
14. Mamula P, Telega GW, Markowitz JE, Brown KA, Russo PA, Piccoli DA, et al. Inflammatory bowel disease in children 5 years of age and younger. Am J Gastroenterol. (2002) 97(8):2005–10. doi: 10.1111/j.1572-0241.2002.05915.x
15. Takeuchi I, Kaburaki Y, Arai K, Shimizu H, Hirano Y, Nagata S, et al. Infliximab for very early-onset inflammatory bowel disease: a tertiary center experience in Japan. J Gastroenterol Hepatol. (2020) 35(4):593–600. doi: 10.1111/jgh.14836
16. Oliva-Hemker M, Hutfless S, Al Kazzi ES, Lerer T, Mack D, LeLeiko N, et al. Clinical presentation and five-year therapeutic management of very early-onset inflammatory bowel disease in a large north American cohort. J Pediatr. (2015) 167(3):527–32.e1-3. doi: 10.1016/j.jpeds.2015.04.045
17. Kelsen JR, Conrad MA, Dawany N, Patel T, Shraim R, Merz A, et al. The unique disease course of children with very early onset-inflammatory bowel disease. Inflamm Bowel Dis. (2020) 26(6):909–18. doi: 10.1093/ibd/izz214
18. Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, et al. Children with early-onset inflammatory bowel disease [IBD]: analysis of a paediatric IBD consortium registry. J Pediatr. (2005) 146(1):35–40. doi: 10.1016/j.jpeds.2004.08.043
19. Uhlig HH, Charbit-Henrion F, Kotlarz D, Shouval DS, Schwerd T, Strisciuglio C, et al. Clinical genomics for the diagnosis of monogenic forms of inflammatory bowel disease: a position paper from the paediatric IBD porto group of European society of paediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. (2021) 72(3):456–73. doi: 10.1097/MPG.0000000000003017
20. Ye Z, Zhou Y, Huang Y, Wang Y, Lu J, Tang Z, et al. Phenotype and management of infantile-onset inflammatory bowel disease: experience from a tertiary care center in China. Inflamm Bowel Dis. (2017) 23(12):2154–64. doi: 10.1097/MIB.0000000000001269
21. Lee WS, Ng RT, Chan KW, Lau YL. Variable outcome in infantile-onset inflammatory bowel disease in an Asian cohort. World J Gastroenterol. (2016) 22(48):10653–62. doi: 10.3748/wjg.v22.i48.10653
22. Kammermeier J, Dziubak R, Pescarin M, Drury S, Godwin H, Reeve K, et al. Phenotypic and genotypic characterization of inflammatory bowel disease presenting before the age of 2 years. J Crohns Colitis. (2017) 11(1):60–9. doi: 10.1093/ecco-jcc/jjw118
23. Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al. 3rd European evidence-based consensus on the diagnosis and management of crohn's disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. (2017) 11(1):3–25. doi: 10.1093/ecco-jcc/jjw168
24. Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, et al. European Society of pediatric gastroenterology, hepatology, and nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. (2014) 58(6):795–806. doi: 10.1097/MPG.0000000000000239
25. Turner D, Griffiths AM, Walters TD, Seah T, Markowitz J, Pfefferkorn M, et al. Mathematical weighting of the pediatric crohn’s disease activity index [PCDAI] and comparison with its other short versions. Inflamm Bowel Dis. (2012) 18(1):55–62. doi: 10.1002/ibd.21649
26. Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. (2007) 133(2):423–32. doi: 10.1053/j.gastro.2007.05.029
27. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. (2011) 17(6):1314–21. doi: 10.1002/ibd.21493
28. Rialon KL, Crowley E, Seemann NM, Fahy AS, Muise A, Langer JC. Long-term outcomes for children with very early-onset colitis: implications for surgical management. J Pediatr Surg. (2018) 53(5):964–7. doi: 10.1016/j.jpedsurg.2018.02.023
