- 1Department of Ultrasonic Medicine, West China Second University Hospital of Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
- 3Department of Pediatric Pulmonology and Immunology, West China Second University Hospital of Sichuan University, Chengdu, China
- 4Key Laboratory of Chronobiology (Sichuan University), National Health Commission of China, Chengdu, China
- 5The Joint Laboratory for Lung Development and Related Diseases of West China Second University Hospital, Sichuan University and School of Life Sciences of Fudan University, West China Institute of Women and Children's Health, West China Second University Hospital of Sichuan University, Chengdu, China
- 6Sichuan Birth Defects Clinical Research Center, West China Second University Hospital of Sichuan University, Chengdu, China
- 7Tibet Autonomous Region Women's and Children's Hospital, West China Second University Hospital of Sichuan University, Lhasa, China
- 8Ziyang Maternal and Child Health Care Hospital, Ziyang, China
- 9Ziyang Women and Children Hospital, West China Second University Hospital of Sichuan University, Ziyang, China
We herein first report the use of conventional echocardiography combined with two-dimensional speckle-tracking to diagnose and monitor the changing process of cardiac involvement in an infant with congenital lipodystrophy. An 8-month-old girl was admitted to our hospital after first presenting at the age of 3 months with abnormal facial features that had been noticed within 4 weeks of birth. Echocardiography performed at the age of 3 months showed only slightly accelerated blood flow in the right ventricular outflow tract. At the age of 5 months, echocardiography showed myocardial hypertrophy; this finding combined with the physical characteristics and other examination results led to the consideration of congenital lipodystrophy. Genetic testing at the age of 9 months confirmed type 2 congenital lipodystrophy caused by BSCL2 gene mutation, and dietary modification was initiated. Conventional echocardiography performed at the ages of 5, 8, and 14 months showed no significant changes and a normal ejection fraction. However, two-dimensional speckle-tracking performed between the ages of 5 and 8 months showed cardiac systolic abnormalities that tended to improve after treatment. This case highlights the value of echocardiography in detecting structural and early functional cardiac changes in infants with congenital lipodystrophy.
Introduction
First reported in 1954, congenital lipodystrophy (CGL) is a very rare autosomal recessive disorder with a heterogeneous presentatio n. CGL has an estimated prevalence of about 1/10,000,000 and is generally associated with parental consanguinity (1). The most extreme phenotype of CGL is severe insulin resistance with the loss of nearly all the body fat at birth and early development of metabolic complications in childhood (1,2). Another four distinct genetic subtypes of CGL have been reported to date, which are associated with mutations of AGPAT2, BSCL2, CAV1, and PTRF, respectively (3). Besides the common clinical manifestations, patients with type 1 CGL might present with acromegaloid features with an enlarged mandible, hands, and feet, and bone cysts as a late complication. Patients with type 2 CGL have an increased prevalence of cardiomyopathy and mild mental retardation and have lower median serum concentrations of leptin and adiponectin than healthy individuals. Patients with type 3 CGL have serum creatine kinase concentrations between 2.5 and 10 times the upper limit of normal. Patients with type 4 CGL have congenital myopathy with high serum concentrations of creatine kinase and a predisposition to serious arrhythmias (1,2). The most common subtypes of CGL are types 1 and 2, and the cardiac abnormalities in patients with CGL usually occur in childhood or later in adulthood (4). In this report, we describe an infant with type 2 CGL in whom early cardiac involvement was diagnosed by echocardiography.
