- Department of Hematology and Immunology, Faculty of Medicine Umm Al-Qura University, Makkah, Saudi Arabia
Introduction: Breast milk is rich in nutrients and immunological factors capable of protecting infants against various immunological diseases and disorders. The current systematic review has been framed with the objective of studying the role of breastfeeding as a protective factor against the development of immune-mediated diseases.
Methods: The database and website searches were performed using PubMed, PubMed Central, Nature, Springer, Nature, Web of Science, and Elsevier. The studies were scrutinized based on the nature of participants and the nature of disease considered. The search was restricted to infants with immune-mediated diseases such as diabetes mellitus, allergic conditions, diarrhoea, and rheumatoid arthritis.
Results: We have included 28 studies, out of which seven deal with diabetes mellitus, two rheumatoid arthritis, five studies about Celiac Disease, twelve studies about allergic/ asthma/wheezing conditions and one study on each of the following diseases: neonatal lupus erythematosus and colitis.
Discussion: Based on our analysis, breastfeeding in association with the considered diseases was found to be positive. Breastfeeding is involved as protective factor against various diseases. The role of breastfeeding in the prevention of diabetes mellitus has been found to be significantly higher than for other diseases.
Introduction
During the first few years of a person's life, the immune system may be readily reshaped, which is important for achieving full protection against infections and the ability to tolerate non-harmful environmental substances to an adequate degree (1). Breastfeeding is geared to the needs of the newborn, and it may compensate for the relative inadequacy of the host defence by delivering substantial quantities of both nonspecific and pathogen-specific secretory IgA (sIgA) (2). Breastfeeding is adapted to the requirements of the infant (1). These antibodies, which are generated as a result of the earlier exposure to infectious agents by the mother, are capable of binding to potentially dangerous pathogens and rendering them inactive (1). Breast milk includes various additional nonspecific components that have antimicrobial properties or give protection to the newborn via different channels (3). These substances are present in addition to the antibodies that are present in breast milk (3). It's possible that the immunological, hormonal, enzymatic, trophic, and/or bioactive substances that are found in breast milk might provide some degree of passive protection (4). Other components, including as macrophages and leukocytes, which are predominantly present at the start of breastfeeding, may have a stronger modulatory influence on the immune system of the neonate and give further protection (5).
Breastfeeding has been regarded as the major protective factor in the lives of infants. The primary milk produced by the mothers is referred to as colostrum, which is found to be rich in immunologically active molecules and various nutrients and vitamins that are absolutely necessary for the growth of the infants (6). Infant's breastfed during their early life have developed immunity against various diseases considerably (7). The infants provided with breastfeeding have also been found to be devoid of malnutrition conditions (8). According to the World Health Organization, breastfeeding helps children attain the necessary nutrients for the first year of their lives (9). Breastfeeding for the initial six months period of life plays an important role in helping the infants to attain optimal growth during their childhood (10).
Breastfeeding aids nutritional benefits and illness protection not only to the infants, but also to the lactating mothers (11). The lactating mothers involved in breastfeeding for longer period are being protected from pregnancy obesity and the risk for cancers in breast and ovaries are observed to be reduced (12). The risk of brittleness in bones leading to osteoporosis was also reported to be lower in mothers who breastfed (3–6 months) their children (13). The risk for immune system mediated diseases and disorders may be decreased by breast milk and breastfed infants, since the breast milk is rich in immunoglobulins that are specific to allergens (14). Thus, we concentrated on reviewing research, especially those including infants with of immune-mediated diseases.
Materials and methods
Study design
The database search was carried out by the reviewer on various publication sites such as PubMed, PubMed Central, Nature, Springer, Nature, Web of Science, and Elsevier. The keywords for searching the studies are: breastfeeding, breast milk, human milk, immunity, diabetes, diabetes mellitus, rheumatoid arthritis, diarrhoea, hypersensitivity, allergens, allergic reactions, erythematosus, colitis, hypoglycemia, hyperglycemia, infantile diabetes and protective factor. The duplicate and irrelevant articles were removed, and the data screening was done.
Inclusion criteria
Only research articles relevant to the current study have been selected. The original research articles, including the in vivo studies, were majorly focused, and the studies involving human participants were given higher priority. The recent studies involving human participants with immune-related diseases diagnosis were considered, along with the in vivo studies involving the management techniques for immune-related clinical conditions.
Review articles, systematic reviews, and meta-analysis reviews were excluded from the study. The research articles that did not deal with the considered clinical conditions as well as the studies involving in vitro analysis were excluded from the study. The articles in which breastfeeding was not associated with the considered immune-related conditions were excluded.
The articles selected on the basis of inclusion and exclusion criteria have been screened manually by the authors for the inter-relationship between breastfeeding and respective clinical conditions. The articles that met the eligible criteria were selected, and data extraction was carried out.
Data extraction
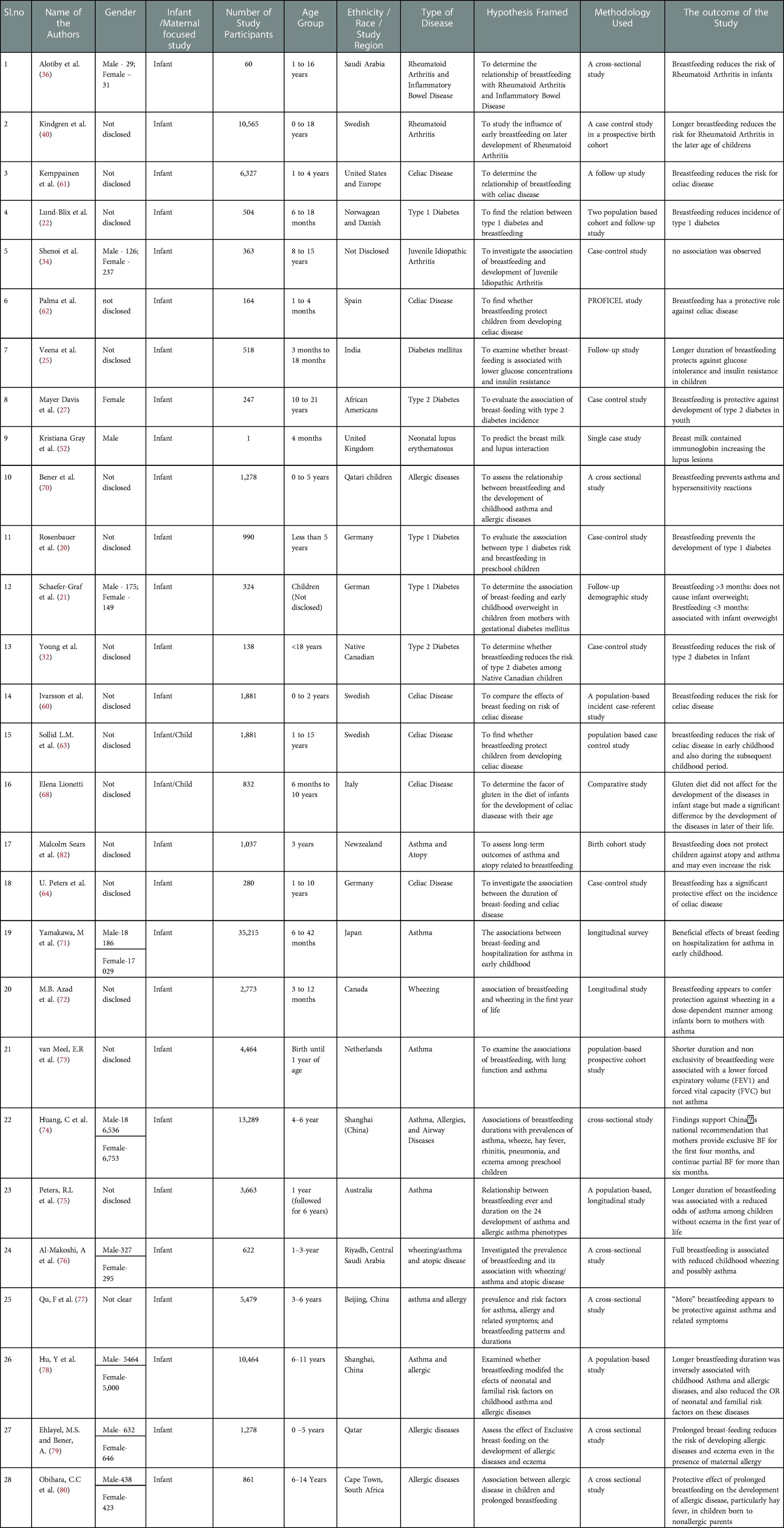
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines Liberati et al. (15) were followed for the data extraction procedure. The details of the eligible articles were extracted from the template obtained from the PRISMA website. The details of the included articles contain: (1) year of publication; (2) number of participants; (3) gender of the included participants; (4) age of the participants; (5) race, ethnicity or religion of the participants; (6) immunological disease considered for the analysis; (7) hypothesis framed for the study; (8) methodology used to test the framed hypothesis; and (9) results obtained from the study. A total of 28 articles were considered and presented in the current review.
