
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 22 March 2023
Sec. Neonatology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1092578
This article is part of the Research Topic New Perspectives of Hypoxic Ischemic Encephalopathy View all 5 articles
 Yasser S. Amer1,2,3,4,5*†
Yasser S. Amer1,2,3,4,5*† Jasim Anabrees6,7*†
Jasim Anabrees6,7*† Mohamed Abdelmawla8
Mohamed Abdelmawla8 Ayman Abdalgader9
Ayman Abdalgader9 Asmaa Almazroei8
Asmaa Almazroei8 Ibrahim Alhifzi10
Ibrahim Alhifzi10 Abdullah Hawash AlOnazi11
Abdullah Hawash AlOnazi11 Yasser Sabr12
Yasser Sabr12 Layal Hneiny13
Layal Hneiny13 Ahmed El-Malky14,15
Ahmed El-Malky14,15 Ayesha Alshalawi16
Ayesha Alshalawi16 Ahmed Alayoubi17
Ahmed Alayoubi17 Iftikhar A. Chaudhry18
Iftikhar A. Chaudhry18 Omar Elkhateeb11
Omar Elkhateeb11
Background and Objective: To systematically review, critically appraise the quality of recent clinical practice guidelines (CPGs) for neonatal hypoxic ischemic encephalopathy (HIE), and map their recommendations.
Data Sources: CPG databases (GIN, ECRI, NICE, SIGN, DynaMed), Bibliographic databases (PubMed, Embase, CINAHL), and related specialized professional societies (e.g., AAP, CPS, BAPM, RCPCH, and SNS).
Study Selection: Original de-novo developed evidence-based CPGs for HIE, group authorship, Arabic or English languages, and international or national scope. The systematic review was drafted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement and Johnston et al methodological guide.
Data Extraction: Quality assessment of the included HIE CPGs by the Appraisal of Guidelines for REsearch & Evaluation II (AGREE II) Instrument and report their characteristics, AGREE II ratings, and recommendations
Data Synthesis: Our search retrieved 2,489 citations, of which two recent HIE CPGs were eligible and appraised: Canadian Paediatric Society (CPS) and Queensland Maternity and Neonatal Services (QMN). The overall assessment of the QMN CPG was superior (83%). Domain 1 (Scope & Purpose) scored (47%, 63%), Domain 2 (Stakeholder Involvement) (72%, 39%), Domain 3 (Rigour of Development) (48%, 43%), Domain 4 (Clarity & Presentation) (100%, 96%), Domain 5 (Applicability) (59%, 9%), and Domain 6 (Editorial Independence) (67%, 17%) for the QMN and CPS CPGs respectively. All appraisers recommended the QMN CPG for use in practice.
Conclusion: The methodological quality of the QMN CPG was superior with the relevant recommendations for its use in neonatal practice.
Limitations: limited to Arabic and English languages.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=258291, identifier: CRD42021258291.
Survivors of hypoxic-ischemic encephalopathy (HIE) have shown reduced neuropsychological functioning, behavioral adjustment, and school outcomes during adolescence among other neonatal morbidities, mortalities, and outcomes (1).
Several initiatives were launched to promote and advance evidence-based neonatal healthcare by national (e.g., American Academy of Pediatrics, British Association of Perinatal Medicine, and Saudi Neonatology Society) and international organizations and professional societies [e.g., International Society for Evidence-Based Neonatology (EBNEO)] (2).
Despite the emphasized potential of Clinical Practice Guidelines (CPGs) to optimize patient outcomes and clinical practice, an increasing volume of CPGs is being published of variable quality in the field of neonatology (3–5).
In 2012, The Saudi Neonatology Society (SNS) published a CPG for Whole Body Cooling for infants with HIE (6). The SNS has recently launched a number of national projects to adapt evidence-based CPGs for the management of high-priority health topics in neonatal healthcare using the “KSU-Modified-ADAPTE” as a formal CPG adaptation methodology, with the goal of providing evidence-based guidance and recommendations to neonatologists and pediatricians across the country (7–11). As newer evidence and CPGs were published overtime, SNS decided to update this CPG and launch a national HIE CPG project.
