
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 17 March 2023
Sec. Obstetric and Pediatric Pharmacology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1087095
This article is part of the Research Topic Emerging Researchers in Frontiers in Pharmacology: Obstetric and Pediatric Pharmacology 2022 View all 13 articles
 Bashayer Mohammed Althobaiti1,2
Bashayer Mohammed Althobaiti1,2 Mahmoud Zaki El-Readi3,4*
Mahmoud Zaki El-Readi3,4* Mohammad Althubiti3
Mohammad Althubiti3 Yosra Zakariyya Alhindi1,2
Yosra Zakariyya Alhindi1,2 Abdullah R Alzahrani1,2
Abdullah R Alzahrani1,2 Saeed S Al-Ghamdi1,2
Saeed S Al-Ghamdi1,2 Nahla Ayoub1,2
Nahla Ayoub1,2 Bassem Refaat5
Bassem Refaat5 Safaa Yehia Eid3*
Safaa Yehia Eid3*
Background: Poisoning occurs when a person is exposed to an external substance at a too high dose for them. It is possible for young children to be exposed to chemicals. Lungs, the heart, CNS, the digestive tract, and kidneys can be poisoned. In 2004, over 45,000 children and teenagers died from acute poisoning, representing 13% of all accidental poisoning deaths worldwide. Poisoning patterns vary by exposure type, age group, poison type, and dose.
Aim: This study assessed the pattern of acute poisoning with drugs, chemicals, and natural toxins among children (<12 years old). The study was done in Makkah region and registered in the poison control center in Makkah, the forensic chemistry center in Haddah during 2020–2021.
Methods: A retrospective cohort study was done on 122 children exposed to toxic substances in Makkah. The children were 12 years old and had good health for a maximum of one year. Stratified random sampling was used to divide cases into groups of similar poisons (pharmaceutical products, household products, plant envenomation, and animal envenomation). Then each group got a random samples. The data were analysed with SPSS software.
Results: The mean age of children was 5.2 years, with 59% being boys. The mean temperature, pulse, systolic, diastolic, and respiratory rates were 36.77, 98.29, 109.1, 69.17, and 21.49. The most documented pharmaceutical products (200 mg) were carbamazepine (5 mg), methanol, risperidone (5 mg), propranolol (5 mg), and olanzapine (5 mg). The most common poison forms were tablets (42.6%), syrups (15.6%), capsules (13.9%), and solutions (13.1%). The most common poisoning routes were ingestion (82.8%), dermal (5.7%), injection (4.9%), and inhalation (6.6%). Accidental poisoning was 83%, with a 30-minute lag for 30.3% of children, and most (69.7%) occurred at home. Benzodiazepines were the most commonly used category class drug (18%), with normal pupils and an ECG of 85.2%. Sixty-seven percent had blood tests. Sickness was 9.48, and the positive result was 213.01. The most common presenting symptoms were GIT and neurological (23.8%). 31.1% had mild, moderate, or severe toxicity. Most cases (68%) were complex. 34.4% were intubated, 9.8% had repeated-dose-activated charcoal for enhanced elimination, and 27.8% were on IV fluids. Children with GIT, CVS, respiratory, dermal, and neurological symptoms had a higher percentage of severe toxicity (p < 0.05). Slight toxicity was associated with whole bowel irrigation, intubation for oxygen therapy, N-acetylcysteine or sedation, fluids, and phenytoin (P < 0.05). Complicated cases had a higher mean AST/IUL than non-complicated cases (75.5 vs. 20.08, p < 0.05). The level of toxicity did not correlate with the mean of all lab tests (p > 0.05). The age of the children correlated positively with their systolic BP (r = 0.22, p < 0.01).
Conclusion: The results show how important it is to teach the public about poisoning and make rules for tracking and dealing with poisonings in Saudi Arabia.
Accidental poisonings take place when a person, typically a child, ingests a poisonous substance without intending to do so (as opposed to purposeful poisoning or overdosing) (1). Children are more likely to experience serious repercussions from poisoning due to the fact that their bodies are smaller, have a faster metabolic rate, and are less capable of neutralizing harmful substances (2). Children who are poisoned might experience psychological and physical repercussions over a long period of time, and the costs to society can be quite high (3).
Poisons ingested can be classified into medications (prescription or non-prescription), household items, and plants Their level of toxicity could be mild, moderate, or severe (4). Poisoning patterns vary depending on the type of exposure, age group, nature, and dose of the poison (5, 6).