Results
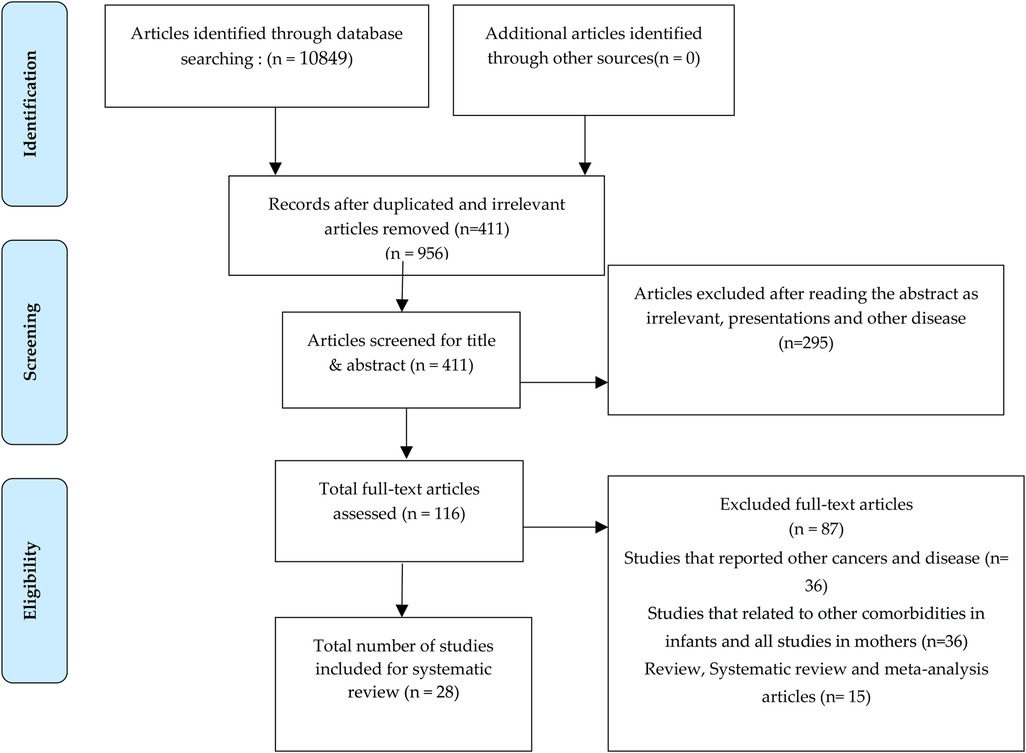
Initial screening of articles included around 10,849 articles from the previously mentioned databases and web-sites, from which 411 articles were considered after removing the duplicated and irrelevant articles. Following that, 295 articles were excluded since the presentation of results was found to be irrelevant after reading, and finally 116 articles were fully screened for the current study. After the exclusion of articles based on inclusion and exclusion criteria, 28 research articles were fully analyzed and considered for the review. After screening the abstracts of 116 full-text articles, 87 articles were removed, and 28 articles were found to be in coherence with the current study (Figure 1).
The 28 articles presented in the current review comprise seven studies on “diabetes mellitus with breastfeeding”, two studies about “rheumatoid arthritis and breastfeeding”, five studies about “ role of breastfeeding in celiac disease, twelve studies about allergic/asthma/wheezing conditions and one study on each of the following diseases in association with breastfeeding: neonatal lupus erythematosus and colitis, and. The characteristics of the 28 articles included in the systematic review are summarized in an Appendix in Table A1.
Breastfeeding and diabetes (type 1 and 2)
Type 1 diabetes is one of the auto-immune diseases that may affect individuals in their early life. It is caused by the autoreactive T cells that ultimately terminate the function of the pancreas's beta cells to produce sufficient insulin. However, its pathological condition may manifest from ten to fourteen years. Nevertheless, its clinical symptoms may occur as early as nine years or as late as 90 years of life (16). Type 2 diabetes is exclusively a metabolic disorder which is meeting the characteristic of type 1 diabetes leads to high blood sugar levels (17). However, the pathogenesis of type 2 diabetes is also documented as an autoimmune disease based on the presence of autoantibodies against beta cells of the pancreas in the blood of people with type 2 diabetes (18, 19).
We have identified six research articles (20) involving the relationship between breastfeeding and diabetes conditions. Three of the studies were reported with type 1 diabetes and rest three studies with type 2 diabetes.
Type 1 diabetes: Two studies were undertaken in Germany, with sample sizes of 990 Rosenbauer et al. (20) and 324 Schaefer-Graf et al. (21). The research that was carried out by Lund-Blix et al. (22) consisted of two population-based cohorts of children who were tracked from the time of their birth (1996–2009) until the year 2014 (in Denmark) or 2015 (Norway), provides evidence in support of the claim that breastfeeding lowers the chance of developing type 1 diabetes.