The systematic review (SR) and quality appraisal of CPGs using the Appraisal of Guidelines for REsearch & Evaluation II (AGREE II) Instrument is a critical step in the KSU-Modified-ADAPTE CPG adaptation process (12, 13).
The overarching CPG adaptation project was registered in the PREPARE (Practice guideline REgistration for trancPAREncy) platform that is hosted by the University of Lanzhou in China http://www.guidelines-registry.org/ (Registration Number: IPGRP-2021CN384) (14).
This study aimed to report the systematic review and quality assessment of HIE CPGs as a part of the HIE CPG adaptation process.
The protocol for this systematic review of CPGs was registered in PROSPERO (International Prospective Register of Systematic Reviews) (ID: CRD42021258291) (15). This systematic review was guided by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement in addition to the methodological guide proposed by Johnston et al. (12, 15).
Our Guidelines Review Group (GRG) included seven expert consultant neonatologists: one of them with expertise in systematic reviews, two consultant Obstetricians and Gynecologists, a senior nurse, a medical and healthcare librarian, and a CPG methodologist with a background in pediatrics.
The medical librarian systematically searched MEDLINE, EMBASE, and CINAHL databases for relevant CPGs using the Ovid platform and hand-searched the relevant CPG databases and repositories for eligible CPGs (Supplementary information).
Two reviewers (MA and OE) conducted the title and abstract screening of the CPGs and articles independently. Two different reviewers double-checked the full-text screening (YSA and JA) and disagreements were resolved through focus group discussions. The full inclusion and exclusion criteria were reported in the PROSPERO protocol (16). The search and screening for eligible HIE CPGs were updated before the submission of the manuscript.
Three members of the GRG attended capacity building training in AGREE II appraisal of CPGs. The AGREE II Instrument (www.agreetrust.org) has 23 items or questions divided into six domains: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence. Using a 7-point Likert scale, each item was scored using its online platform (My AGREE PLUS). Each CPG was critically appraised by four AGREE II raters including four clinicians, and one of them was a CPG methodologist (13).
For each AGREE II domain, we determined standardized scores ranging from 0% to 100% using the techniques advised by the AGREE II instrument's equations. A comparison tabular style was used to summarize the main recommendations of the applicable CPGs (17).
For each item in each area of the two assessed CPGs, we performed inter-rater reliability tests to gauge the degree of agreement between raters (IRR). We did this by utilizing a percent agreement inter-rater reliability assessment test. The consistency of ratings or the capacity for datasets that were gathered as clusters or sorted into clusters using intra-class correlation were also assessed in the second overall assessment (OA2) in addition to the percent agreement in the first overall evaluation (OA1). One common IRR strategy is intra-class correlation (ICC).
When there are more than two raters, we use this. Standards from the same set appeared to be fairly comparable, as shown by a strong intra-class correlation coefficient (kappa) around 1. A low kappa score near 0 denoted a lack of similarity between standards from the same collection. Since our raters and rates varied, we used ANOVA “One-Way Random” on SPSS Statistics, version 21. The extensive range of numerical data from groups or clusters is why we chose ICCC. This allowed us to assess the repeatability and the degree to which peers shared particular characteristics. We looked at how well two categories on an ordinal scale agreed with one another.
Given that the data originated from an ordered scale, we employed weighted kappa (quadratic weights). Following are the weights' calculations: The notation is Cohen's kappa. We decided on linear weights because the difference between the first and second categories was similar to the difference between the second and third categories, and so on. The kappa (K) statistic is used to measure agreement (18, 19): When categorization systems agree completely, K = 1 when there is no agreement greater than chance, and K is negative when there is agreement worse than chance. (Supplementary information) demonstrates possible interpretations of the K value (20).
A total of 4,505 records were retrieved from bibliographic databases and 19 from CPG databases and professional societies. After the title, abstract, and full-text screening using Rayyan https://www.rayyan.ai/ only two source original CPGs were found to be eligible for the AGREE II quality assessment step. Two reviewers conducted the screening (OA, MA) and two additional reviewers (JA, YSA) resolved any discrepancies through discussions (Figure 1).
