
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 03 March 2023
Sec. Neonatology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1078506
This article is part of the Research TopicEndotyping and Phenotyping Prematurity and its ComplicationsView all 5 articles
Introduction: Endotypes leading to very and extremely preterm birth are clustered into two groups: infection/inflammation and dysfunctional placentation. We conducted a systematic review of observational studies exploring the association between these two endotypes and the pharmacological closure of patent ductus arteriosus (PDA) induced by cyclooxygenase (COX) inhibitors. Chorioamnionitis represented the infectious-inflammatory endotype, while dysfunctional placentation proxies were hypertensive disorders of pregnancy (HDP) and small for gestational age (SGA) or intrauterine growth restriction.
Methods: PubMed/Medline and Embase databases were searched. The random-effects odds ratio (OR) and 95% confidence interval (CI) were calculated for each association. We included 30 studies (12,639 infants).
Results: Meta-analysis showed a significant association between exposure to HDP and increased rate of pharmacological closure of PDA (17 studies, OR 1.41, 95% CI 1.10–1.81, p = 0.006). In contrast, neither chorioamnionitis (13 studies, OR 0.75, 95% CI 0.47–1.18, p = 0.211) nor SGA (17 studies, OR 1.20, 95% CI 0.96–1.50, p = 0.115) were significantly associated with the response to therapy. Subgroup analyses showed that the higher response to COX inhibitors in the HDP group was significant for indomethacin (OR 1.568, 95% CI 1.147–2.141, p = 0.005) but not for ibuprofen (OR 1.107, 95% CI 0.248–4.392, p = 0.894) or for the studies using both drugs (OR 1.280, 95% CI 0.935–1.751, p = 0.124). However, meta-regression showed that this difference between the drugs was not statistically significant (p = 0.404).
Discussion/Conclusion: Our data suggest that the pathologic condition that triggers prematurity may alter the response to pharmacological treatment of PDA. The DA of infants exposed to HDP appears to be more responsive to COX inhibitors.
The ductus arteriosus (DA) is a fetal vessel connecting the pulmonary artery to the descending aorta, which is of major functional importance for the integrity of the fetal circulation (1–6). Morphological and functional maturation of the DA during fetal life prepares the vessel for functional and then anatomical postnatal closure. This closure is a key event in the transition to extrauterine life. However, when preterm birth interrupts physiological maturation, infants are exposed to patent ductus DA (PDA) due to underdeveloped ductal closure mechanisms (1–6).
PDA in very and extremely preterm infants (i.e., with gestational age less than 32 weeks) is a persistent dilemma for neonatal medicine, as well as a potential morbidity and mortality contributor when hemodynamically significant (1, 7, 8). Although the therapeutic approach has changed and continues to change over the years, the classical pharmacological treatment of PDA is based on cyclooxygenase (COX) inhibitors, such as indomethacin and ibuprofen (9–12). Paracetamol has been added to these drugs in recent years (9).
Low gestational age (GA) is the main risk factor for both failure of spontaneous and pharmacologic closure of the DA (2, 13–15) but there is a growing recognition that the pathologic conditions that trigger preterm birth may play a relevant role in in the incidence of PDA, as well as in the response to COX inhibitors (16–20). The two main pathophysiologic pathways, or endotypes, leading to very/extremely preterm birth are 1) infection/inflammation and 2) dysfunctional placentation (21–24). Previous studies by our group and other investigators have systematically reviewed the association between these two endotypes and a number of complications of prematurity including PDA (15, 22, 25–31). In these meta-analyses, the infectious-inflammatory endotype was represented by chorioamnionitis, while the placental dysfunction endotype was represented by hypertensive disorders of pregnancy (HDP) and fetal growth restriction (15, 22, 25–31).
Individual studies suggest that prenatal conditions such as chorioamnionitis (16, 17) or HDP (18–20) may affect the therapeutic response to COX inhibitors. However, these findings have not been systematically reviewed. Our aim in the present study is to fill this gap in the literature by conducting a systematic review and meta-analysis on the association between endotype of prematurity and pharmacological closure of PDA in very and extremely preterm infants.
The methodology of the present study is based on that used in our previous meta-analyses on the association of several risk factors and the incidence of PDA and/or the response to pharmacological treatment of PDA (26, 27, 32–34). The study was performed and reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) and meta-analysis of observational studies in epidemiology (MOOSE) guidelines [15]. Review protocol was registered in the PROSPERO international register of systematic reviews (ID = CRD42018095509). The Population, Exposure, Comparison and Outcome (PECO) question was: Do very/extremely preterm infants (P) exposed to chorioamnionitis, HDP, or growth restriction during pregnancy (E) have a different rate of PDA closure in response to treatment with COX inhibitors (O) than infants with no history of exposure (C)?