Type 2 diabetes: Children and adults who received their nutrition from their mothers' breasts rather than from bottles and who were breastfed for longer periods of time throughout infancy had lower rates of type 2 diabetes and lower insulin resistance than those who received their nutrition from bottles (23, 24). It has been hypothesised that variations in the nutritious content of the milk, patterns of baby weight growth, or acquired eating behaviour between infants who are breastfed and babies who are bottle-fed are related to an increased risk of developing diabetes in later life (23). There have only been a few studies that look at diabetes risk in connection to the age at which supplemental meals are introduced to infants (25–27). Longer breast-feeding duration was related with lower fasting insulin concentrations and insulin resistance at 5 years, but not at 9.5 years, according to a research done by S. R. Veena et al. (25). There was no significant relationship found between the age at which a person began eating complementary foods and their glucose or insulin levels. The increased breastfeeding period has been found to be positively associated with the prevention and low risk of type 2 diabetes in lactating mothers Stuebe et al. (26) and children's (27) in the United States population. Similarly, the breastfeeding has also been observed to decrease the risk for type 1 diabetes in females who fed for longer period (22). The susceptibility and possibility of acquiring type 2 diabetes is found to be directly proportional to the period of lactation and breastfeeding in females (28). The presence of a diabetic condition in a breastfeeding female has no effect on the health status of the infants, such as obesity nature or diabetic occurrence (29, 30). Breastfeeding has been reported to be one of the environmental factors that is responsible for children being overweight inversely (30). Reduced breastfeeding has been associated with increased child obesity and type 1 diabetes incidence (31). The risk and occurrence of type 2 diabetes in women may be reduced by suggesting breastfeeding (32). Therefore, breastfeeding plays an important role in protecting infants as well as mothers from the risk of type 1 and type 2 diabetes.
Rheumatoid arthritis and breastfeeding
Rheumatoid arthritis is a chronic and systemic inflammatory illness that causes irreparable damage to cartilage and bones (33). This damage is caused by inflammation in the synovium of the joints revealed that insulin resistance, a significant contributor to the development of diabetes mellitus, is quite common in people with rheumatoid arthritis (33). In genetically sensitive hosts, environmental stressors may trigger Juvenile idiopathic arthritis (JIA). Shenoi et al. (34) found no link between early infection, prenatal factors, or stressful events. Unfortunately, it has been found that the number of studies that link rheumatoid arthritis and breast milk is significantly low, and the studies that report an association between the two have failed to identify the proper underlying aetiology of rheumatoid arthritis in association with breast milk or breastfeeding in infants (35).
Two studies that show a lower risk of rheumatoid arthritis in children who are breastfed have been taken into consideration in the present systematic review (36, 40). Alotiby, A et al. (36) conducted research that shown the relevance of breast milk to neonates in decreasing the risk of Rheumatoid Arthritis (RA) when compared to formula milk consumption. They investigated the differences in the beginning of the disorder in children who were nursed, children who were not breastfed, and children who were given both breast milk and formula (mixed-fed children). Breastfed children (28.3%), formula-fed children (21.7%), and mixed-fed children (50.0%) were the most common. This difference in feeding method was statistically significant. Formula feeding markedly increased the incidence of RAin children. Hence, exclusive breastfeeding may reduce the risk of RA (36).
The immunological memory of the mother is passed on to her child via breast milk, and breast milk includes a range of immune-modulating chemicals, including immune cells and their products such as cytokines (37). Breast milk also allows the mother's immune memory to be passed on to her child. Immunological imprinting and programming of the newborn may be accomplished via breastfeeding (37). Therefore, breastfeeding makes a contribution to the development of the immune system of the newborn (38, 39). According to the findings of Kindgren, E. et al. (40), an in-creased risk of juvenile idiopathic arthritis was related with a shorter overall period of breastfeeding as well as a shorter duration of exclusive breastfeeding.
There was an association found between the early introduction of formula (before the age of 4 months) and an elevated incidence of JIA. When potentially confounding factors were taken into account in the model, none of the correlations lacked their statistical significance (40). Breastfeeding may provide some protection against the development of juvenile idiopathic arthritis, according to one finding (41). According to the findings of another re-search, infants who subsequently developed oligoarticular JIA tended to have shorter nursing durations (42). It is recommended that mothers be encouraged to nurse their newborns exclusively for the first four months Kindgren et al. (40), if at all feasible, and then to maintain partial nursing for a prolonged period of time after the introduction of foreign proteins through food.
Prevention of infantile diarrhea by breastfeeding
It has been shown that beginning breastfeeding as soon as possible and continuing it exclusively protects new-born babies against death due to diarrhea (43). A self-limiting characteristic of the human body that is usually caused by gastroenteritis is termed “diarrhoea.” It is characterized by having loose stools abnormally frequently in a single day (44). The major causes of diarrhoea include dietary habits causing food poisoning or allergies as well as certain medications. We have identified three studies involving the analysis of breastfed infants and their susceptibility to diarrhoea.
We have summarised a study conducted on Qatari children in the year 2009 by Ehlayel et al. (45). The study was targeted at 1,500 mothers and their infants and children aged 1 to 5 years and the response rate was agreed with 1,278 participants. The breastfeeding of the children varied significantly (p < 0.001) from 11.4 ± 6.7 months (longer) to 9.2 ± 4.1 months (shorter). In this study, around 11.4% higher risk and susceptibility were observed in the children who received shorter breastfeeding periods, indicating the protective role of breastfeeding against infantile diarrhoea (45).
The other two studies included 93 mother and infant pairs in the Mexican population in the years 2004 and (46, 47). The mean age of the infants was 6 months. The oligosaccharide content present in breast milk influences the diarrhoea in infants and children. The oligosaccharide contents in breast milk were proved to influence the diarrhea in infants (48). The breast milk contains fucosyl oligosaccharides as its major component, and the fucosyl oligosaccharides have a role in controlling diarrhoea in infants in a positive manner via innate immune response (47).
The effects of ceasing breastfeeding in the early period and the influence of termination on diarrhoea in infants are adverse. The early termination of breastfeeding increases the risk of infantile diarrhoea (49). Reduced breast-feeding in infants has been positively influenced by the mortality of children along with diarrhoea and other clinical conditions (50).
Breastfeeding and neonatal lupus erythematosus
A clinical condition caused in infants due to the presence of autoantibodies in lactating mothers is neonatal lupus erythematosus. It is a rare autoimmune disorder (51). Due to the limited number of studies linking breast-feeding and neonatal lupus erythematosus in infants, we have identified one study involving a male infant of 4–6 months. Intense immunoglobulin levels of IgG and IgA were identified in the breast milk, which induced an erythematosus condition in the infant. The antibodies of the lactating mother were found to induce autoimmunity in the infants, and the lesions in neonatal lupus erythematosus conditions were adverse with an increase in breast-feeding (52). They examined the mother's breast milk from an immunological standpoint for their research. Anti-bodies with significant positive IgG and IgA reactivity against nuclear and Ro targets were found in the mother's breast milk, which was a surprise. After this, the doctor recommended stopping nursing, and three weeks later, the lesions disappeared. Since then, the child has been healthy and has not had any diseases. It is possible to draw the conclusion from this that the illness known as neonatal lupus erythematosus is caused by a passive transfer through the placenta of maternal autoantibodies Vanoni et al. (53), the majority of which are directed against the Ro antigen.
Breastfeeding against colitis
Colitis is a clinical condition in which the large intestine is inflamed. One study has been identified and presented that represents the role of breastfeeding in colitis disease. No studies involving human participants were eligible for the current systematic review, and hence, an in vivo study involving interleukin-10 (IL-10) deficient mice is being considered. The duration of breastfeeding as well as breast milk has an impact on the development and progression of colitis inversely (54). Inflammatory bowel disease, a gastrointestinal inflammatory condition that includes Crohn's disease and colitis, is being reduced in infants who were breastfed for a longer period when com-pared to children who had breastfeeding for a shorter period (55).