Case presentation
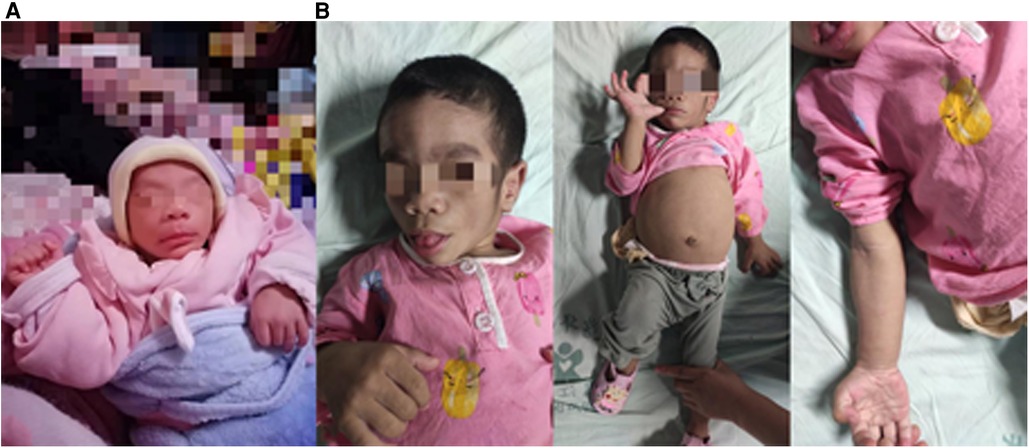
An 8-month-old girl was admitted to the pediatric department of our hospital after first presenting at the age of 3 months with abnormal facial features that had been noticed within 4 weeks of birth (Figure 1A). Her parents were healthy first cousins. Her prenatal ultrasound examination showed no obvious abnormalities. Because the age of the mother at the expected delivery date was more than 35 years, chromosome microarray analysis by amniocentesis had been performed. The chromosome microarray analysis showed no chromosome number abnormalities or pathogenic copy number variation, and detected eight loss of heterozygosity regions of unknown significance. The mother refused whole-exome sequencing of amniotic fluid owing to the high cost. The patient was born after a full-term pregnancy with a birth weight of 2860 g and an Apgar score of 9–10–10. She had no significant family history and had a healthy 9-year-old sister.
At the age of 3 months, physical examination revealed a progeroid facial appearance of coarse facial features with prominent cheekbones and a triangular chin, a low frontal and posterior hairline, and facial hirsutism. She also had hepatomegaly (7 cm in the midclavicular line) with no subcutaneous fat, marked hypertrophy of muscles in the limbs, and acanthosis nigricans. A 3/6 systolic murmur was heard in the precardiac region. Echocardiography showed normal ventricular wall thicknesses (Figure 2A). (interventricular septum (IVS) measured as 4.5 mm, Z score +1.62; left ventricular posterior wall (LVPW) measured as 4 mm, Z score +0.24) and slightly accelerated blood flow in the right ventricular outflow tract (Figure 2B). The preliminary diagnosis was progeria without further treatment.
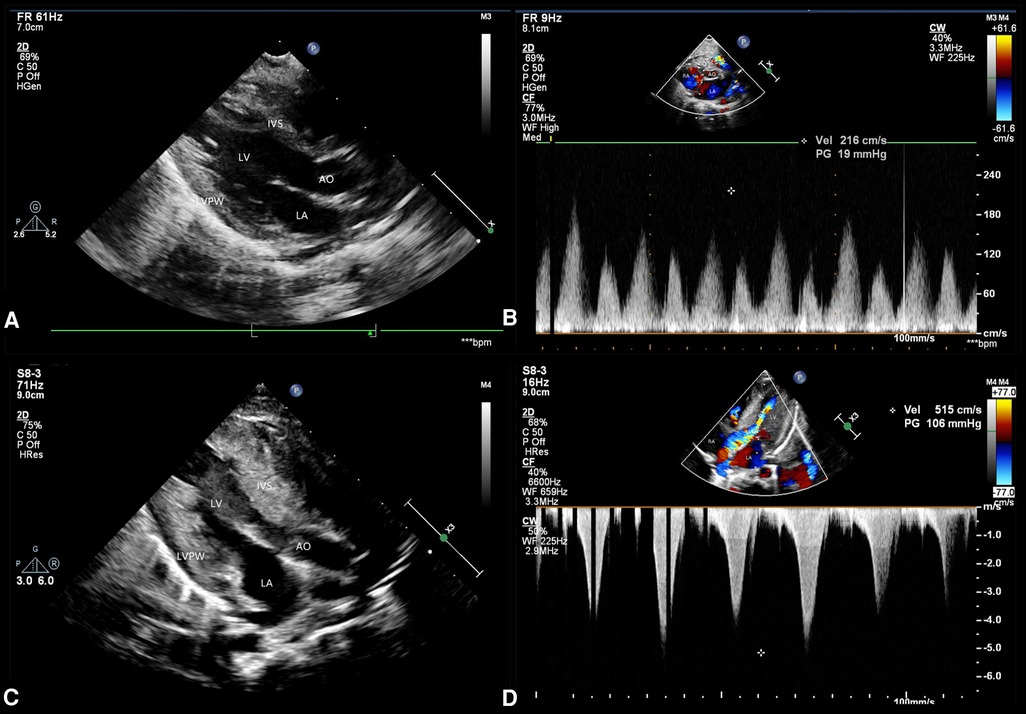
Figure 2. Echocardiographic findings. Echocardiography performed at the age of 3 months shows (A) a normal ventricular wall thickness but (B) slightly accelerated blood flow in the right ventricular outflow tract. Echocardiography performed at 5 months of age shows (C) significant myocardial hypertrophy and (D) an obstructive pattern in the left ventricular outflow tract.