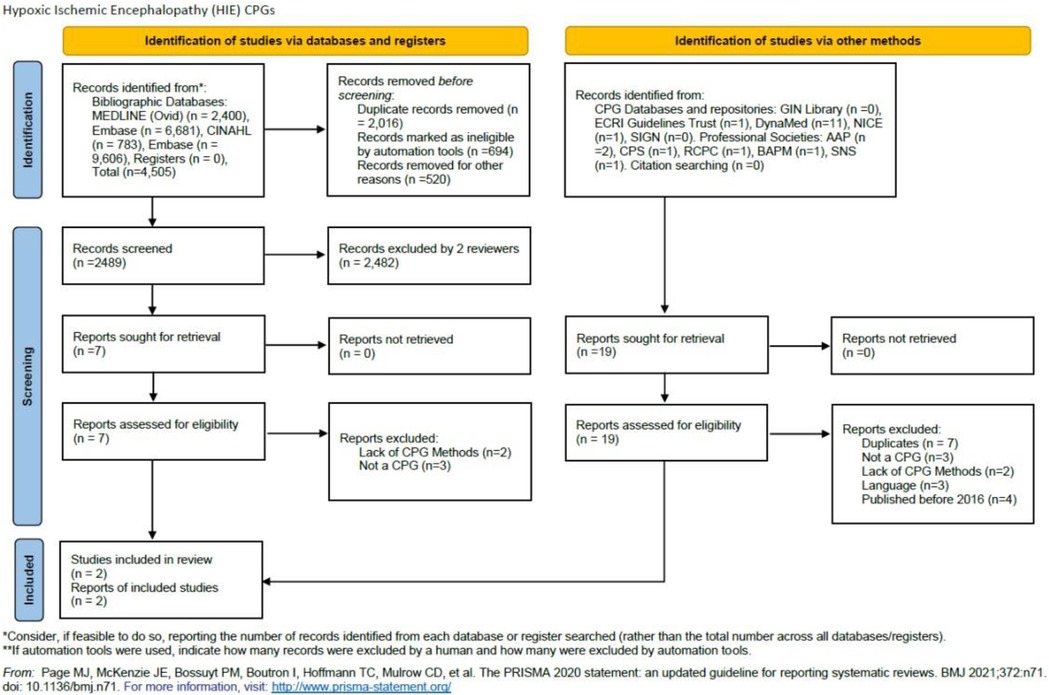
Figure 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers, and other sources.
Table 1 highlights the characteristics of all eligible CPGs. The CPG developer organizations were reference, professional organizations in pediatrics, neonatology, or general non-specialized including CPS, and QH. Both organizations were from high-income countries.
The two CPGs (CPS, QMN) scored (63%, 47%) respectively in domain 1 where the CPS CPG reported its objectives, health questions, and patient population more clearly addressing most of the AGREE II criteria and additional considerations.
CPS and QMN CPGs scored 39% and 72% respectively, where the QMN CPG development group was properly reported and included a multidisciplinary team representing all related specialties to neonatal HIE.
CPS and QMN scored 43% and 48% respectively. There was a large area for improvement in both CPGs in properly and clearly reporting the search methods, evidence selection criteria, strengths and limitations of the evidence, formulation of recommendations, consideration of benefits and harms, link between recommendations and evidence, external review, and updating procedure. Both CPGs reported an overall process of their CPG development the aforementioned criteria need to be reported transparently in clear detail to facilitate the replication of the CPG development process. Both CPGs have not reported using the GRADE (Grading of Recommendations; Assessment; Development and Evaluations) Method.
CPS and QMN CPGs scored 96% and 100% in domain 4 respectively. Both CPGs presented specific and unambiguous recommendations, comprehensive management options, and a set of identifiable key recommendations.
CPS and QMN CPGs scored 9% and 59% in domain 4 respectively. The QMN CPG was superior in its variable set of CPG implementation tools inside the CPG article or on the website including management flowcharts and posters, points for discussion with the parents, checklist for therapeutic hypothermia, assessment of encephalopathy severity (Sarnat scoring), Sarnat and Sarnat staging of HIE, educational material, and patient information on HIE.