Acute poisoning is a common occurrence in emergency rooms worldwide, necessitating extensive medical care and significant financial investment (7). A high number of acute poisoning cases were caused by drug poisoning. Natural poisons, such as toxic plants and animals and acute chemical poisonings in the home, are frequent, especially in children (8). There are various variations in the pattern and etiology of acute poisoning, even within the same geographical region (8).
The pattern and types of poisons vary depending on numerous factors such as demography, education, socioeconomic level, and local beliefs and practices in different parts of the world. As a result, each country needs its epidemiological surveillance to establish the scope and pattern of the disease so that preventative steps can be taken (9).
Knowing the overall trend of poisoning in a certain area can aid in identifying risk factors and enabling early discovery and treatment of such cases, lowering morbidity and mortality (8). Poisoning occurrences were treated differently depending on the patient's condition, the type of poisoning, and the length of exposure (10).
In Saudi Arabia (KSA), acute poisoning in children and adults has been reported in several Saudi cities, including Jeddah, Hafr Al Batin, Abha, and Al Riyadh (11). In Abha, there were 114 acute poisoning incidents in children between January 2000 and October 2003 (12). At King Khaled Hospital in the Al Majmaah region of Saudi Arabia, a study done in 2014 found that most instances were caused by animal envenomation (13). Alghadeer et al., 2018 found that most instances were asymptomatic, and most of the youngsters arrived at the hospital in under three hours (5).
Another perspective study conducted in Riyadh in 2019 found that toxic household goods were the most implicated substance class in children under the age of six (13), and recently in 2020, a study conducted at East Jeddah Hospital in Jeddah city found that unintentional poisoning occurred in 56.5% of recorded instances and 92.8% of incidents occurred at home (14).
This study aimed to assess the pattern of acute poisoning with drugs, chemicals, and natural toxins of children (≤12) in the Makkah region and registered in the poison control center in Makkah, Saudi Arabia. This study can provide additional information about the agents most commonly involved in poisoning in this region and prevention and management guidelines to avoid them.
A retrospective cohort study was done.
122 children with toxicity from the Children's Hospital in Makkah region of Saudi Arabia and registered in the poison control center in Makkah, forensic chemistry center in Haddah during 2020–2021 were included. The inclusion criteria were all children taking toxic substances any way route, of both genders, and children who live in Makkah. The exclusion criteria were forensic toxicology children with poisoning, adults, those that do not need medical management or recommendation, and children from regions other than Makkah.
Stratified Random Sampling was done by dividing cases into groups according to the type of poisons with similar attributes (Pharmaceutical products-Household products-plant envenomation-animal envenomation). Then, from each group, a random sample was drawn. Children were classified into having mild, moderate, and severe toxicity as follows: (1) Mild; transient and spontaneously resolving symptoms, (2) Moderate; pronounced or prolonged symptoms, and (3) Severe or life-threatening symptoms. The sample size was calculated by this website (Qualtrics XM of sample size calculator). This equation was done at a confidence level of (95%) and a (5%) margin of error. The ideal sample size was (one hundred twenty-two).
No issues regarding animal subjects. This study has had the approval of the research ethics committee at the Department of the deanship of postgraduate studies at Umm al Qura University in the Makkah region and the poison control center in Makkah forensic chemistry center. Written informed consent for the research was not required in accordance with National legislation and institutional requirements. No identifiable human images or data was present in the study.
The data were analyzed using a statistical package for social sciences (SPSS) version 26. (Armonk, NY: IBM Corp.). Qualitative data were expressed as numbers and percentages to test the relationship between variables, and the Chi-squared test (χ2) was used. Quantitative data were expressed as mean and standard deviation (Mean ± SD), and non-parametric variables were tested using the Mann-Whitney (U) and Kruskal Wallis tests. Correlation analysis was performed using the Spearman's test, and a p-value of less than 0.05 was considered statistically significant.
The mean age of the studied children was 5.2 ± 3.74 years, and the mean BMI was 26.67 ± 24.86 kg/m2, respectively. Of the children, 59% were males, and 58.2% had a Saudi nationality. 38.5% of children had mild toxicity, while 31.1% and 30.3% had moderate and severe toxicity. And most of the cases (68%) were complicated. However, 32% of cases were not complicated. For 30.3% of children, the duration since poisoning was 30 min, 26.2% was one hour, 21.3% was 2 h, 8.2% was 3–12 h, and for 13.9%, the duration was 12 h. For most children (69.7%), the poisoning happened at home.