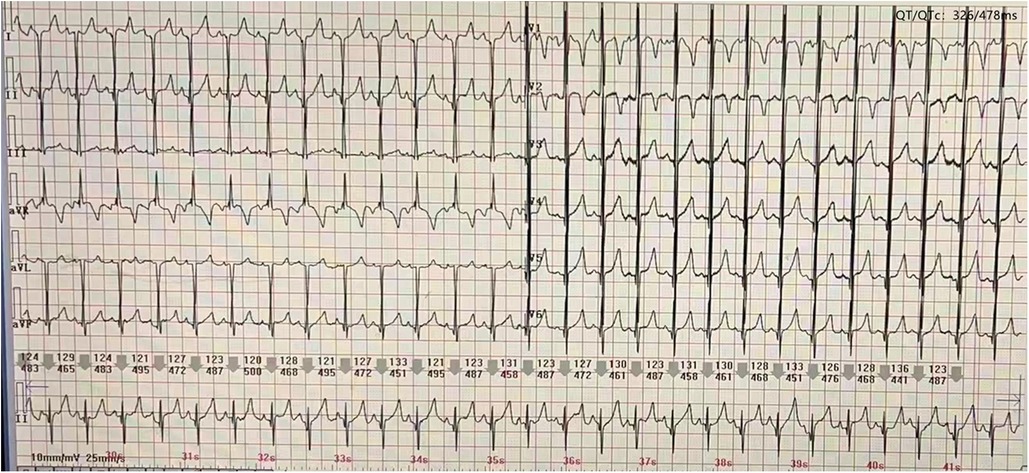
At the age of 5 months, the physical examination findings were similar to the findings at 3 months (Figure 1B). Standard conventional echocardiography performed at the age of 5 months showed myocardial hypertrophy (Figure 2C). (IVS measured as 13 mm, Z score +23.21; LVPW measured as 8.5 mm, Z score +9.1) and an obstructive pattern in the left ventricular outflow tract (Figure 2D) but a normal ejection fraction of 70%. Because the ventricular wall thickness was significantly thicker at the age of 5 months than it was at the age of 3 months, we ruled out the diagnosis of classic hypertrophic cardiomyopathy and considered that the ventricular wall hypertrophy was caused by metabolic factors. Two-dimensional speckle-tracking (2D-STE) was performed to assess strain parameters in the left ventricle and revealed a global longitudinal strain of −16.79% (Figure 3A), a global radial strain of 65.67%, and a global circumferential strain of −14.04%. Abdominal ultrasound showed hepatic steatosis with hepatomegaly. An electrocardiogram revealed sinus rhythm and a prolonged QTc interval (Figure 4). Her blood pressure was 123/65 mmHg. Additional fasting blood testing showed an increased free fatty acid concentration (1.39 mmol/L), normoglycemic hyperinsulinemia (glycemia concentration, 4.60 mmol/L; insulin concentration, 58.53 µIU/mL), dyslipidemia (increased triglycerides [10.14 mmol/L], total cholesterol [5.33 mmol/L], and low-density lipoprotein cholesterol [3.8 mmol/L]), and decreased high-density lipoprotein cholesterol (0.69 mmol/L) indicating insulin resistance. CGL was considered based on the physical characteristics and investigations.