CPS and QMN CPGs scored 17% and 67% respectively where QMN CPG documented the funding body and the conflicts of interest more clearly.
The AGREE II standardized domain scores for the first overall assessment was higher for the QMN CPG (83%) than the CPS CPG (63%).
The second overall assessment revealed a consensus between the four reviewers on recommending the use of the QMN CPG while half of the reviewers recommended using the CPS CPG and half of them recommended its use with modifications.
In terms of Classification of the strength of agreement among the four raters against the two guidelines; the assessment was classified according to strength into Poor, Fair, Good, Very Good, and Excellent. With an emphasis on the sum of scores, the sum of OA scores, and the first overall assessment. Regarding the CPS 2018 Position Statement; it was arranged as follows; 0, 0, 12, 6, 6, 333, and 19 however, QMNCG 2021 Queensland Maternity and Neonatal Clinical Guideline was 0, 0, 2, 6, 16, 431 and 24. Supplementary Tables S2.1 and S2.2. shows the intraclass correlation coefficient (Kappa value) among raters for the two CPGs for the second Overall Assessment. The number of observed agreements is six (77.16% of the observations). 7.0 agreements are predicted by chance (85.00 percent of the observations). Kappa = 0.912; kappa SE = 0.586; 95% confidence interval: Weighted Kappa = 0.081 for values ranging from 0.752 to 1.642 (Figures 2, 3).
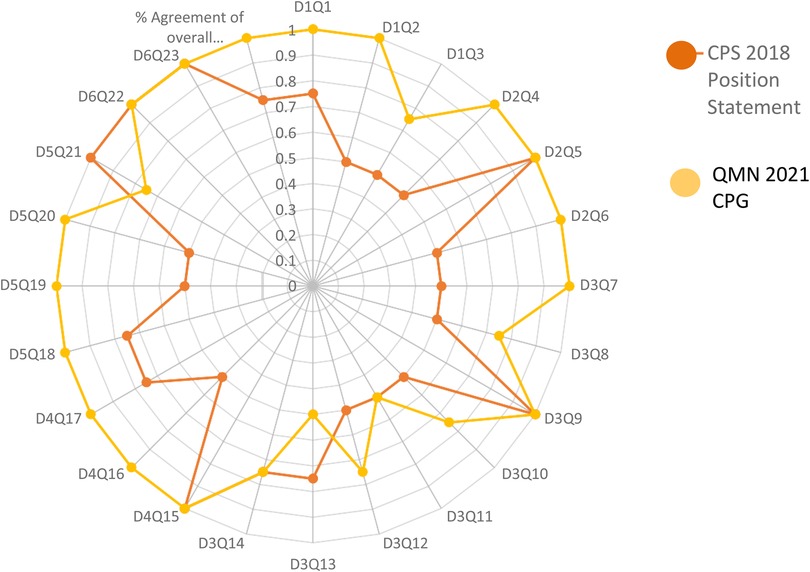
Figure 3. Showed percent agreement among raters for the two HIE practice guidelines focusing on every question in every domain.
Table 2 shows a map of the recommendations from both HIE CPGs (21, 22).
The difficulty of variation in their quality and evidence base continues despite the significant volume of national and international neonatal CPGs that are regularly released. To our knowledge, this evaluation is unique in that it uses AGREE II to comprehensively assess the quality of recently published HIE CPGs as a part of a nationwide CPG adaptation program (11).
The methodological rigor of the two HIE CPGs was evaluated using the AGREE II instrument, which identified a number of opportunities for improvement. Although the evaluation of the overall guideline quality and the usage of the recommendation are essential sections of AGREE II, it's likely that they are not clearly communicated in the published CPGs' methodology.
A recently published network meta-analysis compared the effectiveness and safety of different neuroprotective interventions for neonates with HIE (23). This study supports the current CPGs that recommend hypothermia for neonates with HIE, regardless of setting. Its findings support whole-body hypothermia as a first-line treatment option due to its ease of use, improved mortality, and positive neurodevelopmental outcomes (23).