A comprehensive literature search was undertaken using the PubMed and EMBASE databases. The search terms involved various combinations of the following key words: ductus arteriosus, patent ductus arteriosus, PDA, Patency of the Ductus Arteriosus, Ductus Botalli, treatment, pharmacologic(al) closure, indomethacin, ibuprofen, paracetamol, acetaminophen, cyclooxygenase, COX, chorioamnionitis, intrauterine infection, intrauterine inflammation, antenatal infection, antenatal inflammation, preeclampsia, IUGR, growth restriction, growth retardation, restricted growth, fetal growth, fetus growth, placental dysfunction, placental insufficiency, chronic hypoxia, chronic hypoxemia, small for gestational age, small for date, SGA, gestational hypertension, maternal hypertension, hellp syndrome, hypertensive disorders, toxemia, hypertensive disorders of pregnancy. No language limit was applied. The literature search was updated up to March 2022. Narrative reviews, systematic reviews, case reports, letters, editorials, and commentaries were excluded, but read to identify potential additional studies. Additional strategies to identify studies included manual review of reference lists from key articles that fulfilled our eligibility criteria, use of “related articles” feature in PubMed, and use of the “cited by” tool in Web of Science and Google scholar.
Studies were included if they had a prospective or retrospective design, examined infants with GA below 32 weeks and reported primary data that could be used to measure the association between pharmacological closure of PDA and exposure to chorioamnionitis (clinical or histological), HDP (including pregnancy-induced hypertension, preeclampsia and eclampsia) or fetal growth restriction. As we did in our previous meta-analyses, we accepted small for gestational age (SGA) as a proxy for fetal growth restriction (22, 27). Regarding response to drug treatment, when a study reported on several treatment courses, only the final response was taken into account. To identify relevant studies, two reviewers (GG-L and MB-L) independently screened the results of the searches and applied inclusion criteria using a structured form. Discrepancies were resolved by the third reviewer (EV).
Two investigators (GG-L and MB-L) extracted data on study design, demographics, and response to treatment. Another investigator (EV) checked the data extraction for completeness and accuracy. Methodological quality was assessed using the Newcastle-Ottawa Scale (NOS) for cohort studies (15). This scale assigns a maximum of 9 points (4 for selection, 2 for comparability, and 3 for outcome). NOS scores ≥ 7 were considered high-quality studies (low risk of bias), and scores of 5 to 6 denoted moderate quality (moderate risk of bias) (15).
Studies were combined and analyzed using comprehensive meta-analysis V3.0 software (Biostat Inc., Englewood, NJ, USA). The odds ratio (OR) with 95% confidence interval (CI) was calculated with a random-effects model and subgroups were combined with a mixed-effects model (35). Statistical heterogeneity was assessed by Cochran's Q statistic and by the I2 statistic. I2 was interpreted on the basis of Higgins and Thompson criteria, where 25%, 50%, and 75% correspond to low, moderate, and high heterogeneity, respectively (36). Potential sources of heterogeneity were assessed through subgroup analysis and/or random effects (method of moments) univariate meta-regression analysis as previously described (16, 17). For both categorical and continuous covariates, the R2 analog, defined as the total between-study variance explained by the moderator, was calculated based on the meta-regression matrix. Predefined sources of heterogeneity included the following characteristics of cohorts: definition of exposure (clinical or histological chorioamnionitis, HDP definition, and growth restriction or SGA definition), mean or median GA, median year of birth, and drug used for PDA treatment. We used the Egger's regression test and funnel plots to assess publication bias. Subgroup analyses, meta-regression, and publication bias assessment were performed only when there were at least ten studies in the meta-analysis. A probability value of less than 0.05 (0.10 for heterogeneity) was considered statistically significant.
The flow diagram of the search process is shown in Supplementary Figure S1. Of 964 potentially relevant studies, 30 (including 12,639 infants) were included (13, 16–20, 37–60). Their characteristics are summarized in Supplementary Table S1. Focusing on exposure, 13 studies provided data on chorioamnionitis, 17 on HDP, and 17 on SGA. We found no studies that evaluated IUGR (i.e., assessment of growth during fetal period). Nineteen studies reported on indomethacin (16–20, 37–50), five on ibuprofen (51–55) and in six studies both drugs were used (13, 56–60). The quality score of each study according to the Newcastle-Ottawa Scale is depicted in Supplementary Table S1. All studies received at least 7 points indicating a low risk of bias.