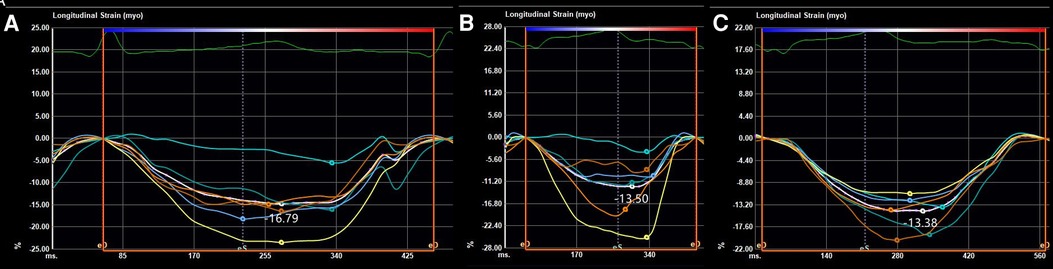
Figure 3. Strain parameters on two-dimensional speckle-tracking of the global longitudinal strain at the ages of (A) 5 months, (B) 8 months, and (C) 14 months.
At the age of 8 months, 2D-STE showed a decrease in several left ventricular strain parameters (global longitudinal strain, −13.50% (Figure 3B); global radial strain, 37.63%; and global circumferential strain, −14.76%) compared with the values recorded at the age of 5 months despite the findings on conventional echocardiography showing no significant change (IVS measured as 13.5 mm, Z score +23.34; LVPW measured as 8.5 mm, Z score +9.7). Combined with the typical clinical manifestations (severe insulin resistance with lipoatrophy and hypertrophic cardiomyopathy, and hirsutism), exon group of single gene disease-customized capture sequencing test at the age of 9 months identified a homozygous mutation in the BSCL2 gene: c.166_184del (p.Tyr56fs*) (reference sequence NM_032667.6) on 11q12.3, confirming a diagnosis of type 2 CGL (OMIM # 269700).. Both her mother and sister were confirmed to be heterozygous carriers. Dietary modification was introduced to restrict her fat intake to 25% of the total dietary energy. Follow-up biochemistry at the age of 14 months showed progressive improvement in the fasting lipid profile (triglycerides, 4.13 mmol/L; total cholesterol, 5.10 mmol/L; insulin concentration, 29.49 µIU/mL) with normal glycemia concentration [4.5 mmol/L]. Her blood pressure at the age of 14 months was normal. Conventional echocardiography showed no significant changes (IVS measured as 13 mm, Z score +20.55; LVPW measured as 8.5 mm, Z score +7.75) from the findings at the age of 8 months, and the left ventricular strain parameters measured by 2D-STE tended to remain stable (global longitudinal strain, −13.38% (Figure 3C); global radial strain, 34.60%; global circumferential strain, −14.41%).
Discussion
A diagnosis of CGL is based on three major criteria or two major criteria plus more than two minor criteria and/or genetic testing (2). The major criteria are: lipoatrophy affecting the trunk, limbs, and face; acromegaloid features; hepatomegaly; elevated serum triglycerides; and insulin resistance. The minor criteria are hypertrophic cardiomyopathy, psychomotor retardation or mild to moderate intellectual impairment, hirsutism, precocious puberty (female), bone cysts, and phlebomegaly.
Although cardiac involvement in CGL was first reported in 1959 (5), the pathophysiological mechanism is still not completely clear; however, it may involve the combined effects of abnormal metabolism, dysfunction in the autonomic nervous system, and reduction or loss of epicardial adipose tissue (4, 6). A variety of cardiac problems have been described, ranging from cardiomyopathy, impaired cardiac function, myocardial infarction, and arrhythmia to heart failure and sudden death (2, 3). Hypertrophic cardiomyopathy is reported to be present in 20%–25% of individuals with CGL (2), is found mainly in type 2 but occasionally in type 1 (4), and is a significant cause of morbidity associated with cardiac failure and with early mortality at around 30 years of age (2). Arrhythmias are mostly seen in type 4 CGL (3), while a long QT interval is commonly seen in types 2 and 4 (4). QT prolongation is an independent predictor of all-cause and cardiovascular mortality (3).