Table 1 shows that the most common documented pharmaceutical products were Depakine, Olanzapine (5 mg), Cannabinoids, Carbamazepine, Hydrogen peroxide (6%), Methanol, Risperidone lorazepam, Propranolol, and valproate sodium (200 mg). A non-significant relationship was found between the level of toxicity and the taken pharmaceutical products (p > 0.05).
Table 2 shows that the most common category class drug used were Benzodiazepine (18%), followed by Analgesic non opioid (15.6%), senna leaves rhubarb root (7.4%), and alcohol or NSAID (4.1%). A non-significant relationship was found between the level of toxicity and major category class drugs used (p > 0.05).
Table 3 shows that the most common presenting symptoms were both GIT and neurological symptoms (23.8%), followed by only neurological symptoms (16.4%) and only GIT symptoms (13.1%). The table shows that children who were presented with (CVS, dermal and neurological symptoms) or (CVS and neurological symptoms) or (GIT, CVS, respiratory, dermal and neurological symptoms) or (GIT, CVS, dermal and neurological) or (Respiratory, dermal and neurological) had a significantly higher 100% of having severe toxicity (p < 0.05).
Table 4 shows that almost one-quarter of children had activated charcoal (25.4%), 8.2% received N-acetylcysteine, (34.4%) were intubated, and (9.8%) had repeated-dose-activated charcoal for enhancement elimination, 9% had IV fluids as pre-hospital management, and 3.3% were ventilated. Almost one-third of children were on IV fluid (27.8%), 4.1% were on Activated charcoal or N-acetylcysteine or antibiotics, 7.4% were on Flumazenil, and 1.6% were on omeprazole injection. Children who had whole bowel irrigation were intubated for oxygen therapy and had N-acetylcysteine or sedation, fluids, and phenytoin had a significantly higher % of having severe toxicity (p < 0.05).
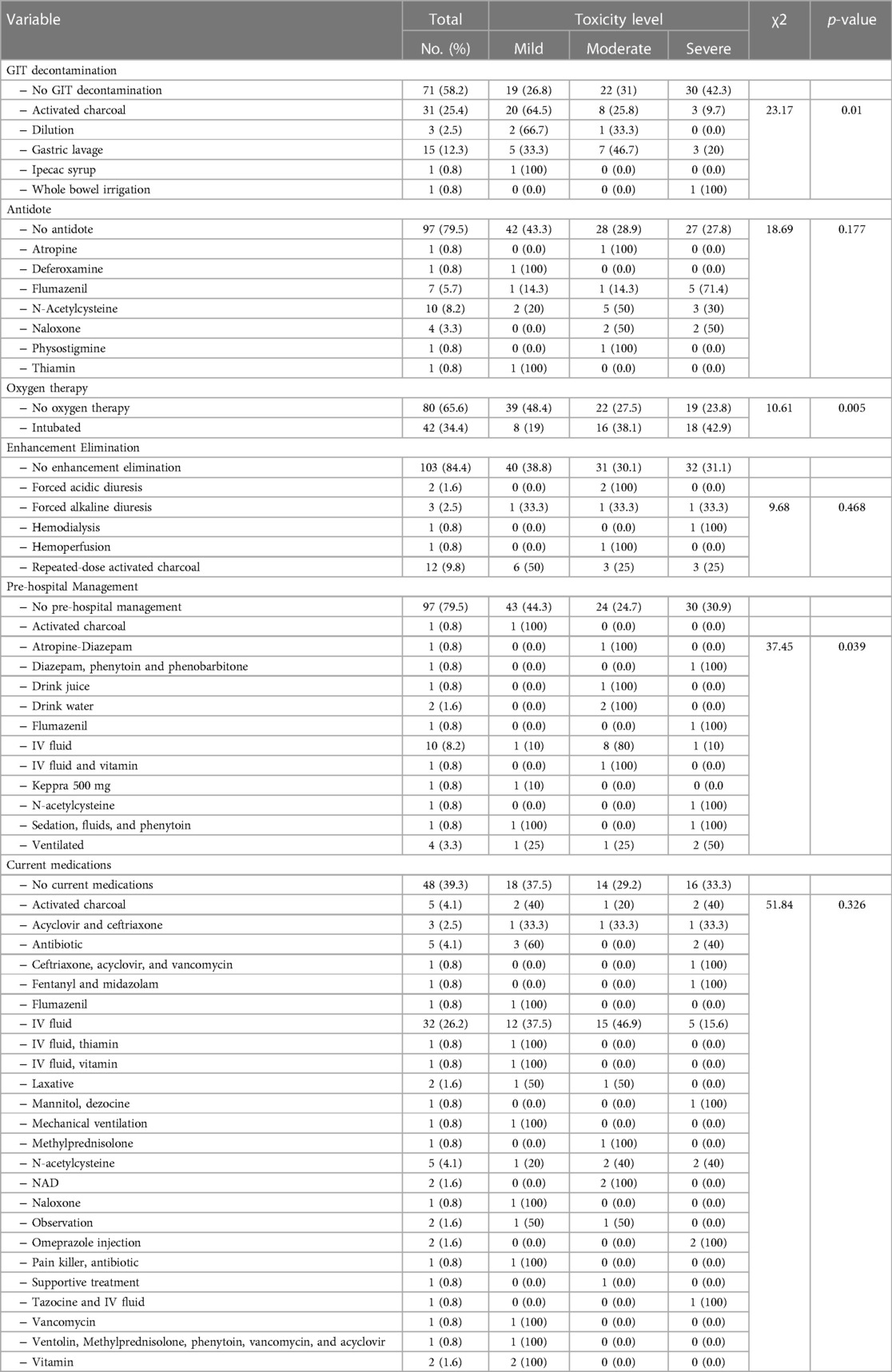
Table 4. Relationship between GIT decontamination, antidote, oxygen therapy, enhancement elimination, pre-hospital management, and current medications and level of toxicity.
Table 5 demonstrated a non-significant relationship between the level of toxicity among studied children and their age, height, weight BM, gender, nationality, pharmaceutical products, or poison forms (p > 0.05).
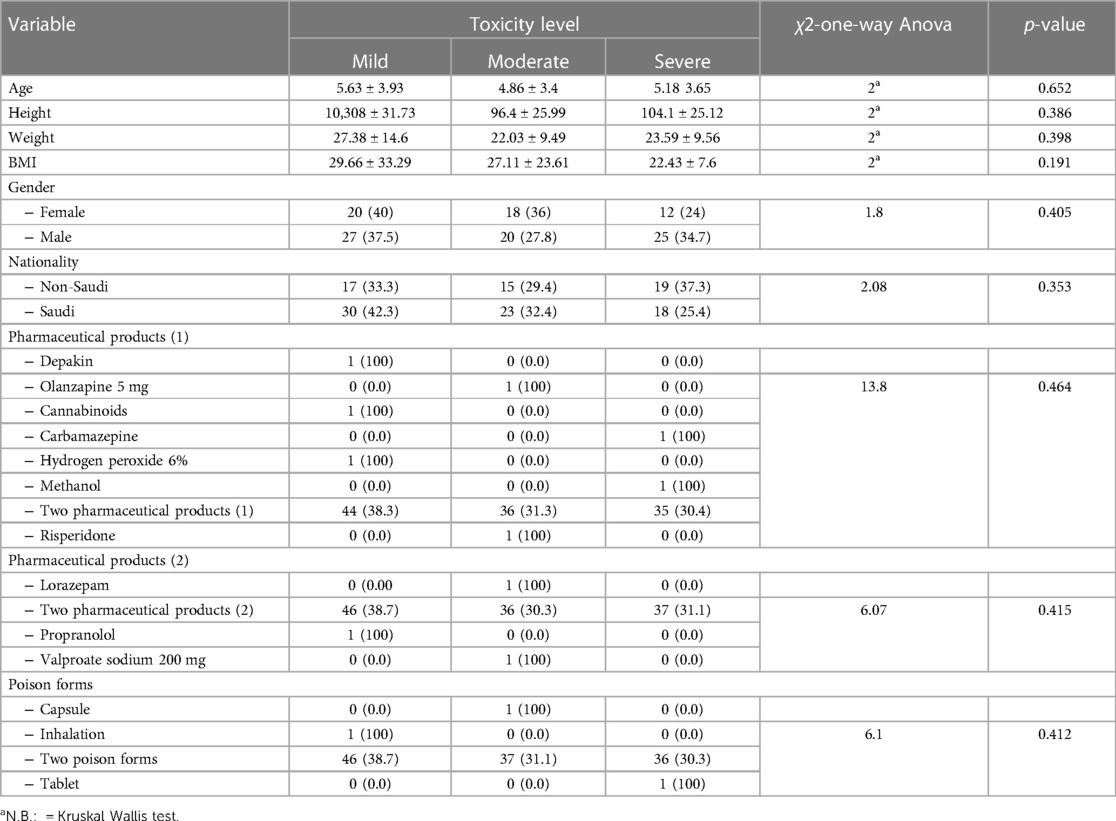
Table 5. Relationship between level of the toxicity and the children's characters, weight, BMI. pharmaceutical products and poison forms (n = 122).
