
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 13 February 2023
Sec. Neonatology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1081802
Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD) is an autosomal recessive condition of impaired beta-oxidation. Traditionally, treatment included restriction of dietary long-chain fatty acids via a low-fat diet and supplementation of medium chain triglycerides. In 2020, triheptanoin received FDA approval as an alternative source of medium chain fatty acids for individuals with long-chain fatty acid oxidation disorders (LC-FAOD). We present a case of a moderately preterm neonate born at 33 2/7 weeks gestational age with LCHADD who received triheptanoin and developed necrotizing enterocolitis (NEC). Prematurity is known as a major risk factor for NEC, with risk increasing with decreasing gestational age. To our knowledge, NEC has not previously been reported in patients with LCHADD or with triheptanoin use. While metabolic formula is part of the standard of care for LC-FAOD in early life, preterm neonates may benefit from more aggressive attempts to use skimmed human milk to minimize exposure to formula during the risk period for NEC during feed advancement. This risk period may be longer in neonates with LC-FAOD compared to otherwise healthy premature neonates.
Long-chain fatty acid oxidation disorders (LC-FAOD) are rare, autosomal recessive, conditions of impaired beta-oxidation (1–4). Isolated long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD) is the most common mitochondrial trifunctional protein (MTP) complex deficiency with an estimated incidence of 1:363,768 for LCHADD and 1:1,822,568 for MTP deficiency in the United States (1, 5, 6). The c.1528G > C variant in HADHA is the most frequently associated allele in LCHADD with 87%–90% of symptomatic European-ancestry individuals carrying at least one copy (1, 7). Its presentation can include hypoketotic hypoglycemia, lactic acidosis, liver dysfunction, encephalopathy, rhabdomyolysis, fatigue, and cardiac arrhythmias (1, 2, 4, 5, 8–10). It is also associated with prematurity and maternal hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome (1, 11). LCHADD is part of the newborn screen in many North American and European countries, including the United States (1, 5, 8). Treatment includes restriction of dietary long-chain fatty acids, supplementation with medium chain triglycerides (MCT), and low-fat diet (1, 2, 3, 4, 8). In 2020, triheptanoin, a synthetic MCT, received FDA approval as an alternative source of medium chain fatty acids for children and adults with LC-FAOD (2). Triheptanoin directly enters mitochondria for beta-oxidation with resulting metabolites providing energy as well as replenishing key Krebs cycle intermediates and has proven to be more effective than MCT oil in reducing risk of cardiomyopathy and rhabdomyolysis (3, 10, 12).
Necrotizing enterocolitis (NEC) is a known complication of prematurity and significant cause of morbidity and mortality (13, 14). We present a case of NEC in a 9-day-old, moderately preterm male with confirmed LCHADD who received triheptanoin. Triheptanoin treatment is described in neonates as young as 2 and 3 days old (15, 16). However, the youngest age of reported use in an infant born prematurely is 13 months (11). While NEC has been reported as the presentation of MTP deficiency in a 3-day-old female born at 35 weeks, to our knowledge, there have been no published, peer-reviewed reports of NEC developing in patients with LCHADD or after the initiation of triheptanoin (5).