29. Atia O, Benchimol EI, Ledderman N, Greenfeld S, Kariv R, Weisband YL, et al. Incidence, management, and outcomes of very early onset inflammatory bowel diseases and infantile-onset disease: an epi-IIRN study. Clin Gastroenterol Hepatol. (2022). [Epub ahead of print]. doi: 10.1016/j.cgh.2022.10.026
30. Bramuzzo M, Arrigo S, Romano C, Filardi MC, Lionetti P, Agrusti A, et al. Efficacy and safety of infliximab in very early onset inflammatory bowel disease: a national comparative retrospective study. United European Gastroenterol J. (2019) 7(6):759–66. doi: 10.1177/2050640619847592
31. Kelsen JR, Grossman AB, Pauly-Hubbard H, Gupta K, Baldassano RN, Mamula P. Infliximab therapy in pediatric patients 7 years of age and younger. J Pediatr Gastroenterol Nutr. (2014) 59(6):758–62. doi: 10.1097/MPG.0000000000000533
32. Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe crohn’s disease in children. Gastroenterology. (2007) 132:863–73. doi: 10.1053/j.gastro.2006.12.003
33. Corica D, Romano C. Biological therapy in pediatric inflammatory bowel disease: a systematic review. J Clin Gastroenterol. (2017) 51(2):100–10. doi: 10.1097/MCG.0000000000000696
34. Collen LV, Kim DY, Field M, Okoroafor I, Saccocia G, Whitcomb SD, et al. Clinical phenotypes and outcomes in monogenic versus non-monogenic very early onset inflammatory bowel disease. J Crohns Colitis. (2022) 16(9):1380–96. doi: 10.1093/ecco-jcc/jjac045
35. Nelson AD, Elkins JR, Stocchi L, Farraye FA, Hashash JG. Use and misuse of parenteral putrition in patients with inflammatory bowel disease. Inflamm Bowel Dis. (2022) 28(10):1592–1602. doi: 10.1093/ibd/izac085; Online ahead of print.
36. Schaefer ME, Machan JT, Kawatu D, Langton CR, Markowitz J, Crandall W, et al. Factors that determine risk for surgery in pediatric patients with crohn's disease. Clin Gastroenterol Hepatol. (2010) 8(9):789–94. doi: 10.1016/j.cgh.2010.05.021
37. Patton D, Gupta N, Wojcicki JM, Garnett EA, Nobuhara K, Heyman MB. Postoperative outcome of colectomy for pediatric patients with ulcerative colitis. J Pediatr Gastroenterol Nutr. (2010) 51(2):151–4. doi: 10.1097/MPG.0b013e3181c99290
38. Dore M, Triana Junco P, Sánchez Galán A, Prieto G, Ramos E, Muñoz Romo M, et al. Pitfalls in diagnosis of early-onset inflammatory bowel disease. Eur J Pediatr Surg. (2018) 28(1):39–43. doi: 10.1055/s-0037-1604428
Keywords: inflammatory bowel disease, infantile onset, remission, surgery, long-term outcome
Citation: Krauthammer A, Weintraub I, Shaoul R, Lev-Tzion R, Broide E, Wilschanski M, Lerner A, Yerushalmi B, Shouval DS, Shamaly H, Haberman-Ziv Y and Weiss B (2023) Infantile-onset inflammatory bowel disease has variable long-term outcomes. Front. Pediatr. 11:1097779. doi: 10.3389/fped.2023.1097779
Received: 14 November 2022; Accepted: 13 February 2023;
Published: 1 March 2023.
Edited by:
Joseph Meredith, University of Edinburgh, United KingdomReviewed by:
Corentin Babakissa, Université de Sherbrooke, Canada© 2023 Krauthammer, Weintraub, Shaoul, Lev-Tzion, Broide, Wilschanski, Lerner, Yerushalmi, Shouval, Shamaly, Haberman-Ziv and Weiss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alex Krauthammer a3JhdXRhbGV4QGdtYWlsLmNvbQ==
Specialty Section: This article was submitted to Pediatric Gastroenterology, Hepatology and Nutrition, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.