Table 6 shows that the most common poison forms were tablets (42.6%), syrups (15.6%), capsules (13.9%), solutions (13.1%) poisoning by pharmaceutical products (80.3%), household products accounts (12.3%), plant envenomation (5.7%,) and by animal envenomation (1.6%) as for the route of poisoning, ingestion accounts for (82.8%), dermal route (5.7%), injection (4.9%), inhalation (6.6%). Accidental poisoning was (83%), and intentional poisoning accounts for (39%) of cases. A non-significant relationship was found between the level of toxicity and the taken pharmaceutical products, poison forms, route or mode of poisoning (p => 0.05).
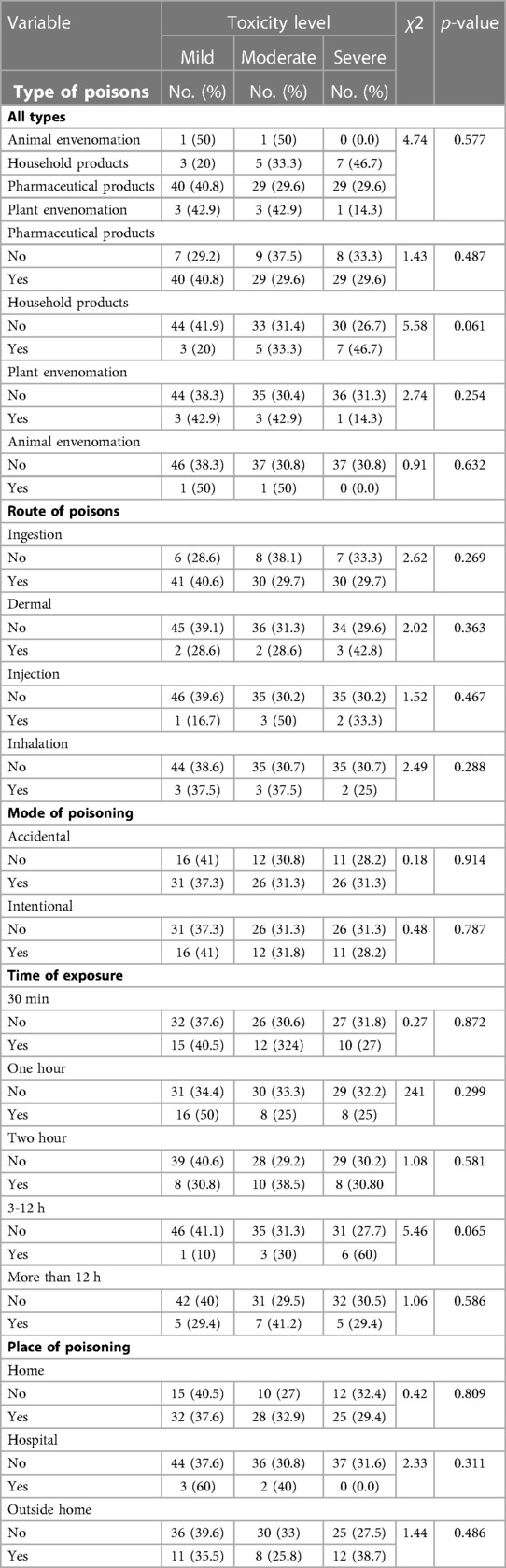
Table 6. Relationship between level of toxicity and poison forms, route and mode of poisoning, time of exposure, and place of poisoning (No.:122).
Table 7 shows that complicated cases had a significantly higher mean level of AST (IU/l) than non-complicated cases (75.5 vs. 20.08 (p < 0.05). On the other hand, a non-significant relationship was found between the presence of complicated cases and all other laboratory test results of the studied children (p > 0.05).