This baby boy was born at 33 2/7 weeks gestational age to a 36-year-old gravida 3, para 3 mother with negative serologies, except for Group B Streptococcus unknown, whose pregnancy was complicated by medication-controlled gestational diabetes and gestational hypertension, and a family history significant for a sibling with LCHADD. The patient was delivered by cesarean section at an outside facility due to concern for serious maternal morbidity associated with preeclampsia, specifically HELLP syndrome. Neonatal resuscitation was unremarkable and the Appearance, Pulse, Grimace, Activity, and Respiration (APGAR)s were 8 and 9 at 1 and 5 min, respectively. The birth weight was 2.46 kg, length 47.5 cm, and head circumference 32.5 cm, which were all appropriate for gestational age. The patient was admitted to the level III NICU at the birth facility. A peripheral intravenous line was placed and 10% dextrose fluids at 150 ml/kg/day were initiated. At six hours of life, based on the significant family history and the maternal HELLP syndrome frequently seen in mothers with gestations complicated by LCHADD, our patient was transported to our level IV NICU for metabolic consultation and further evaluation and management. On admission, serum acylcarnitine profile was consistent with LCHADD given elevation of C16-OH and C18-OH levels with the ultimate diagnosis based on perinatal and family history in conjunction with biochemical and molecular studies (Table 1). Echocardiogram shortly after arrival showed a small patent ductus arteriosus and patent foramen ovale. Enteral nutrition with donor human milk was initiated at 12 h of life. Triheptanoin was initiated at 14 h of life at a dose of 0.5 g/kg/day and advanced 0.5 g/kg every 5–7 days. As feeds were advanced by unit standard of 20 ml/kg/day, he was limited to 23 ml/kg/day of human milk with the remainder of his enteral diet consisting of Enfaport (Chicago, IL, United States), SolCarb (Westbury, NY, United States), and Similac liquid protein fortifier (Abbott Park, IL, United States) formulas (Table 2). His feeds were fortified to 24 kcal/oz on day of life 6. On day 7 of life, the patient started to have new bradycardia and desaturation episodes with two small emeses but a reassuring abdominal examination. Enteral nutrition was continued. On day 9 of life, he had one episode of emesis and then hematochezia. An abdominal x-ray (Figure 1) demonstrated pneumatosis intestinalis and he was subsequently diagnosed with NEC (modified Bell Stage IIa). He was managed conservatively with bowel rest, ampicillin–sulbactam and gentamicin for 7 days, and bowel decompression for 5 days. He was maintained on total parental nutrition (TPN) with 4 g/kg/day TrophAmine, glucose infusion rate ∼15 mg/kg/min, and 2 g/kg/day 20% Intralipid. Diet following re-initiation of enteral nutrition consisted of a combination of skimmed and full fat maternal and donor human milk, Enfaport, MCT oil instead of triheptanoin (per parental request), SolCarb, and Similac liquid protein fortifier (Figure 2). He recovered uneventfully and was discharged home on day 33 of life.
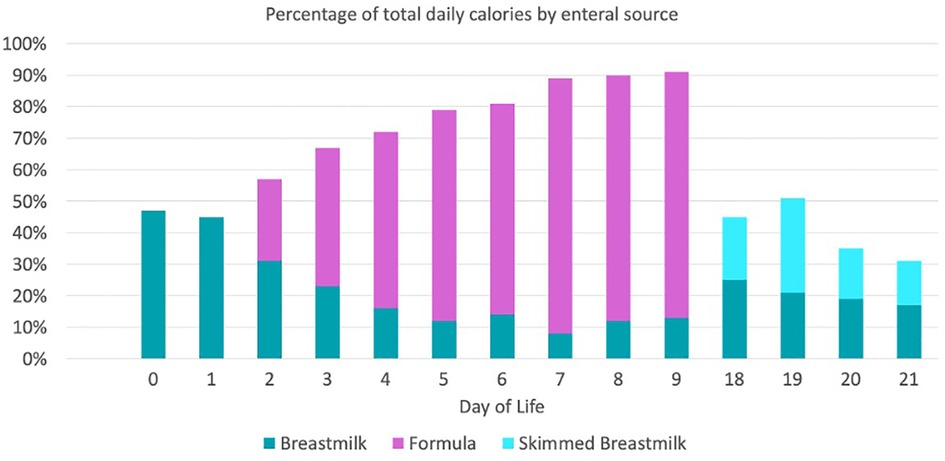
Figure 2. Enteral feeding advancement regimen prior to developing NEC. *SolCarb was added starting on day of life 5. *Similac liquid protein fortifier was added starting on day of life 7.