Breast feeding and celiac disease
The impact of childhood infections on the development of celiac disease is debatable. Although frequent infections during the first 18 months of life have been linked to an increased risk of celiac disease later in life (56–58), acute infections at the time of gluten introduction have no effect on disease risk in the general population (59). Coeliac disease is multifactorial, resulting from genetic and environmental factors (59). HLA and non-HLA genes are involved, and gluten is a key environmental factor because the disease remits when gluten is eliminated. The important case-control study by Ivarsson et al. 2002 concludes that breast milk protects under-2-year-olds from coeliac disease (60).Different studies such as case control, follow-up studies, comparative studies showed a significant correlation between breast feeding and coeliac disease (60–63). Breastfeeding (62, 64) and later gluten introduction (61, 63) reduced celiac disease incidence. Different populations had delayed celiac disease onset (65–67). Elena Lionetti (68) reported the administration of gluten in the early of life was linked to a development of illness in the later stage of life.
Role of breastfeeding in hypersensitivity and allergic conditions
An abnormal or altered immunological reaction that is in response to the untimely response of the immune system is termed hypersensitivity (69). The hypersensitivity or allergic reactions is majorly targeted towards harmless foreign substances resulting in damage of tissues (69). One study regarding hypersensitive and allergic reactions has been included in this current review, which includes the screening of 1,278 lactating Qatari mothers and their infants and children (70). The mean age of the participating mothers was 32.5 years, and the children were 2.5 years old. The period of the study was from around the years 2006 to 2007. More than 59% of infants were exclusively breastfed, 28% were partially breastfed, and the remaining infants were not breastfed. The study report revealed a significant variation (p < 0.01) in the occurrence of allergic reactions (70). Allergy and hypersensitive reactions are prevented in infants receiving breast milk, indicating the protective nature of breast milk against hypersensitivity and allergic reactions (71–80).
A respiratory condition called asthma is being triggered by the immune cells as the result of allergic response to certain environmental factors (70). The breastfeeding influences the risk of developing asthma (81). According to Malcolm Sears et al. (82), nursing does not prevent children from atopy or asthma and may potentially increase the risk. Breast milk has been shown to transport food molecules intact from the maternal body to the infants (81). A study has reported hypersensitive allergic reactions towards fish by infants due to increased dietary fish intake by the mother (83). Similar allergic reactions have also been observed in infants in response to egg intake as well as peanut intake by lactating and breastfeeding mothers (84).
Discussion
This structured and systematic review on breastfeeding as a protective role against the development of auto-immune diseases identified around 20 relevant and appropriate articles related to breastfeeding and autoimmune diseases. Most of the considered investigations were cohort and follow-up studies on lactating mothers and breast-feeding infants. Many research articles were published in accordance with breastfeeding and its protective role against immune-mediated diseases. The objectives of interest were breastfeeding, ingestion of breast milk, and immune-related diseases in infants. It has been reported that the breast milk of diabetic mothers fed to their infants for a longer period as well as in larger volumes induces childhood obesity (85). But, in this review, we have suggested and provided evidence that childhood overweight as well as childhood obesity is prevented by longer breastfeeding than partial breastfeeding. The authors would also like to add the fact that the glucose content in breast milk of diabetic and non-diabetic mothers is similar (86).
Few studies have been reported in favour of breastfeeding as a protective factor against rheumatoid arthritis, which is currently being discussed in this review. Children's exposure to breastfeeding for less than four months increases the risk of rheumatoid arthritis during their childhood stage (87). Middle-aged and elderly women who had been breastfeeding their children for an extended period were less prone to rheumatoid arthritis, and breast-feeding is observed to be positively associated with the prevention of rheumatoid arthritis in infants during their childhood and in mothers at their elderly age (35). The relationship between breastfeeding and rheumatoid arthritis have concluded that the breastfeeding eventually reduces the susceptibility and risk of rheumatoid arthritis irrespective of feeding period (88).
A skin condition called eczema has been reported as one of the allergic conditions prevalent in early childhood as well as in infants and has been associated with the food intake of the lactating mother (89). Asthma and other respiratory infections in the early stages of a child's life have been found to be associated with breastfeeding inversely, i.e., the longer the breastfeeding, the lower the risk of respiratory infections (90, 91). An allergic reaction in the nose resulting in rhinitis has been reported to be reduced in breastfed children Bloch et al. (92). A hypothetical suggestion is to be provided in the case of allergic and hypersensitivity reactions to breastfeeding, since breastfeeding may induce hypersensitive reactions in some cases as well as protection in a few cases, as reported in this review.
A few studies have reported that the diabetic condition, accompanied by a gluten intolerance clinical condition, celiac disease, is being influenced by breastfeeding (93). The risk for developing autoimmune nature in the infants increases when the microbial infections are diagnosed before 9th month of their life (94). Here, the authors present a contrasting review that shows autoimmune conditions are prevented and protected in children who were breastfed for more than 4 months. Breastfeeding favours the immune system of infants to produce necessary immunity (95). Infants fed with breast milk are being observed to attain support for their immune systems that has yet to be matured. The mechanism involves the components of breast milk like anti-inflammatory cytokines, which is an immune-modulating compound (96).
At birth, a newborn infant is immediately exposed to a vast array of microbes from the environment, but primarily from the mother (97). However, breast milk then “feeds” the gastrointestinal tract both directly (maternal milk microbes) and indirectly (through the birth process) (97). In breastfed infants, the microbiome predominantly consists of Bifidobacterium (B breve, B longum, and B bifidum) (98). In contrast, the microbiome of formula-fed infants is more diverse, with the increased relative abundance of Bacteroidetes and Firmicutes, and with increased Clostridium difficile (98). In contrast, the microbiome of formula-fed infants is more diverse, with an increased relative abundance of Bacteroidetes and Firmicutes (99). There is a complex relationship between breast milk and the infant's microbiome (98). This relationship involves the transfer of immunoglobulins, bacteria, viruses, and bacteriophages (viruses that parasitize a bacterium by infecting it and reproducing inside it) from the mother to the infant through the mother's only milk. The microbiome begins to resemble that of an adult by the third year of life, and the sequential acquisition of gut microbes early in life has a long-lasting effect on gut health. This occurs as the micro-biome gradually transforms to match that of an adult. A disruption in the establishment of this microbiome has been linked to increased risks of obesity, diabetes, and mental health disorders, as well as immune-mediated and inflammatory conditions such as inflammatory bowel disease and atrophy. Breastfeeding an infant is related to a lower risk of diarrhoeal illness than formula-feeding an infant. The variations in the microbiota of breastfed and formula-fed infants continue to exist beyond six months (100, 101). The current review also reported a similar suggestion that breastfeeding favours the maturation of the immune system in infants.