This study aimed to assess and analyse the patterns of acute poisoning cases with drugs, chemicals, and natural toxins for children in the Makkah region of Saudi Arabia registered in the Makkah poison control center and the Haddah forensic chemistry center.
In this study, male children accounted for 59% of the affected cases compared to 41% females. And the mean age was 5.2 ± 3.74 years. A previous Saudi study done in 2018 in Riyadh city found a prevalence of 49.7% among females, and the mean age of children was 2.7 ± 2.1 years (8).
Between 2010 and 2016, a review study conducted in the Riyadh region found that more than half of poisoning cases (62%) happened in youngsters under two (5). Another study conducted in the Jeddah region discovered that most cases occurred in male children (1), which was comparable with international studies (15, 16). Previously, the study that conducted in Sri Lanka reported that the majority of children who ingested poisons were under the age of five years (16). The WHO also reported an overall higher rate of poisoning in boys than girls in different world regions (15).
Paracetamol was shown to be the most dangerous pharmaceutical product (94%), followed by other pharmaceuticals such as Depakine, Olanzapine 5 mg, and Risperidone lorazepam. In the present study, the most common category class drug used were Benzodiazepine (18%), followed by analgesic non opioid (15.6%), senna leaves rhubarb root (7.4%), and alcohol or NSAID (4.1%). A study conducted in Jeddah in 2020 found that ingested medicines were the leading cause of acute poisoning (73.9%), a finding that was consistent with a previous study (14) in which medicinal products were the leading cause of poisoning. Previously, the most frequently involved drug class was weak analgesics dominated by paracetamol (n = 91, 35%), followed by opioids and benzodiazepines (17).
Many reports, especially from Saudi Arabia, support this finding, highlighting medicine's role in self-poisoning (5, 18). The drug administration providing a reason this to delivering medication in envelopes rather than child-resistant containers, easy access to drugs without prescriptions, and irresponsible home drug storage (19). Furthermore, Saudi Arabia's higher rating for unintended drug poisoning may be related to Saudi families' habit of storing unused prescriptions for future usage (5). The ready availability of medications and chemicals in various forms at home and a lack of parental monitoring to keep these materials in a safe place and out of reach of children were the most common causes of childhood poisoning.
In the present work, it was observed that the most common dosage forms were tablets (41.8%). According to a previous study conducted in Abha (20), tablets' most common poison types (19). The present work found that ingestion was the most common rote of poisoning (93.4%), followed by cutaneous poisoning (13.9%), inhalation (9.8%), and injection (9.8%). This result agrees with previous studies done in KSA (5, 20).
This work observed that accidental poisoning accounted for (68%) of cases compared to 31.1% for intentional poisoning. The same result was found in a previous study done in KSA (1, 8) and others (21). The study showed that for (30.3%) of children, the time of exposure was 30 min, for (26.2%) it was one hour, and for (21.3%) it was 2 h. A study done in India found that the time interval between exposure to poison and admission to the hospital was less than 3 h in 73 (35.96%) cases, 3 to 6 h in 92 (45.32%) cases, and more than 6 h in 38 (18.71%) cases (22). Another study in Iran discovered that the average time from incident to hospitalization was 144.3171 min (23). The poisoning occurred at home for the majority of the youngsters (69.7%). The same result was revealed from other studies (1, 21).
In the present study, most symptoms appear as GIT symptoms. In the study done in Majmaah, 25.6% of children had GIT symptoms (8). In Abha, the most common symptoms of poisoning were nausea, vomiting (40.4%), and (16.7%) abdominal pain (18). In an Iranian study, 28.2 percent of youngsters experienced gastrointestinal symptoms (23).
In the current study, 38.5 percent of children experienced mild toxicity, whereas 31.1% and 30.3% had moderate and severe toxicity, respectively. Furthermore, most cases (68%) were complicated. However, 32% of cases were uncomplicated. The high prevalence of mild cases was found in previous studies (1, 16, 24).
Almost one-quarter of children had activated charcoal (25.4%), (12.3%) had gastric lavage, and (2.5%) had dilution. Activated charcoal was found to limit the absorption of various toxins and medications in the stomach and intestine, including antipsychotics, antiepileptics, and salicylates (5, 25). Clinical trials have shown that multi-dose administration of activated charcoal can prevent severe intoxication from carbamazepine, quinine, phenobarbital, and theophylline (26).