Shipley et al. emphasized the importance of training, equipping, and supporting neonatal centers that lack immediate access to cooling services with active therapeutic hypothermia should be prioritized, reducing interruptions in initiating and achieving appropriate target temperature before transfer to specialized tertiary cooling neonatal centers (24).
We continue to recommend compiling the findings of this study with similar quality appraisals of neonatology CPGs to set up a CPGs hub or Recommendation map that would be of the utmost value for healthcare providers caring for newborn babies in selecting and implementing high-quality evidence-based CPGs and recommending them to their colleagues (11, 25–29).
Moreover, we are looking forward to having new evidence-based recommendations in the next editions of these CPGs based on the mounting evidence addressing the new options of care like the use of conventional electroencephalography (EEG) or Amplitude-integrated Electroencephalogram (aEEG) monitoring in neonatal HIE (30, 31).
The findings of our review can be further used to guide all relevant CPG development or adaptation project for neonatal HIE.
Implementation of the results of this systematic review through identifying and selecting high-quality CPGs will decrease variation in the management of neonatal HIE babies which might lead to improved clinical outcomes and decrease legal litigations.
We recommend monitoring the implementation of the process and auditing the process for quality improvement. Reporting adverse outcomes or variance of practice might help for future updates.
The methodological quality of the QMN CPG was superior followed by CPS. Recommendations addressed inclusion and exclusion criteria, fluid management, feeding, resuscitation, anticonvulsant drugs, sedation, allopurinol, xenon, melatonin, erythropoietin, neural stem cells, erythropoietin, magnesium sulphate, therapeutic hypothermia and target temperature, rewarming, brain MRI timing, and follow up.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
YA and JA conceptualized and designed the study. LH, YA, OA, MA, and JA contributed to the search, screening, review, and critical appraisal of guidelines. YA, JA, and OA wrote the first draft of the manuscript. YA and AE analyzed and interpreted the data. YA and JA supervised the procedures in the study and reviewed the drafts and final version of this manuscript. All authors have made substantial contributions and provided final approval for the conception, drafting, and final version of this manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the Saudi Neonatology Society (SNS).
We would like to thank the Saudi Neonatology Society (SNS) for the logistic support in the parallel training workshops conducted as part of this project and the financial support for the publication fees of this article. The SNS did not influence any phase of this research project. This study was supported by King Saud University, Deanship of Scientific Research, Research Chair for Evidence-Based Health Care and Knowledge Translation, Riyadh, Saudi Arabia. Furthermore, we would like to extend our thanks to King Saud University Medical City for logistics, database access, and relevant resources.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1092578/full#supplementary-material.
1. Halpin S, McCusker C, Fogarty L, White J, Cavalière E, Boylan G, et al. Long-term neuropsychological and behavioral outcome of mild and moderate hypoxic ischemic encephalopathy. Early Hum Dev. (2022) 165:105541. doi: 10.1016/j.earlhumdev.2022.105541
2. Keir A, Bamat N, Boyle E, ElKhateeb O, Wright C. EBNEO Commentaries: an ongoing collaboration advancing evidence-based neonatal care. Acta Paediatr. (2021) 110(5):1398–400. doi: 10.1111/apa.15750
3. Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet. (2017) 390(10092):415–23. doi: 10.1016/S0140-6736(16)31592-6
4. Chorath K, Garza L, Tarriela A, Luu N, Rajasekaran K, Moreira A. Clinical practice guidelines on newborn hearing screening: a systematic quality appraisal using the AGREE II instrument. Int J Pediatr Otorhinolaryngol. (2021) 141:110504. doi: 10.1016/j.ijporl.2020.110504
5. Renesme L, Bedu A, Tourneux P, Truffert P. How to assess clinical practice guidelines with AGREE II: the example of neonatal jaundice. Arch Pediatr. (2015) 23(3):241–8. doi: 10.1016/j.arcped.2015.11.021
6. Mosalli R. Whole body cooling for infants with hypoxic-ischemic encephalopathy. J Clin Neonatol. (2012) 1(2):101–6. doi: 10.4103/2249-4847.96777