The reported mean age at the time of diagnosis of cardiomyopathy in patients with CGL is 20 years (7), with cardiovascular manifestations occurring earlier in type 2 CGL (4). Most of the published data on cardiac involvement in infants with CGL come from case reports, in which hypertrophic cardiomyopathy is the most common cardiac manifestation (7–11). The literature includes the following cases: a boy with type 1 CGL in whom concentric thickening of the left ventricle, a mild obstructive pattern in the left ventricular outflow tract, and tachycardia were noted at 2 months of age (7) and four infants with type 2 CGL (a girl who showed asymmetric hypertrophic cardiomyopathy at the age of 6 months (8), a boy with asymmetric hypertrophic cardiomyopathy, an obstructive pattern in the left ventricular outflow tract, and tachycardia at 4 months of age (9), a boy who showed hypertrophic cardiomyopathy at the age of 4 months and worsening of left ventricular wall thickness at the age of 6.5 months (10), and a boy who was mentioned to have cardiomyopathy at 2 months of age with no further clinical details provided) (11). In our case, the patient was initially diagnosed with progeria, but the cardiac changes and unusual clinical features led us to consider the diagnosis of CGL. Cardiac involvement was first detected as slightly accelerated blood flow in the right ventricular outflow tract at the age of 3 months that developed into hypertrophic cardiomyopathy combined with a prolonged QTc interval by the age of 5 months. To our knowledge, this is the first report in which echocardiography observed the thickening process of the ventricular wall from normal thickness to asymmetric hypertrophy, and the simultaneous occurrence of cardiomyopathy and this type of arrhythmia has not been reported previously in infants with type 2 CGL. However, the slightly accelerated blood flow in the right ventricular outflow tract may have resulted from slight myocardial hypertrophy of the basal anterior septum, which may have been ignored during the examination because the basal segment of the anterior ventricular septum is more likely to be involved in patients with CGL (12). Furthermore, using 2D-STE, we observed progressive decreases in global longitudinal strain and global radial strain between the ages of 5 and 8 months with a normal ejection fraction. This finding suggests subclinical impairment of myocardial systolic function and is in accordance with the results of the only previous study that has used 2D-STE to detect cardiac alterations in patients with CGL (13). A possible explanation of these findings is the histopathological changes that occur in these patients, including diffuse distribution of interstitial deposits of collagen, severe subendocardial fibrosis, hypertrophy of myocytes, and coronary atherosclerosis (14). The higher sensitivity of 2D-STE allows early detection of cardiac systolic abnormalities, and our finding that 2D-STE parameters tended to improve when dietary modification was introduced into the treatment plan confirms that metabolic abnormalities contribute to cardiac involvement in CGL. Therefore, we recommend 2D-STE for early evaluation of myocardial dysfunction in children with CGL and for decision-making regarding an appropriate treatment strategy. If the patient's 2D-STE parameters are improved after just restricting the total fat intake, there is no need to use drugs; however, once the cardiac function starts worsening, even if the ejection fraction is normal, we recommend the addition of drugs such as leptin.
Conclusion
We have encountered a case of cardiac involvement in an infant with type 2 CGL as a result of BSCL2 mutation. Echocardiography is helpful for real-time detection of cardiac changes in patients with CGL and can be used for follow-up examinations. This is the first report of the simultaneous occurrence of cardiomyopathy and a prolonged QTc interval in an infant with CGL, and the thickening process of ventricular wall was observed by echocardiography. There is only one previous report on the use of 2D-STE for the detection of cardiac alterations in patients with CGL, with our case report being the first report to use 2D-STE to detect cardiac alterations and monitor myocardial function in an infant.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the ethics committee of West China Second University Hospital of Sichuan University. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Fundamental Research Funds for the Central Universities, the National Key R & D Program of China (grant numbers: 2017YFC0211705, 2017YFC0113905), the Key R & D Program of Science and Technology Department of Sichuan Province (grant numbers: 2019YFS0403, 2019YFS0037), and the Popularization and Application Project of the Sichuan Health and Family Planning Commission (grant numbers: 17PJ415).
Acknowledgments
The authors are very grateful to Wen Zhang and Song Yan, who contributed the images accompanying this article. They also thank Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1087833/full#supplementary-material.
References
1. Patni N, Garg A. Congenital generalized lipodystrophies–new insights into metabolic dysfunction. Nat Rev Endocrinol. (2015) 11(9):522–34. doi: 10.1038/nrendo.2015.123
2. Van Maldergem L. Berardinelli-Seip congenital lipodystrophy. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, et al., editors. Genereviews®. Seattle (WA): University of Washington, Seattle; (2003). p. 1–17.