In a previous study, only 6% of cases received a specific antidote, which could be attributed to the short time between poisoning and arrival at the hospital (25). Approximately 28% of children were treated by gut decontamination with activated charcoal in Riyadh, and only 1.8% were given specific antidotes (5).
The limitation of this study was having a retrospective study design and singular location. Incomplete and missing data may further affect the study's findings' generalization.
Based on the present study results, acute poisoning among children is a major public health issue in Saudi Arabia, so implementing a national policy for adequate and prompt management will result in a favorable outcome. In addition, children's exposure to harmful chemicals necessitates more attention, particularly among families, to raise their awareness of safety requirements within the home. Future studies are needed to clarify the role of various factors involved in childhood poisoning.
Due to its retrospective design and single-center setting, the study has limited general validity. The small sample size may also reduce the study's generalizability; therefore, we intend to conduct additional research involving multiple centres in different geographic regions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the medical research ethics committee at the Faculty of medicine, Umm al Qura University and the poison control center in Makkah forensic chemistry center. Written informed consent from the participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
All authors contributed to the article and approved the submitted version.
Thank the Deanship of Scientific Research, Umm Al-Qura University for supporting this work Grant Code: 22UQU4350123DSR03.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Al Hazmi AM. Patterns of accidental poisoning in children in Jeddah, Saudi Arabia. Ann Saudi Med. (1998) 18:457–9. doi: 10.5144/0256-4947.1998.457
2. Afifi RM, Zaytoun SS. Distribution Patterns of Severe Pediatric Trauma: Mandated vs. Non-Mandated Trauma Systems. Surg. Sci. (2013) 4(9):385–92. doi: 10.4236/ss.2013.49076
3. Alazab RM, Elmougy MT, Fayad RA, Abdelsalam HF, Mohamed AS. Risk factors of acute poisoning among children: a study at a poisoning unit of a university hospital in Egypt. South East Asia J Public Health. (2012) 2:41–7. doi: 10.3329/seajph.v2i2.15943
4. Branche C, Ozanne-Smith J, Oyebite K, Hyder AA. World report on child injury prevention. Geneva, Switzerland: World Health Organization, 2008 - Business & Economics - 211 (2008).
5. Alghadeer S, Alrohaimi M, Althiban A, Kalagi NA, Balkhi B, Khan AA. The patterns of children poisoning cases in community teaching hospital in Riyadh, Saudi Arabia. Saudi Pharm J. (2018) 26:93–7. doi: 10.1016/j.jsps.2017.10.007
6. Rahimzadeh MR, Rahimzadeh MR, Kazemi S, Moghadamnia AA. Cadmium toxicity and treatment: an update. Caspian J Intern Med. (2017) 8:135. doi: 10.22088/cjim.8.3.135
7. Mehrpour O, Akbari A, Jahani F, Amirabadizadeh A, Allahyari E, Mansouri B, et al. Epidemiological and clinical profiles of acute poisoning in patients admitted to the intensive care unit in eastern Iran. BMC Emerg Med. (2018) 18:1–9. doi: 10.1186/s12873-018-0181-6
8. Abd-Elhaleem ZAE, Al Muqhem BA. Pattern of acute poisoning in Al Majmaah region, Saudi Arabia. Am J Clin Exp Med. (2014) 2:79–85. doi: 10.11648/j.ajcem.20140204.15
9. Yadav S, Yadav SP, Agrawal J, Shah G. Pattern of acute poisoning in children in a tertiary care hospital in eastern Nepal. Inter J Contempor Pediatr. (2016) 3:1001–5. doi: 10.18203/2349-3291.ijcp20162380