Meta-analysis could not demonstrate a significant association between chorioamnionitis and response to pharmacological treatment of PDA (OR 0.746, 95% CI 0.472–1.181, p = 0.211) (Figure 1). This lack of significant effect of chorioamnionitis was observed for both clinical (OR 0.838, 95% CI 0.492–1.428, p = 0.516) and histological chorioamnionitis (OR 0.536, 95% CI 0.217–1.319, p = 0.175). Meta-regression showed that the difference in effect size between clinical and histological chorioamnionitis was not statistically significant (p = 0.400). In contrast, meta-analysis showed a significant association between exposure to HDP and pharmacological closure of PDA (OR 1.413, 95% CI 1.102–1.811, p = 0.006) (Figure 2). Subgroup analysis showed that this significant association was maintained in the any HDP group (OR 1.392, 95% CI 1.004–1.931, p = 0.006) but not in the preeclampsia group (OR 1.441, 95% CI 0.984–2.109, p = 0.061). However, meta-regression showed that the difference in effect size between any HDP and preeclampsia was not statistically significant (p = 0.962). Finally, the meta-analysis could not demonstrate a significant association between being SGA and response to pharmacological treatment of PDA (OR 1.197, 95% CI 0.957–1.497, p = 0.115) (Figure 3). This lack of significant association was consistent for all SGA definitions (Figure 3). Meta-regression showed that the difference in effect size between the different SGA definitions was not statistically significant (p = 0.852). Neither visual inspection nor Egger's test suggested the presence of publication or selection bias for none of the three meta-analyses (Supplementary Figure S2).
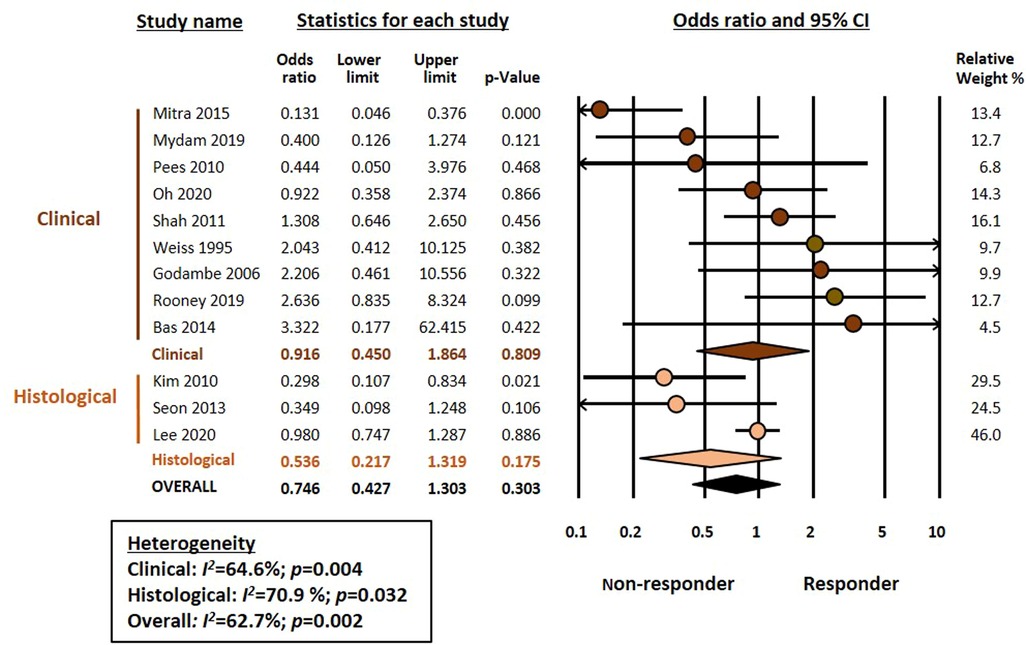
Figure 1. Meta-analysis on the association between chorioamnionitis and pharmacological closure of patent ductus arteriosus.