7. Home - Saudi Neonatology Society (SNS). (2022). Available at: https://sns.med.sa/?lang=en [cited 18 August 2022].
8. Amer YS, Wahabi HA, Abou Elkheir MM, Bawazeer GA, Iqbal SM, Titi MA, et al. Adapting evidence- based clinical practice guidelines at university teaching hospitals: a model for the eastern Mediterranean Region. J Eval Clin Pract. (2019) 25(4):550–60. doi: 10.1111/jep.12927
9. Amer YS, Elzalabany MM, Omar TI, Ibrahim AG, Dowidar NL. The “adapted ADAPTE”: an approach to improve utilization of the ADAPTE guideline adaptation resource toolkit in the Alexandria Center for Evidence-Based Clinical Practice Guidelines. J Eval Clin Pract. (2015) 21(6):1095–106. doi: 10.1111/jep.12479
10. Wang Z, Norris SL, Bero L. The advantages and limitations of guideline adaptation frameworks. Implement Sci. (2018) 13(1):72. doi: 10.1186/s13012-018-0763-4
11. Amer Y, Shaiba L, Hadid A, Anabrees J, Almehery A, AAssiri M, et al. Quality assessment of clinical practice guidelines for neonatal sepsis using the appraisal of guidelines for research and evaluation (AGREE) II instrument: a systematic review of neonatal guidelines. Front Pediatr. (2022) 10:1–9. doi: 10.3389/fped.2022.891572
12. Johnston A, Kelly SE, Hsieh SC, Skidmore B, Wells GA. Systematic reviews of clinical practice guidelines: a methodological guide. J Clin Epidemiol. (2019) 108:64–76. doi: 10.1016/j.jclinepi.2018.11.030
13. Brouwers MC, Spithoff K, Lavis J, Kho ME, Makarski J, Florez ID. What to do with all the AGREEs? The AGREE portfolio of tools to support the guideline enterprise. J Clin Epidemiol. (2020) 125:191–7. doi: 10.1016/j.jclinepi.2020.05.025
14. Chen Y, Guyatt GH, Munn Z, Florez ID, Marušić A, Norris SL, et al. Clinical practice guidelines registry: toward reducing duplication, improving collaboration, and increasing transparency. Ann Intern Med. (2021) 174:705–7. doi: 10.7326/m20-7884
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
16. Amer Y, Anabrees J, Elkhateeb O, Abdelmawla M, Alhifzi I, AlOnazi A, et al. A critical appraisal of clinical practice guidelines for diagnosis and management of Hypoxic Ischemic Encephalopathy: Systematic review and Assessment. PROSPERO 2021 CRD42021258291. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021258291 (Cited 17/08/2022).
17. Login - AGREE Enterprise website. Agreetrust.org. Available at: https://www.agreetrust.org/my-agree/ (Accessed August 17, 2022).
18. Cohen JA. Coefficient of agreement for nominal scales. Educ Psychol Measur. (1960) 20:37–46. doi: 10.1177/001316446002000104
19. Fleiss JL, Shrout PE. Approximate interval estimation for a certain intraclass correlation coefficient. Psychometrika. (1978) 43:259–62. doi: 10.1007/BF02293867
20. Bland JM, Altman DG. Statistics notes: measurement error and correlation coefficients. Br Med J. (1996) 313:41–2. doi: 10.1136/bmj.313.7048.41
21. Queensland Clinical Guidelines. Hypoxic-Ischemic Encephalopathy (HIE). Guideline No. MN21.11-V10- R26. Queensland Health. (2021). Available from: http://www.health.qld.gov.au/qcg or https://www.health.qld.gov.au/qcg/publications#neonatal [Accessed 16/08/2022].