NEC is a spectrum of gastrointestinal disease with incompletely understood pathophysiology (17, 18). NEC occurs in a gut compromised by intestinal immaturity with an inciting mucosal injury, disruption to the microvascular supply, alteration in the microbial colonization, activation of inflammatory processes, and genetic predisposition (14, 17, 19). NEC occurs in 1%–5% of all NICU admissions and 5%–10% of all very low birthweight infants (<1,500 g) (20). Validated NEC risk factors include being small for gestational age, low gestational age, sepsis, assisted ventilation, premature rupture of membranes, black race, out born status, and delayed gut priming or initiation of feeds (17, 21, 22). However, there are several pre- and post-natal NEC risk factors beyond this list specific to our case. Data for a link between maternal preeclampsia, as seen with HELLP syndrome, and NEC have been mixed with some studies showing an increased NEC risk while other studies attributing this trend to delivery at an earlier gestational age for maternal wellbeing (19, 23). Research has shown long-chain 3-hydroxyacyl-CoA dehydrogenase to be expressed during fetal development in fetal heart, eye, liver, brain, and gut epithelium (9). It has also been suggested that fatty acid oxidation enzymes in the gut play a role in mucus synthesis and intracellular junction integrity (5). These findings indicate that gut development could already be impaired in neonates with LCHADD, leading them to be more susceptible to NEC. Given that LCHADD is rare and neonates with LCHADD are typically born at gestational ages when NEC is infrequent, it may be challenging, if not impossible, to quantify this risk using traditional epidemiologic methods.
Postnatally, NEC prevention has focused on human milk feeding. The strong support for early human milk feedings arises from bioactive substances such as lactoferrin and human milk oligosaccharides with various bactericidal, immunomodulating, and intestinal maturation-inducing properties found in human milk (13, 17, 20, 24–26). NEC rates were found to be six times more common in premature infants exclusively formula fed compared to those fed exclusively human milk and 3-times more common in those fed formula exclusively compared to those fed a mixture of formula and human milk (26, 27). There has been an increasing amount of literature looking specifically at the role fatty acids in human milk play in gut development, microbial colonization, immune function, and inflammatory response in in vitro, in vivo, and in human cohort studies (28–30). One recent example found less NEC among a cohort of preterm neonates that received supplementation of the specific long-chain polyunsaturated fatty acid (LCPUFA) docosahexaenoic acid (DHA) compared to controls (31). While this supplementation is exciting for future work, current expert-consensus NEC prevention targets include supporting antenatal corticosteroids before delivery, prioritizing mother's own milk, a standardized unit-based feeding protocol, use of programmatic approach to reducing NEC with quality improvement methodology, discussing risks and benefits of probiotic administration with parents, and skin-to-skin care (24). Modest evidence supports other standards of care with donor human milk as a substitute for mother's own milk over formula, limiting prolonged empiric antibiotic courses, and limiting the use of histamine-2 antagonists (22, 24). As our knowledge of NEC pathophysiology grows, it is natural to target preventative and treatment strategies to the patient's specific circumstances and disease (32).
In almost all cases, preference for a human milk-based diet seems prudent. We used formula early in this patient's course due to his metabolic needs specific to his LC-FAOD. However, upon reintroduction of the feeds after NEC treatment, we utilized skimmed milk to maximize its NEC protection benefits while minimizing its metabolic risk to our patient. Skimmed human milk to eliminate long-chain triglycerides has been used in the treatment of infants with chylothorax (33). Milk skimming techniques include gravity, centrifuge, top loading washing machine acting as a centrifuge, and a commercially available cream separator (16, 33, 34). The effectiveness of milk skimming has been compared between centrifuged refrigerated milk, centrifuged non-refrigerated milk, and non-centrifuged refrigerated milk stored for 24 h. The centrifuged refrigerated method was most effective at skimming while retaining protein and immunoglobulins (33). While centrifuge-based methods are most effective, non-centrifuge methods do not require additional equipment and may be more logistically feasible for families. However, our patient's family needed to cautiously transport the refrigerated mother's milk to the hospital while maintaining gravity separation and staff needed to budget time for skimming to the already short shelf life of fresh human milk. In the end, our patient received primarily donor milk skimmed by centrifuge and a minority of gravity skimmed mother's milk during the initial hospitalization due to these logistical challenges.