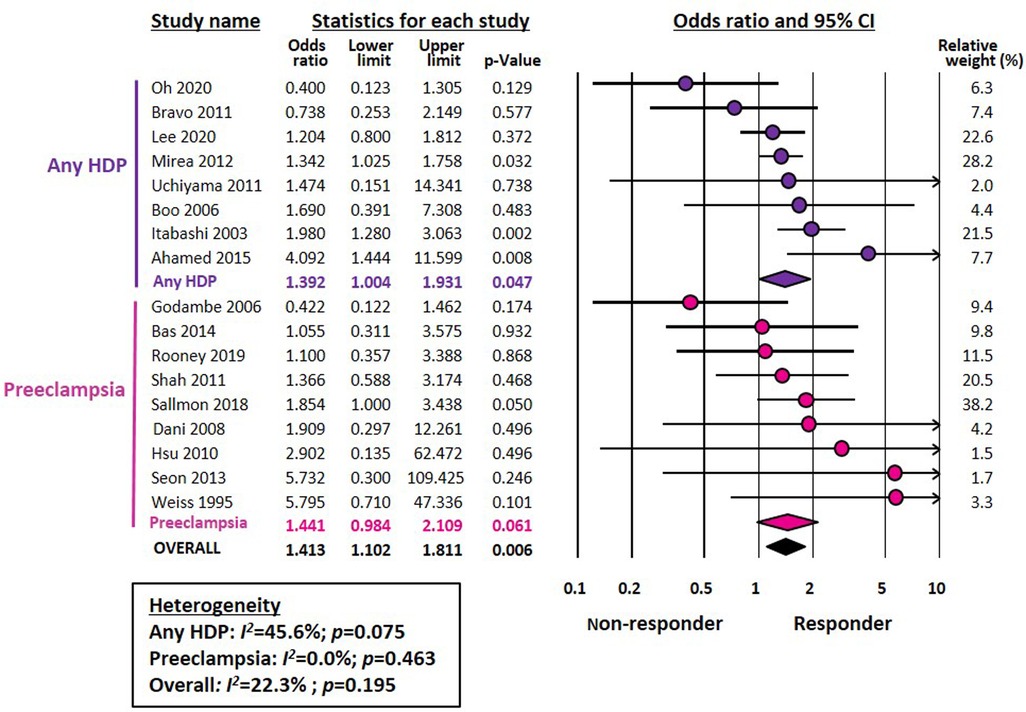
Figure 2. Meta-analysis on the association between hypertensive disorders of pregnancy (HDP) and pharmacological closure of patent ductus arteriosus.
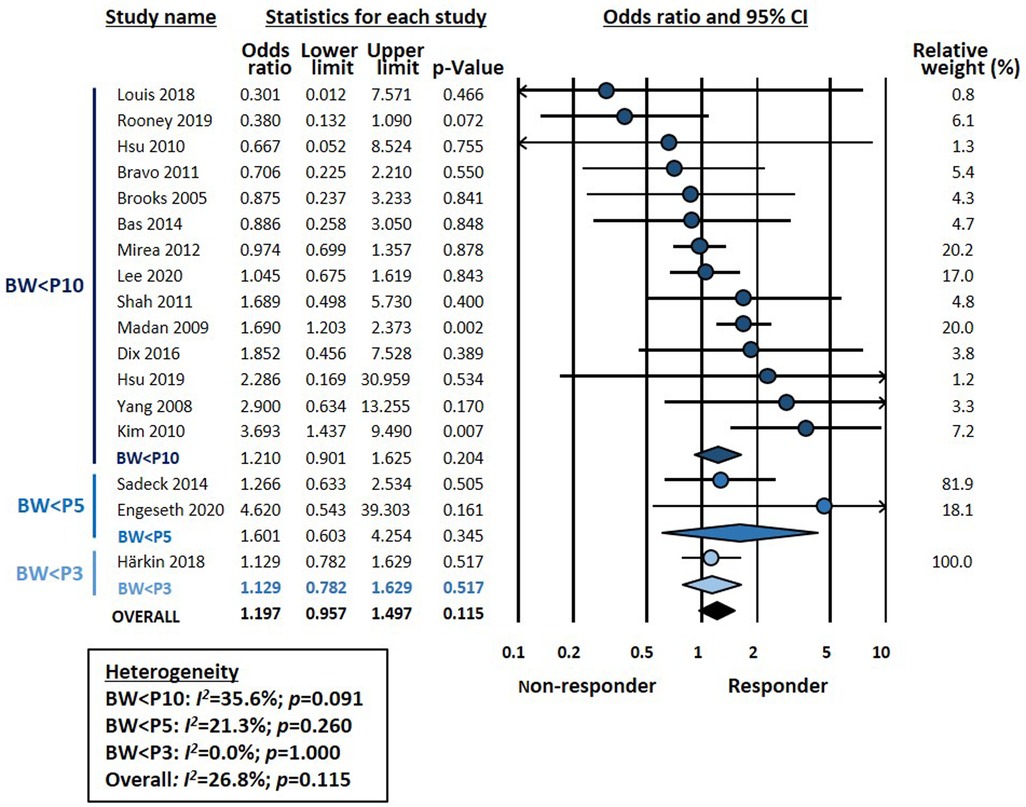
Figure 3. Meta-analysis on the association between being small for gestational age (SGA) and pharmacological closure of patent ductus arteriosus.