10. Abbas SK, Tikmani SS, Siddiqui NT. Accidental poisoning in children. Mercury. (2012) 3:7.0. PMID: 22755274.
11. Saddique A. Poisoning in Saudi Arabia: ten-year experience in king khaled university hospital. Ann Saudi Med. (2001) 21:88–91. doi: 10.5144/0256-4947.2001.88
12. Alruwaili ND, Halimeh B, Al-Omar M, Alhatali B, Sabie II, Alsaqoub M. An epidemiological snapshot of toxicological exposure in children 12 years of age and younger in Riyadh. Ann Saudi Med. (2019) 39:229–35. doi: 10.5144/0256-4947.2019.229
13. Naseem A, Khurram D, Khan D, Gari S, Lalani N. Accidental poisoning its magnitude and implications in children. Int J Pediatr Res. (2016) 3:400–09. doi: 10.17511/ijpr.2016.i06.06
14. Tobaiqy M, Asiri BA, Sholan AH, Alzahrani YA, Alkatheeri AA, Mahha AM, et al. Frequency and management of drug and chemical poisoning among children attending an emergency department in a single hospital in Saudi Arabia. medRxiv. (2020) 8(4):189. doi: 10.3390/pharmacy8040189
15. Jullien S. Prevention of unintentional injuries in children under five years. BMC Pediatr. (2021) 21:1–11. doi: 10.1186/s12887-020-02457-3
16. Dayasiri M, Jayamanne S, Jayasinghe CY. Patterns and outcome of acute poisoning among children in rural Sri Lanka. BMC Pediatr. (2018) 18:1–8. doi: 10.1186/s12887-018-1246-0
17. Andersen C.U., Nielsen L.P., Møller J.M., Olesen A.E., Acute drug poisonings leading to hospitalization. Basic Clin Pharmacol Toxicol 130 (2022) 328–36. doi: 10.1111/bcpt.13688
18. Khan LA, Khan SA, AI-Hateeti HS, Bhat AR, Bhat KS, Sheikh FS. Clinical profile and outcome of poisoning in najran. Ann Saudi Med. (2003) 23:205–7. doi: 10.5144/0256-4947.2003.205
19. Al-Shehri MA. Pattern of childhood poisoning in abha city–southwestern Saudi Arabia. J Family Community Med. (2004) 11:59. PMID: 23012050.23012050
20. Kandeel F, El-Farouny R. Study of acute poisoning cases in children admitted to menoufia poison control center (MPCC) during the year (2016). Ain Shams J Forensic Med Clin Toxicol. (2017) 29:89–99. doi: 10.21608/ajfm.2017.18213
21. Fang J, Wang M, Gong S, Cui N, Xu L. Increased 28-day mortality due to fluid overload prior to continuous renal replacement in sepsis associated acute kidney injury. Ther Apher Dial. (2022) 26:288–96. doi: 10.1111/1744-9987.13727
22. bdulaziz Al-Sekait M. Accidental poisoning of children in Riyadh, Saudi Arabia. J R Soc Health. (1989) 109:204–5. doi: 10.1177/146642408910900609
23. Shirkosh S, Esmaeilidooki M, Nakhjavani N, Hadipour A, Osia S, Hajiahmadi M. Epidemiological and clinical pattern of acute poisoning in children: a hospital based study in northern Iran. Caspian J Pediatr. (2019) 5:334–41. doi: 10.22088/CJP.BUMS.5.1.334
24. Elshoura AIA, Sherif MM, Noor El-Deen TM, Ali MA, Abbod MA, Ghanem MA. Assessment of acute poisoning among children in damietta governorate. Al-Azhar Med J. (2016) 45:631–44. doi: 10.12816/0033129
25. Zhu Y, Q WU. The current situation of acute poisoning in children. Chin Pediatr Emerg Med. (2018) 12:81–3. doi: 10.3760/cmajissn1673-4912.2018.02.001
Keywords: patterns, acute, poisoning, children, outbreak, Corona virus, makkah
Citation: Althobaiti BM, El-Readi MZ, Althubiti M, Alhindi YZ, Alzahrani Abdullah R, Al-Ghamdi Saeed S, Ayoub N, Refaat B and Eid SY (2023) Patterns of acute poisoning for children during outbreak of Corona virus in Makkah region Saudi Arabia. Front. Pediatr. 11:1087095. doi: 10.3389/fped.2023.1087095
Received: 1 November 2022; Accepted: 24 February 2023;
Published: 17 March 2023.
Edited by:
Katia Candido Carvalho, University of São Paulo, BrazilReviewed by:
Srinivas Dannaram, Banner Health, United States© 2023 Althobaiti, El-Readi, Althubiti, Alhindi, Alzahrani, Al-Ghamdi, Ayoub, Refaat and Eid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahmoud Zaki El-Readi bXpyZWFkaUB1cXUuZWR1LnNh Safaa Yehia Eid c3llaWRAdXF1LmVkdS5zYQ==
Specialty Section: This article was submitted to Obstetric and Pediatric Pharmacology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.