Conclusions
Based on all the literature surveyed for the current systematic review, we conclude that breastfeeding infants anonymously helps them to build a mature, strong, and healthy immune system against immunological conditions. The breastfeeding helps the infants to protect against certain acquired immunological conditions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
Amna Alotiby is a solo author for this paper. All authors contributed to the article and approved the submitted version.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc R Soc B Biol Sci. (2015) 282(1821):20143085. doi: 10.1098/rspb.2014.3085
2. Gianni ML, Bettinelli ME, Manfra P, Sorrentino G, Bezze E, Plevani L, et al. Breastfeeding difficulties and risk for early breastfeeding cessation. Nutrients. (2019) 11(10):2266.doi: 10.3390/nu11102266
3. Ballard O, Morrow AL. Human milk composition. Pediatr Clin North Am. (2013) 60(1):49–74. doi: 10.1016/j.pcl.2012.10.002
4. Hamosh M. Bioactive factors in human milk. Pediatr Clin North Am. (2001) 48(1):69–86. doi: 10.1016/S0031-3955(05)70286-8
5. Xanthou M. Human milk cells. Acta Paediatr. (1997) 86(12):1288–90. doi: 10.1111/j.1651-2227.1997.tb14899.x
6. Donovan SM. The role of lactoferrin in gastrointestinal and immune development and function: a preclinical perspective. J Pediatr. (2016) 173:S16–28. doi: 10.1016/j.jpeds.2016.02.072
7. Cacho NT, Lawrence RM. Innate immunity and breast milk. Front Immunol. (2017) 8:584. doi: 10.3389/fimmu.2017.00584
8. Jarpa MC, Cerda LJ, Terrazas MC, Cano CC. Lactancia materna como factor protector de sobrepeso y obesidad en preescolares. Rev Chil Pediatría. (2015) 86(1):32–7. doi: 10.1016/j.rchipe.2015.04.006
9. World health organisation. “Breastfeeding,” 2022. https://www.who.int/health-topics/breastfeeding#tab=tab_1
10. World Health Organization. “World Breastfeeding Week 2022: Step up for Breastfeeding,” 2022. https://www.paho.org/en/campaigns/world-breastfeeding-week-2022-step-breastfeeding#:∼:text=1-7 AUGUST 2022, an important breastfeeding promotion strategy.
11. Kuhn L, Aldrovandi G. Survival and health benefits of breastfeeding versus artificial feeding in infants of HIV-infected women: developing versus developed world. Clin Perinatol. (2010) 37(4):843–62. doi: 10.1016/j.clp.2010.08.011
12. Anstey EH, Shoemaker ML, Barrera CM, O’Neil ME, Verma AB, Holman DM. Breastfeeding and breast cancer risk reduction: implications for black mothers. Am J Prev Med. (2017) 53(3):S40–6. doi: 10.1016/j.amepre.2017.04.024
13. Mukherjee A, Parashar R. Longitudinal trends in the health outcomes among children of the North Eastern States of India: a comparative analysis using national DHS data from 2006 to 2020. Eur J Clin Nutr. (2022) 76(11):1528–35. Available at: https://www.nature.com/articles/s41430-022-01147-w35444272
14. Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol. (2015) 15(5):308–22. Available at: https://www.nature.com/articles/nri3830 doi: 10.1038/nri3830
15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006
16. Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. (2010) 39(3):481–97. doi: 10.1016/j.ecl.2010.05.011
17. Wentworth JM, Fourlanos S, Harrison LC. Reappraising the stereotypes of diabetes in the modern diabetogenic environment. Nat Rev Endocrinol. (2009) 5(9):483–9. Available at: https://www.nature.com/articles/nrendo.2009.149 doi: 10.1038/nrendo.2009.149
18. Sladek R. The many faces of diabetes: addressing heterogeneity of a complex disease. Lancet Diabetes Endocrinol. (2018) 6(5):348–9. doi: 10.1016/S2213-8587(18)30070-6
19. de Candia P, Prattichizzo F, Garavelli S, De Rosa V, Galgani M, Di Rella F, et al. Type 2 diabetes: how much of an autoimmune disease? Front Endocrinol (Lausanne). (2019) 10:451. doi: 10.3389/fendo.2019.00451
20. Rosenbauer J, Herzig P, Giani G. Early infant feeding and risk of type 1 diabetes mellitusa nationwide population-based case control study in pre-school children. Diabetes Metab Res Rev. (2008) 24(3):211–22. doi: 10.1002/dmrr.791
21. Schaefer-Graf UM, Pawliczak J, Passow D, Hartmann R, Rossi R, Bührer C, et al. Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care. (2005) 28(7):1745–50. doi: 10.2337/diacare.28.7.1745
22. Lund-Blix NA, Dydensborg Sander S, Størdal K, Nybo Andersen AM, Rønningen KS, Joner G, et al. Infant feeding and risk of type 1 diabetes in two large scandinavian birth cohorts. Diabetes Care. (2017) 40(7):920–7. doi: 10.2337/dc17-0016
23. Horta B, Bahl R, Martines J, Victora C. Evidence on the long-term effects of breastfeeding: systematic reviews and meta-analyses. World Heal Organ. (2007) 3:1–52. Available at: https://www.dors.it/latte/docum/Breastfeeding and Maternal and Infant Health- review.pdf
24. Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. (2006) 84(5):1043–54. doi: 10.1093/ajcn/84.5.1043
25. Veena SR, Krishnaveni GV, Wills AK, Hill JC, Karat SC, Fall CHD. Glucose tolerance and insulin resistance in Indian children: relationship to infant feeding pattern. Diabetologia. (2011) 54(10):2533–7. doi: 10.1007/s00125-011-2254-x
26. Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. (2005) 294(20):2601–10. doi: 10.1001/jama.294.20.2601
27. Mayer-Davis EJ, Dabelea D, Lamichhane AP, D'Agostino RB Jr, Liese AD, Thomas J, et al. Breast-feeding and type 2 diabetes in the youth of three ethnic groups: the SEARCh for diabetes in youth case-control study. Diabetes Care. (2008) 31(3):470–5. Available at: https://diabetesjournals.org/care/article-abstract/31/3/470/26076 doi: 10.2337/dc07-1321
28. Schwarz EB, Brown JS, Creasman JM, Stuebe A, McClure CK, Van Den Eeden SK, et al. Lactation and maternal risk of type 2 diabetes: a population-based study. Am J Med. (2010) 123(9):863.e1–6. doi: 10.1016/j.amjmed.2010.03.016
29. Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care. (2006) 29(10):2231–7. Available at: https://diabetesjournals.org/care/article-abstract/29/10/2231/23523 doi: 10.2337/dc06-0974
30. Schaefer-Graf UM, Hartmann R, Pawliczak J, Passow D, Abou-Dakn M, Vetter K, et al. Association of breast-feeding and early childhood overweight in children from mothers with gestational diabetes mellitus. Diabetes Care. (2006) 29(5):1105–7. doi: 10.2337/diacare.2951105
31. Xie L, Huang Z, Li H, Liu X, Zheng S, Su W. IL-38: a new player in inflammatory autoimmune disorders. Biomolecules. (2019) 9(8):345. doi: 10.3390/biom9080345