3. Sanon VP, Handelsman Y, Pham SV, Chilton R. Cardiac manifestations of congenital generalized lipodystrophy. Clin Diabetes. (2016) 34(4):181–6. doi: 10.2337/cd16-0002
4. Ponte CMM, Fernandes VO, Liberato CBR, Montenegro APDR, Batista LA, Gurgel MHC, et al. Association between cardiovascular autonomic neuropathy and left ventricular hypertrophy in young patients with congenital generalized lipodystrophy. Diabetol Metab Syndr. (2019) 11:53. doi: 10.1186/s13098-019-0444-8
5. M SEIP. Lipodystrophy and gigantism with associated endocrine manifestations. A new diencephalic syndrome?. Acta Paediatr (Stockh). (1959) 48:555–74.14444642
6. Nelson MD, Victor RG, Szczepaniak EW, Simha V, Garg A, Szczepaniak LS. Cardiac steatosis and left ventricular hypertrophy in patients with generalized lipodystrophy as determined by magnetic resonance spectroscopy and imaging. Am J Cardiol. (2013) 112(7):1019–24. doi: 10.1016/j.amjcard.2013.05.036
7. Debray FG, Baguette C, Colinet S, Van Maldergem L, Verellen-Dumouin C. Early infantile cardiomyopathy and liver disease: a multisystemic disorder caused by congenital lipodystrophy. Mol Genet Metab. (2013) 109(2):227–9. doi: 10.1016/j.ymgme.2013.04.011
8. Bhayana S, Siu VM, Joubert GI, Clarson CL, Cao H, Hegele RA. Cardiomyopathy in congenital complete lipodystrophy. Clin Genet. (2002) 61(4):283–7. doi: 10.1034/j.1399-0004.2002.610407.x
9. Friguls B, Coroleu W, del Alcazar R, Hilbert P, Van Maldergem L, Pintos-Morell G. Severe cardiac phenotype of berardinelli-seip congenital lipodystrophy in an infant with homozygous E189X BSCL2 mutation. Eur J Med Genet. (2009) 52(1):14–6. doi: 10.1016/j.ejmg.2008.10.006
10. Jeninga EH, de Vroede M, Hamers N, Breur JM, Verhoeven-Duif NM, Berger R, et al. A patient with congenital generalized lipodystrophy due to a novel mutation in BSCL2: indications for secondary mitochondrial dysfunction. JIMD Rep. (2012) 4:47–54. doi: 10.1007/8904_2011_86
11. Miranda DM, Wajchenberg BL, Calsolari MR, Aguiar MJ, Silva JM, Ribeiro MG, et al. Novel mutations of the BSCL2 and AGPAT2 genes in 10 families with berardinelli-seip congenital generalized lipodystrophy syndrome. Clin Endocrinol (Oxf). (2009) 71(4):512–7. doi: 10.1111/j.1365-2265.2009.03532.x
12. Scatteia A, Pagano C, Pascale C, Guarini P, Marotta G, Perrone-Filardi P, et al. Asymmetric hypertrophic cardiomyopathy in generalized lipodystrophy. Int J Cardiol. (2016) 202:724–5. doi: 10.1016/j.ijcard.2015.10.032
13. Liberato CBR, Olegario NBDC, Fernandes VO, Montenegro APDR, Lima GEDCP, Batista LAA, et al. Early left ventricular systolic dysfunction detected by two-dimensional speckle-tracking echocardiography in young patients with congenital generalized lipodystrophy. Diabetes Metab Syndr Obes. (2020) 13:107–15. doi: 10.2147/DMSO.S233667
Keywords: congenital generalized lipodystrophy, cardiac involvement, echocardiography, infant, systolic dysfunction
Citation: Zhou J, Liu H, Chen J and He X (2023) Case report: Echocardiographic diagnosis of cardiac involvement caused by congenital generalized lipodystrophy in an infant. Front. Pediatr. 11:1087833. doi: 10.3389/fped.2023.1087833
Received: 2 November 2022; Accepted: 6 March 2023;
Published: 23 March 2023.
Edited by:
Harvey Ho, University of Auckland, New ZealandReviewed by:
Haifeng Zong, Southern Medical University, ChinaAndrea Vergara, University of Campania Luigi Vanvitelli, Italy
Víctor A. Cortés, Pontificia Universidad Católica de Chile, Chile
© 2023 Zhou, Liu, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiao Chen Y2hlbmppYW9Ac2N1LmVkdS5jbg==
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
 Jie Zhou
Jie Zhou Hanmin Liu
Hanmin Liu Jiao Chen
Jiao Chen Xiaolan He8,9
Xiaolan He8,9