Skimmed term human milk contains 12.4 kcal/oz and 0.4 g/dl fat compared to unskimmed term human milk that contains 20 kcal/oz and 3.6 g/dl. Skimmed milk would, therefore, require fortification to increase calories and any nutritional deficiencies not provided in the skimmed milk. The specific metabolic needs leading to formula use need to be balanced with the needs of a premature infant. Earlier adoption of a diet maximizing human milk usage and minimizing formula may have helped prevent NEC with the protective factors of human milk while meeting the specific dietary restrictions of our patient. Based on the research supporting LCPUFA, this skimming may decrease some of the NEC-protective benefits of human milk. However, we believe the remaining properties of human milk likely make it a superior choice to formula in this unusual clinical setting. Further research would be needed to assess this theory.
A review of the literature failed to identify other reports of NEC after early neonatal use of triheptanoin. Our decision to begin standard MCT oil following the episode of NEC was driven by parental preference and paucity of data on triheptanoin in this setting. From a medical standpoint, MCT has been used extensively without known complications of NEC although it is less effective in reducing adverse events of LC-FAODs compared to triheptanoin (4, 35). With the parental concerns regarding triheptanoin and our patient's complication, the decision was made to switch to MCT with its better described safety profile despite known differences in efficacy. Given the challenge of studying an uncommon complication (NEC) of a rare group of disorders (LC-FAODs) and the relatively limited data on triheptanoin (FDA approved in 2020), case reports such as this are likely to comprise a significant portion of the evidence basis for assessing a possible link between triheptanoin and NEC. Making that assessment also requires consideration of alternative causes of NEC in our patient and potential opportunities for NEC prevention in similarly situated neonates.
In summary, we present a case of a moderately preterm neonate with LCHADD who developed NEC. We do not think the triheptanoin was the cause of NEC. Limited use of human milk in the setting of metabolic management for LC-FAOD may have contributed to the development of NEC. It is important to consider skimmed human milk to minimize exposure to formula in the NEC risk period during feeding advancement, which may be longer in neonates with LC-FAOD than in otherwise healthy premature neonates.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
MM contributed to the conception of the work, drafted the initial manuscript, oversaw acquisition and interpretation of data, and revised the final manuscript. SN contributed to study design, drafted the initial manuscript, and reviewed and revised the final submission. BPC contributed to the conception of the work, interpretation of data, and revised the manuscript critically for important intellectual content. WB contributed to the acquisition, analysis and interpretation of data, and revision of the manuscript critically for important intellectual content. CM contributed to the acquisition, analysis and interpretation of data, and revision for the work. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
BPC receives salary support in part by the Steve and Cindy Rasmussen Institute for Genomic Medicine and the Nationwide Foundation Pediatric Innovation Fund. The funder had no role in the conception, design or conduct of the work described.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lotz-Havla AS, Röschinger W, Schiergens K, Singer K, Karall D, Konstantopoulou V, et al. Fatal pitfalls in newborn screening for mitochondrial trifunctional protein (MTP)/long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency. Orphanet J Rare Dis. (2018) 13(1):122. doi: 10.1186/s13023-018-0875-6