32. Young TK, Martens PJ, Taback SP, Sellers EA, Dean HJ, Cheang M, et al. Type 2 diabetes mellitus in children: prenatal and early infancy risk factors among native Canadians. Arch Pediatr Adolesc Med. (2002) 156(7):651–5. doi: 10.1001/archpedi.156.7.651
33. Alwarith J, Kahleova H, Rembert E, Yonas W, Dort S, Calcagno M, et al. Nutrition interventions in rheumatoid arthritis: the potential use of plant-based diets. A review. Front Nutr. (2018) 6:141. doi: 10.3389/fnut.2019.00141
34. Shenoi S, Shaffer ML, Wallace CA. Environmental risk factors and early-life exposures in juvenile idiopathic arthritis: a case-control study. Arthritis Care Res (Hoboken). (2016) 68(8):1186–94. doi: 10.1002/acr.22806
35. Pikwer M., Bergström U., Nilsson J.-Å., Jacobsson L., Berglund G., Turesson C., “Breast feeding, but not use of oral contraceptives, is associated with a reduced risk of rheumatoid arthritis,” Ann RHeum Dis., 68, 4, pp. 526–30, 2009, doi: 10.1136/ard.2007.084707
36. Alotiby A, Bagadood R, Bazuhayr R, Shabanah L, Qurbi N. The relationship between breastfeeding and autoimmune diseases among children in makkah city. India: Health Sciences (2021). 107–13. Available at: https://www.ijmrhs.com/medical-research/the-relationship-between-breastfeeding-and-autoimmune-diseases-among-children-in-makkah-city-74378.html
37. Lemke H, Lange H. Is there a maternally induced immunological imprinting phase ala Konrad Lorenz. Scand J Immunol. (1999) 50:348–54. Available at: http://labs.icb.ufmg.br/lbcd/pages2/bernardo/artigos/Lemke.pdf doi: 10.1046/j.1365-3083.1999.00620.x
38. M’Rabet L, Vos AP, Boehm G, Garssen J. Breast-feeding and its role in early development of the immune system in infants: consequences for health later in life. J Nutr. (2008) 138(9):1782S–90S. Available at: https://academic.oup.com/jn/article-abstract/138/9/1782S/4750857 doi: 10.1093/jn/138.9.1782S
39. Björkstén B. Environmental influences on the development of the immune system: consequences for disease outcome. Nestle Nutr Workshop Ser Pediatr Program. (2008) 61:243–54. doi: 10.1159/000113498.
40. Kindgren E, Fredrikson M, Ludvigsson J. Early feeding and risk of juvenile idiopathic arthritis: a case control study in a prospective birth cohort. Pediatr Rheumatol. (2017) 15(1):1–9. Available at: https://link.springer.com/article/10.1186/s12969-017-0175-z doi: 10.1186/s12969-017-0175-z
41. Mason T, Rabinovich CE, Fredrickson DD, Amoroso K, Reed AM, Stein LD, et al. Breast feeding and the development of juvenile rheumatoid arthritis. J Rheumatol. (1995) 22(6):1166–70. Available at: https://europepmc.org/article/med/7674248. PMID: 7674248
42. Kasapçopur O, Taşdan Y, Apelyan M, Akkuş S, Calişkan S, Sever L, et al. Does breast feeding prevent the development of juvenile rheumatoid arthritis? J Rheumatol. (1998) 25(11):2286–7. Available at: https://pubmed.ncbi.nlm.nih.gov/9818684/. PMID: 9818684
43. Hamer DH, Solomon H, Das G, Knabe T, Beard J, Simon J, et al. Importance of breastfeeding and complementary feeding for management and prevention of childhood diarrhoea in low-and middle-income countries. J Glob Health. (2022) 12:10011. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9344980/ doi: 10.7189/jogh.12.10011
44. Feldman M, Friedman LS, Brandt LJ. Sleisenger and fordtran’s gastrointestinal and liver disease E-book: pathophysiology, diagnosis, management. Amsterdam: Elsevier health sciences (2020). Available at: https://books.google.com/books?hl=en&lr=&id=gIrtDwAAQBAJ&oi=fnd&pg=PP1&dq=M.+Feldman,+L.+S.+Friedman,+and+L.+J.+Brandt,+Sleisenger+and+Fordtran’s+gastrointestinal+and+liver+disease+E-book:+path-ophysiology,+diagnosis,+management.+Elsevier+health+sciences
45. Ehlayel MS, Bener A, Abdulrahman HM. Protective effect of breastfeeding on diarrhea among children in a rapidly growing newly developed society. Turk J Pediatr. (2009) 51(6):527–33. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20196384. PMID: 20196384
46. Morrow R, Birol F, Griffin D, Sudre J. Divergent pathways of cyclonic and anti cyclonic ocean eddies. Geophys Res Lett. (2004) 31:24. Available at: https://agupubs.onlinelibrary.wiley.com/doi/abs/10.1029/2004GL020974 doi: 10.1029/2004GL020974
47. Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Meinzen-Derr J, Guerrero MdeL, et al. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology. (2004) 14(3):253–63. doi: 10.1093/glycob/cwh020
48. Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, et al. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. (2004) 145(3):297–303. doi: 10.1016/j.jpeds.2004.04.054
49. Diallo AF, McGlothen-Bell K, Lucas R, Walsh S, Allen C, Henderson WA, et al. Feeding modes, duration, and diarrhea in infancy: continued evidence of the protective effects of breastfeeding. Public Health Nurs. (2020) 37(2):155–60. doi: 10.1111/phn.12683
50. Mulatu T, Yimer NB, Alemnew B, Linger M, Liben ML. Exclusive breastfeeding lowers the odds of childhood diarrhea and other medical conditions: evidence from the 2016 Ethiopian demographic and health survey. Ital J Pediatr. (2021) 47(1):166. doi: 10.1186/s13052-021-01115-3
51. Lun Hon K, Leung AKC. Neonatal lupus erythematosus. Autoimmune Dis. (2012) 2012:1–6. doi: 10.1155/2012/301274
52. Gray K. Neonatal lupus erythematosus associated with breast milk autoantibodies. J Am Acad Dermatol. (2007) 56(2):AB155. doi: 10.1016/j.jaad.2006.10.716
53. Vanoni F, Lava SAG, Fossali EF, Cavalli R, Simonetti GD, Bianchetti MG, et al. Neonatal systemic lupus erythematosus syndrome: a comprehensive review. Clin Rev Allergy Immunol. (2017) 53(3):469–76. Available at: https://link.springer.com/article/10.1007/s12016-017-8653-0 doi: 10.1007/s12016-017-8653-0
54. Madsen KL, Fedorak RN, Tavernini MM, Doyle JS. Normal breast milk limits the development of colitis in IL-10-deficient mice. Inflamm Bowel Dis. (2002) 8(6):390–8. doi: 10.1097/00054725-200211000-00003
55. Davis MK. Breastfeeding and chronic disease in childhood and adolescence. Pediatr Clin North Am. (2001) 48(1):125–41. doi: 10.1016/S0031-3955(05)70289-3
56. Myléus A, Hernell O, Gothefors L, Hammarström ML, Persson LÅ, Stenlund H, et al. Early infections are associated with increased risk for celiac disease: an incident case-referent study. BMC Pediatr. (2012) 12(1):1–8. Available at: https://link.springer.com/article/10.1186/1471-2431-12-194 doi: 10.1186/1471-2431-12-1