22. Lemyre B, Chau V. Hypothermia for newborns with hypoxic-ischemic encephalopathy. Paediatr Child Health. (2018) 23(4):285–91. doi: 10.1093/pch/pxy028
23. Lee CYZ, Chakranon P, Lee SWH. Comparative efficacy and safety of neuroprotective therapies for neonates with hypoxic ischemic encephalopathy: a network meta-analysis. Front Pharmacol. (2019) 10:1221. doi: 10.3389/fphar.2019.01221
24. Shipley L, Mistry A, Sharkey D. Outcomes of neonatal hypoxic-ischaemic encephalopathy in centres with and without active therapeutic hypothermia: a nationwide propensity score-matched analysis. Arch Dis Child Fetal Neonatal Ed. (2022) 107:6–12. doi: 10.1136/archdischild-2020-320966
25. Zhang M, Tang J, He Y, Li W, Chen Z, Xiong T, et al. Systematic review of global clinical practice guidelines for neonatal hyperbilirubinemia. BMJ Open. (2021) 11(1):e040182. doi: 10.1136/bmjopen-2020-040182
26. Balice-Bourgois C, Zumstein-Shaha M, Vanoni F, Jaques C, Newman CJ, Simonetti GD. A systematic review of clinical practice guidelines for acute procedural pain on neonates. Clin J Pain. (2020) 36(5):390–8. doi: 10.1097/AJP.0000000000000808
27. Hough JL, Barton J, Jardine LA. A quality appraisal using the AGREE II instrument of endotracheal tube suction guidelines in neonatal intensive care units. Aust Crit Care. (2021) 34(6):524–9. doi: 10.1016/j.aucc.2021.02.001
28. Chorath K, Garza L, Tarriela A, Luu N, Rajasekaran K, Moreira A. Clinical practice guidelines on newborn hearing screening: a systematic quality appraisal using the AGREE II instrument. Int J Pediatr Otorhinolaryngol. (2021) 141:110504. doi: 10.1016/j.ijporl.2020.110504
29. Renesme L, Bedu A, Tourneux P, Truffert P. Évaluation de la qualité d’élaboration d’une recommandation pour la pratique clinique avec la grille AGREE II: exemple de l’ictère néonatal [how to assess clinical practice guidelines with AGREE II: the example of neonatal jaundice]. Arch Pediatr. (2016) 23(3):241–8. French. doi: 10.1016/j.arcped.2015.11.021
30. Bourel-Ponchel E, Querne L, Flamein F, Ghostine-Ramadan G, Wallois F, Lamblin MD. The prognostic value of neonatal conventional-EEG monitoring in hypoxic-ischemic encephalopathy during therapeutic hypothermia. Dev Med Child Neurol. (2023) 65(1):58–66. doi: 10.1111/dmcn.15302
Keywords: hypoxic ischemic encephalopathy, HIE, pediatrics, neonatology, clinical practice guidelines, systematic review, AGREE II instrument, quality assessment
Citation: Amer YS, Anabrees J, Abdelmawla M, Abdalgader A, Almazroei A, Alhifzi I, AlOnazi AH, Sabr Y, Hneiny L, El-Malky A, Alshalawi A, Alayoubi A, Chaudhry IA and Elkhateeb O (2023) Clinical practice guidelines for neonatal hypoxic-ischemic encephalopathy: A systematic review using the appraisal of guidelines for research and evaluation (AGREE) II instrument. Front. Pediatr. 11:1092578. doi: 10.3389/fped.2023.1092578
Received: 8 November 2022; Accepted: 27 February 2023;
Published: 22 March 2023.
Edited by:
Emel Okulu, Ankara University, TürkiyeReviewed by:
Cheng Guoqiang, Fudan University, China© 2023 Amer, Anabrees, Abdelmawla, Abdalgader, Almazroei, Alhifzi, AlOnazi, Sabr, Hneiny, El-Malky, Alshalawi, Alayoubi, Chaudhry and Elkhateeb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasser S. Amer eWFtZXJAa3N1LmVkdS5zYQ== eWFzc2Vyc2FtaWFtZXJAZ21haWwuY29t Jasim Anabrees amFzaW0xODAwQHlhaG9vLmNvbQ== amFuYWJyZWVzQGtzdS5lZHUuc2E=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Abbreviations AGREE II, Appraisal of Guidelines for REsearch and Evaluation (Version 2); CPG, Clinical Practice Guideline; CPS, Canadian Paediatric Society; GRADE, Grading of Recommendations; Assessment; Development and Evaluations; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses Statement; PROSPERO, International Database of Prospectively Registered Systematic Reviews with a Health Related Outcome; QMN, Queensland Statewide Maternity and Neonatal Clinical Network.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.