2. Shirley M. Triheptanoin: first approval. Drugs. (2020) 80(15):1595–600. doi: 10.1007/s40265-020-01399-5
3. Vockley J, Charrow J, Ganesh J, Eswara M, Diaz GA, McCracken E, et al. Triheptanoin treatment in patients with pediatric cardiomyopathy associated with long chain-fatty acid oxidation disorders. Mol Genet Metab. (2016) 119(3):223–31. doi: 10.1016/j.ymgme.2016.08.008
4. Vockley J, Burton B, Berry G, Longo N, Phillips J, Sanchez-Valle A, et al. Effects of triheptanoin (UX007) in patients with long-chain fatty acid oxidation disorders: results from an open-label, long-term extension study. J Inherit Metab Dis. (2021) 44(1):253–63. doi: 10.1002/jimd.12313
5. Diekman EF, Boelen CC, Prinsen BH, Ijlst L, Duran M, de Koning TJ, et al. Necrotizing enterocolitis and respiratory distress syndrome as first clinical presentation of mitochondrial trifunctional protein deficiency. JIMD Rep. (2013) 7:1–6. doi: 10.1007/8904_2012_128
6. Therrell BL, Lloyd-Puryear MA, Camp KM, Mann MY. Inborn errors of metabolism identified via newborn screening: ten-year incidence data and costs of nutritional interventions for research agenda planning. Mol Genet Metab. (2014) 113(1–2):14–26. doi: 10.1016/j.ymgme.2014.07.009
7. Nedoszytko B, Siemińska A, Strapagiel D, Dąbrowski S, Słomka M, Sobalska-Kwapis M, et al. High prevalence of carriers of variant c.1528G>C of HADHA gene causing long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD) in the population of adult Kashubians from North Poland. PLoS One. (2017) 12(11):e0187365. doi: 10.1371/journal.pone.0187365
8. Fraser H, Geppert J, Johnson R, Johnson S, Connock M, Clarke A, et al. Evaluation of earlier versus later dietary management in long-chain 3-hydroxyacyl-CoA dehydrogenase or mitochondrial trifunctional protein deficiency: a systematic review. Orphanet J Rare Dis. (2019) 14(1):258. doi: 10.1186/s13023-019-1226-y
9. Oey NA, den Boer ME, Wijburg FA, Vekemans M, Augé J, Steiner C, et al. Long-chain fatty acid oxidation during early human development. Pediatr Res. (2005) 57(6):755–9. doi: 10.1203/01.PDR.0000161413.42874.74
10. Sklirou E, Alodaib AN, Dobrowolski SF, Mohsen AA, Vockley J. Physiological perspectives on the use of triheptanoin as anaplerotic therapy for long chain fatty acid oxidation disorders. Front Genet. (2020) 11:598760. doi: 10.3389/fgene.2020.598760
11. Karall D, Brunner-Krainz M, Kogelnig K, Konstantopoulou V, Maier EM, Möslinger D, et al. Clinical outcome, biochemical and therapeutic follow-up in 14 Austrian patients with long-chain 3-hydroxy acyl CoA dehydrogenase deficiency (LCHADD). Orphanet J Rare Dis. (2015) 10:21. doi: 10.1186/s13023-015-0236-7
12. Zöggeler T, Stock K, Jörg-Streller M, Spenger J, Konstantopoulou V, Hufgard-Leitner M, et al. Long-term experience with triheptanoin in 12 Austrian patients with long-chain fatty acid oxidation disorders. Orphanet J Rare Dis. (2021) 16(1):28. doi: 10.1186/s13023-020-01635-x
13. Cacho NT, Parker LA, Neu J. Necrotizing enterocolitis and human milk feeding: a systematic review. Clin Perinatol. (2017) 44(1):49–67. doi: 10.1016/j.clp.2016.11.009
14. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. (2011) 364(3):255–64. doi: 10.1056/NEJMra1005408
15. Norris MK, Scott AI, Sullivan S, Chang IJ, Lam C, Sun A, et al. Tutorial: triheptanoin and nutrition management for treatment of long-chain fatty acid oxidation disorders. JPEN J Parenter Enteral Nutr. (2021) 45(2):230–8. doi: 10.1002/jpen.2034
16. Kritzer A, Tarrant S, Sussman-Karten K, Barbas K. Use of skimmed breast milk for an infant with a long-chain fatty acid oxidation disorder: a novel therapeutic intervention. JIMD Rep. (2020) 55(1):44–50. doi: 10.1002/jmd2.12152
17. Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. (2016) 13(10):590–600. doi: 10.1038/nrgastro.2016.119
18. Mϋller MJ, Paul T, Seeliger S. Necrotizing enterocolitis in premature infants and newborns. J Neonatal Perinatal Med. (2016) 9(3):233–42. doi: 10.3233/NPM-16915130