57. Canova C, Zabeo V, Pitter G, Romor P, Baldovin T, Zanotti R, et al. Association of maternal education, early infections, and antibiotic use with celiac disease: a population-based birth cohort study in northeastern Italy. Am J Epidemiol. (2014) 180(1):76–85. Available at: https://academic.oup.com/aje/article-abstract/180/1/76/2739114 doi: 10.1093/aje/kwu101
58. Morild K, Kahrs CR, Tapia G, Stene LC, Stordal K. Infections and risk of celiac disease in childhood: a prospective nationwide cohort study. Off J Am Coll Gastroenterol ACG. (2015) 110(10):1475–84. Available at: https://journals.lww.com/ajg/fulltext/2015/10000/infections_and_risk_of_celiac_disease_in.21.aspx doi: 10.1038/ajg.2015.287
59. Sandberg Bennich S, Dahlquist G, Kollon B. Coeliac disease is associated with intrauterine growth and neonatal infections. Acta Paediatr. (2002) 91(1):30–3. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1651-2227.2002.tb01635.x doi: 10.1111/j.1651-2227.2002.tb01635.x
60. Ivarsson A, Hernell O, Stenlund H, Persson LA. Breast-feeding protects against celiac disease. Am J Clin Nutr. (2002) 75(5):914–21. Available at: https://academic.oup.com/ajcn/article-abstract/75/5/914/4689407 doi: 10.1093/ajcn/75.5.914
61. Kemppainen KM, Lynch KF, Liu E, Lönnrot M, Simell V, Briese T, et al. Factors that increase risk of celiac disease autoimmunity after a gastrointestinal infection in early life. Clin Gastroenterol Hepatol. (2017) 15(5):694–702.e5. doi: 10.1016/j.cgh.2016.10.033
62. Palma GD, Capilla A, Nova E, Castillejo G, Varea V, Pozo T, et al. Influence of milk-feeding type and genetic risk of developing coeliac disease on intestinal Microbiota of infants: the PROFICEL study. PLoS One. (2012) 7(2):e30791. doi: 10.1371/journal.pone.0030791
63. Sollid LM. Breast milk against coeliac disease. Gut. (2002) 51(6):767–8. Available at: https://gut.bmj.com/content/51/6/767.short doi: 10.1136/gut.51.6.767
64. Peters U, Schneeweiss S, Trautwein EA, Erbersdobler HF. A case-control study of the effect of infant feeding on celiac disease. Ann Nutr Metab. (2001) 45(4):135–42. Available at: https://www.karger.com/Article/Abstract/46720 doi: 10.1159/000046720
65. Maki M, Kallonen K, Lahdeaho ML, Visakorpi JK. Changing pattern of childhood coeliac disease in Finland. Acta Paediatr. (1988) 77(3):408–12. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1651-2227.1988.tb10668.x doi: 10.1111/j.1651-2227.1988.tb10668.x
66. Greco L, Tozzi AE, Mayer M, Grimaldi M, Silano G, Auricchio S. Unchanging clinical picture of coeliac disease presentation in campania, Italy. Eur J Pediatr. (1989) 148(7):610–3. Available at: https://link.springer.com/article/10.1007/BF00441511 doi: 10.1007/BF00441511
67. Maki M, Holm K. Incidence and prevalence of coeliac disease in tampere coeliac disease is not disappearing. Acta Paediatr. (1990) 79(10):980–2. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1651-2227.1990.tb11367.x doi: 10.1111/j.1651-2227.1990.tb11367.x
68. Lionetti E, Castellaneta S, Francavilla R, Pulvirenti A, Tonutti E, Amarri S, et al. Introduction of gluten, HLA Status, and the risk of celiac disease in children. N Engl J Med. (2014) 371(14):1295–303. doi: 10.1056/NEJMoa1400697
69. Momtazmanesh S, Rezaei N. “Hypersensitivity,” in reference module in biomedical sciences. Amsterdam: Elsevier (2020). doi: 10.1016/B978-0-12-818731-9.00032-X.
70. Bener A, Ehlayel MS, Alsowaidi S, Sabbah A. Role of breast feeding in primary prevention of asthma and allergic diseases in a traditional society. Eur Ann Allergy Clin Immunol. (2007) 39(10):337–43. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18386435. PMID: 18386435
71. Yamakawa M, Yorifuji T, Kato T, Yamauchi Y, Doi H. Breast-feeding and hospitalization for asthma in early childhood: a nationwide longitudinal survey in Japan. Public Health Nutr. (2015) 18(10):1756–61. Available at: https://www.cambridge.org/core/journals/public-health-nutrition/article/breastfeeding-and-hospitalization-for-asthma-in-early-childhood-a-nationwide-longitudinal-survey-in-japan/535D8505561ECAEBB5842E2548731E07 doi: 10.1017/S1368980014002407
72. Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. (2015) 45(3):632–43. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/cea.12487 doi: 10.1111/cea.12487
73. Evelien Rvan Meel, Mandy de Jong, Niels JElbert, Herman T, et al. Duration and exclusiveness of breastfeeding and school-age lung function and asthma. Ann Allergy Asthma Immunol. (2017) 119(1):21–6. Available at: https://www.sciencedirect.com/science/article/pii/S1081120617303563 doi: 10.1016/j.anai.2017.05.002
74. Huang C, Liu W, Cai J, Weschler LB, Wang X, Hu Y, et al. Breastfeeding and timing of first dietary introduction in relation to childhood asthma, allergies, and airway diseases: a cross-sectional study. J Asthma. (2017) 54(5):488–97. Available at: https://www.tandfonline.com/doi/abs/10.1080/02770903.2016.1231203 doi: 10.1080/02770903.2016.1231203
75. Peters RL, Kay T, McWilliam VL, Lodge CJ, Ponsonby AL, Dharmage SC, et al. The interplay between eczema and breastfeeding practices may hide breastfeeding’s protective effect on childhood asthma. J Allergy Clin Immunol Pract. (2021) 9(2):862–71. Available at: https://www.sciencedirect.com/science/article/pii/S2213219820309521 doi: 10.1016/j.jaip.2020.09.006
76. Al-Makoshi A, Al-Frayh A, Turner S, Devereux G. Breastfeeding practice and its association with respiratory symptoms and atopic disease in 13-year-old children in the city of Riyadh, central Saudi Arabia. Breastfeed Med. (2013) 8(1):127–33. Available at: https://www.liebertpub.com/doi/abs/10.1089/bfm.2011.0137 doi: 10.1089/bfm.2011.0137
77. Qu F, Weschler LB, Sundell J, Zhang Y. Increasing prevalence of asthma and allergy in Beijing pre-school children: is exclusive breastfeeding for more than 6 months protective? Chinese Sci Bull. (2013) 58(34):4190–202. Available at: https://link.springer.com/article/10.1007/s11434-013-5790-6 doi: 10.1007/s11434-013-5790-6
78. Hu Y, Chen Y, Liu S, Jiang F, Wu M, Yan C, et al. Breastfeeding duration modified the effects of neonatal and familial risk factors on childhood asthma and allergy: a population-based study. Respir Res. (2021) 22(1):1–11. Available at: https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-021-01644-9 doi: 10.1186/s12931-020-01578-8