19. Nair J, Longendyke R, Lakshminrusimha S. Necrotizing enterocolitis in moderate preterm infants. Biomed Res Int. (2018) 2018:4126245. doi: 10.1155/2018/4126245
20. Thompson AM, Bizzarro MJ. Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs. (2008) 68(9):1227–38. doi: 10.2165/00003495-200868090-00004
21. Samuels N, van de Graaf RA, de Jonge RCJ, Reiss IKM, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr. (2017) 17(1):105. doi: 10.1186/s12887-017-0847-3
22. Drenckpohl D, Knaub L, Schneider C, McConnell C, Wang H, Macwan K. Risk factors that may predispose premature infants to increased incidence of necrotizing enterocolitis. ICAN: Infant, Child, & Adolescent Nutrition. (2010) 2(1):37–44. doi: 10.1177/1941406409359195
23. Rose AT, Patel RM. A critical analysis of risk factors for necrotizing enterocolitis. Semin Fetal Neonatal Med. (2018) 23(6):374–9. doi: 10.1016/j.siny.2018.07.005
24. Gephart SM, Underwood MA, Rosito S, Kim JH, Caplan MS. Grading the evidence to identify strategies to modify risk for necrotizing enterocolitis. Pediatr Res. (2020) 88(Suppl 1):41–7. doi: 10.1038/s41390-020-1079-z
25. Cotten CM. Modifiable risk factors in necrotizing enterocolitis. Clin Perinatol. (2019) 46(1):129–43. doi: 10.1016/j.clp.2018.10.007
26. Eaton S, Rees CM, Hall NJ. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology. (2017) 111(4):423–30. doi: 10.1159/000458462
27. Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. (1990) 336(8730):1519–23. doi: 10.1016/0140-6736(90)93304-8
28. Ramiro-Cortijo D, Singh P, Liu Y, Medina-Morales E, Yakah W, Freedman SD, et al. Breast milk lipids and fatty acids in regulating neonatal intestinal development and protecting against intestinal injury. Nutrients. (2020) 12(2):534. doi: 10.3390/nu12020534
29. Alshaikh BN, Reyes Loredo A, Knauff M, Momin S, Moossavi S. The role of dietary fats in the development and prevention of necrotizing enterocolitis. Nutrients. (2021) 14(1):145. doi: 10.3390/nu14010145
30. Wijendran V, Brenna JT, Wang DH, Zhu W, Meng D, Ganguli K, et al. Long-chain polyunsaturated fatty acids attenuate the IL-1β-induced proinflammatory response in human fetal intestinal epithelial cells. Pediatr Res. (2015) 78(6):626–33. doi: 10.1038/pr.2015.154
31. Abou El Fadl DK, Ahmed MA, Aly YA, Darweesh EAG, Sabri NA. Impact of docosahexaenoic acid supplementation on proinflammatory cytokines release and the development of necrotizing enterocolitis in preterm neonates: a randomized controlled study. Saudi Pharm J. (2021) 29(11):1314–22. doi: 10.1016/j.jsps.2021.09.012
32. Neu J. Necrotizing enterocolitis: the future. Neonatology. (2020) 117(2):240–4. doi: 10.1159/000506866
33. Drewniak MA, Lyon AW, Fenton TR. Evaluation of fat separation and removal methods to prepare low-fat breast milk for fat-intolerant neonates with chylothorax. Nutr Clin Pract. (2013) 28(5):599–602. doi: 10.1177/0884533613497763
34. Gilgan H, Deveau D, O’Leary G. Skimming mother’s breast milk at home: a novel approach to the treatment of chylothorax. ICAN Childhood Obesity and Nutrition. (2014) 7(1):24–8. doi: 10.1177/1941406414556507
Keywords: necrotizing enterocolitis, triheptanoin, preterm, human milk, LCHADD
Citation: Metzler M, Burns W, Mitchell C, Napolitano S and Chaudhari BP (2023) A case report of necrotizing enterocolitis in a moderately preterm neonate with LCHADD—A call to focus on the basics while utilizing advanced new therapies. Front. Pediatr. 11:1081802. doi: 10.3389/fped.2023.1081802
Received: 27 October 2022; Accepted: 16 January 2023;
Published: 13 February 2023.
Edited by:
Nishad Plakkal, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), IndiaReviewed by:
Noa Ofek-shlomai, Hadassah Medical Center, Israel© 2023 Metzler, Burns, Mitchell, Napolitano and Chaudhari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bimal P. Chaudhari YmltYWwuY2hhdWRoYXJpQG5hdGlvbndpZGVjaGlsZHJlbnMub3Jn
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.