79. Ehlayel MS, Bener A. Duration of breast-feeding and the risk of childhood allergic diseases in a developing country. Allergy Asthma Proc. (2008) 29(4):386–91. Available at: https://www.researchgate.net/profile/Abdulbari-Bener/publication/23170607_Duration_of_breast-feeding_and_the_risk_of_childhood_allergic_diseases_in_a_developing_country/links/57e507ed08aef5d0161b5cda/Duration-of-breast-feeding-and-the-risk-of-childhood-al doi: 10.2500/aap.2008.29.3138
80. Obihara CC, Marais BJ, Gie RP, Potter P, Bateman ED, Lombard CJ, et al. The association of prolonged breastfeeding and allergic disease in poor urban children. Eur Respir J. (2005) 25(6):970–7. Available at: https://erj.ersjournals.com/content/25/6/970.short doi: 10.1183/09031936.05.00116504
81. Vieira Borba V, Sharif K, Shoenfeld Y. Breastfeeding and autoimmunity: programing health from the beginning. Am J Reprod Immunol. (2018) 79(1):e12778. doi: 10.1111/aji.12778
82. Sears MR, Greene JM, Willan AR, Taylor DR, Flannery EM, Cowan JO, et al. Long-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet. (2002) 360(9337):901–7. doi: 10.1016/S0140-6736(02)11025-7
83. Arima T, Campos-Alberto E, Funakoshi H, Inoue Y, Tomiita M, Kohno Y, et al. Immediate systemic allergic reaction in an infant to fish allergen ingested through breast milk. Asia Pac Allergy. (2016) 6(4):257. doi: 10.5415/apallergy.2016.6.4.257
84. Des Roches A, Paradis L, Singer S, Seidman E. An allergic reaction to peanut in an exclusively breastfed infant. Allergy. (2005) 60(2):266–7. doi: 10.1111/j.1398-9995.2005.00681.x
85. Plagemann A, Harder T, Kohlhoff R, Fahrenkrog S, Rodekamp E, Franke K, et al. Impact of early neonatal breast-feeding on psychomotor and neuropsychological development in children of diabetic mothers. Diabetes Care. (2005) 28(3):573–8. Available at: https://diabetesjournals.org/care/article-abstract/28/3/573/27668 15735190
86. Achong N, Duncan EL, McIntyre HD, Callaway L. The physiological and glycaemic changes in breastfeeding women with type 1 diabetes mellitus. Diabetes Res Clin Pract. (2018) 135:93–101. doi: 10.1016/j.diabres.2017.11.005
87. Hyrich KL, Baildam E, Pickford H, Chieng A, Davidson JE, Foster H, et al. Influence of past breast feeding on pattern and severity of presentation of juvenile idiopathic arthritis. Arch Dis Child. (2016) 101(4):348–51. Available at: https://adc.bmj.com/content/101/4/348.short doi: 10.1136/archdischild-2014-308117
88. Chen H, Wang J, Zhou W, Yin H, Wang M. Breastfeeding and risk of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. (2015) 42(9):1563–9. doi: 10.3899/jrheum.150195
89. Nuzzi G, Di Cicco ME, Peroni DG. Breastfeeding and allergic diseases: what’s new? Child (Basel, Switzerland). (2021) 8(5):330. doi: 10.3390/children8050330
90. Kull I, Almqvist C, Lilja G, Pershagen G, Wickman M. Breast-feeding reduces the risk of asthma during the first 4 years of life. J Allergy Clin Immunol. (2004) 114(4):755–60. doi: 10.1016/j.jaci.2004.07.036
91. Raheem OA, Khandwala YS, Sur RL, Ghani KR, Denstedt JD. Burden of urolithiasis: trends in prevalence, treatments, and costs. Eur Urol Focus. (2017) 3(1):18–26. Available at: https://www.sciencedirect.com/science/article/pii/S240545691730086X doi: 10.1016/j.euf.2017.04.001
92. Bloch AM, Mimouni D, Mimouni M, Gdalevich M. Does breastfeeding protect against allergic rhinitis during childhood? A meta-analysis of prospective studies. Acta Paediatr. (2007) 91(3):275–9. doi: 10.1111/j.1651-2227.2002.tb01714.x
93. Hogg-Kollars S, Al Dulaimi D, Tait K, Rostami K. Type 1 diabetes mellitus and gluten induced disorders. Gastroenterol Hepatol Bed Bench. (2014) 7(4):189. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4185872/. PMID: 25289132
94. Arrieta M-C, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. (2014) 5:427. Available at: https://www.frontiersin.org/articles/10.3389/fimmu.2014.00427/full. doi: 10.3389/fimmu.2014.00427
95. Lewis ED, Richard C, Larsen BM, Field CJ. The importance of human milk for immunity in preterm infants. Clin Perinatol. (2017) 44(1):23–47. doi: 10.1016/j.clp.2016.11.008
96. Miettinen ME, Honkanen J, Niinistö S, Vaarala O, Virtanen SM, Knip M. Breastfeeding and circulating immunological markers during the first 3 years of life: the DIABIMMUNE study. Diabetologia. (2022) 65(2):329–35. doi: 10.1007/s00125-021-05612-2
97. Stewart CJ, Ajami NJ, O'Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. (2018) 562(7728):583–8. Available at: https://www.nature.com/articles/s41586-018-0617-x doi: 10.1038/s41586-018-0617-x
98. Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. Cmaj. (2013) 185(5):385–94. Available at: https://www.cmaj.ca/content/185/5/385.short doi: 10.1503/cmaj.121189
99. Berrington JE, Stewart CJ, Cummings SP, Embleton ND. The neonatal bowel microbiome in health and infection. Curr Opin Infect Dis. (2014) 27(3):236–43. Available at: https://journals.lww.com/co-infectiousdiseases/Fulltext/2014/06000/The_neonatal_bowel_microbiome_in_health_and.5.aspx doi: 10.1097/QCO.0000000000000061
100. Rautava S. Early microbial contact, the breast milk microbiome and child health. J Dev Orig Health Dis. (2016) 7(1):5–14. Available at: https://www.cambridge.org/core/journals/journal-of-developmental-origins-of-health-and-disease/article/early-microbial-contact-the-breast-milk-microbiome-and-child-health/BAC428C08779F804DB06D55426AC1DAC doi: 10.1017/S2040174415001233
101. Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. (2018) 562(7728):589–94. Available at: https://www.nature.com/articles/s41586-018-0620-2 doi: 10.1038/s41586-018-0620-2
Appendix A
Keywords: allergy, breastfeeding, immunity, diabetes, infants, lactation
Citation: Alotiby AA (2023) The role of breastfeeding as a protective factor against the development of the immune-mediated diseases: A systematic review. Front. Pediatr. 11:1086999. doi: 10.3389/fped.2023.1086999
Received: 1 November 2022; Accepted: 26 January 2023;
Published: 16 February 2023.
Edited by:
Reema Fayez Tayyem, Qatar University, Qatar© 2023 Alotiby. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amna A. Alotiby YWFtb2dhdHlAdXF1LmVkdS5zYQ==
Specialty Section: This article was submitted to Children and Health, a section of the journal Frontiers in Pediatrics
 Amna A. Alotiby
Amna A. Alotiby