In addition to the subgroup analysis based on the definition of the different exposures (Figures 1–3), we conducted a second analysis based on the drug used to treat PDA. As shown in Table 1 and Supplementary Figure S3, the association between HDP and pharmacologic closure of PDA was significant only for indomethacin but not for ibuprofen or for studies using either indomethacin or ibuprofen. However, meta-regression showed that this difference between the drugs was not statistically significant (P = 404, Supplementary Figure S3). In the chorioamnionitis and SGA meta-analyses, no significant association with any of the drugs was detected (Table 1, Supplementary Figure S3).
We also analyzed by meta-regression how the median year of the cohort (Table 2, Supplementary Figure S4) or the gestational age of the included infants (Table 2, Supplementary Figure S5) could influence the effect size of the different meta-analyses. The only significant finding from these meta-regressions (Table 2, Supplementary Figure S4) was the correlation between the median year of the cohort and the effect size of the association between SGA and drug response (p = 0.008). As the cohort became more modern, the effect size of the association significantly decreased (Table 2, Supplementary Figure S4).
To the best of our knowledge, the present study is the first systematic review and meta-analysis examining the effect of endotype of prematurity on the response to pharmacological treatment of PDA in very or extremely preterm infants. We analyzed three pathological conditions (chorioamnionitis, HDP, and SGA) and observed that HDP was associated with a higher rate of pharmacologic closure of PDA. Moreover, subgroup analysis showed that the association between HDP and response to treatment was particularly significant for indomethacin. However, the relatively small number of studies and the moderate degree of heterogeneity that was present in some of the meta-analyses limit our results.
As mentioned in the introduction, the different endotypes of prematurity are not only the trigger for preterm birth but also induce a different pathophysiological environment for the development of fetal organs and systems. Thus, the infectious-inflammatory endotype is characterized by the development of a systemic inflammatory response with elevated cytokine levels (21, 23, 24). In turn, the placental dysfunction endotype is characterized by chronic hypoxia and imbalance of pro- and anti-angiogenic factors (21–24). Both pathophysiological pathways could plausibly affect the normal development of the DA resulting in delay or failure of its spontaneous or pharmacological closure.
Peri- and postnatal infection is classically considered a key risk factor for PDA (16, 17, 61–64). Two previous meta-analyses showed a significant association between chorioamnionitis and risk of developing PDA in very preterm infants (26, 31). However, a significant proportion of the risk appears to be associated with the lower GA of infants with chorioamnionitis compared to those not exposed to the insult (26). Although intrauterine infection is associated with increased COX expression and prostaglandin production (17, 61, 63, 65), the present meta-analysis could not demonstrate an association between chorioamnionitis and response to COX inhibitors. Nevertheless, the low number of studies reporting on histological chorioamnionitis limits our results. Moreover, histological chorioamnionitis have been dichotomized without taking into account that there are different grades of the condition (66, 67). In our previous meta-analysis on the association between chorioamnionitis and risk of developing PDA, we could perform a sub-analysis comparing fetal inflammation (i.e., funisitis) with maternal inflammation (i.e., chorioamnionitis without funisitis). We did not observe an increased risk of PDA in the funisitis group (26). Unfortunately, for the present analysis we could only find three studies that reported on histological chorioamnionitis (17, 55, 59) and, of these, only one reported data on funisitis (55). The data from that single study do not suggest that funisitis significantly increases the risk of non-response to pharmacological treatment of PDA (OR 0.46, 95% CI 0.14–1.52) (55).
The only antenatal pathology for which we have found an association with pharmacological closure of the DA was HDP. That HDP may affect the incidence of PDA has been demonstrated in several cohort studies (68–70). However, a recent meta-analysis, including eight studies, found no significant association between HDP and the risk of developing PDA (15). There are several potential explanations for the higher rate of spontaneous and pharmacologic ductal closure in preterm infants with intrauterine exposure to HDP. Women with HDP are likely to deliver before the onset of natural labor and therefore the production of prostaglandins is lower than that of pregnant women in labor (71). In addition, preterm infants born due to HDP tend to have higher gestational ages than those born due to chorioamnionitis (22). This leads to higher clinical stability and lower incidence of respiratory complications (22, 23). Nevertheless, preterm birth is by definition a pathological condition. Therefore, there is no “healthy control group” to compare with. The positive effects of HDP on pharmacological closure of the DA may reflect not the direct action of HDP but the absence, or at least attenuated presence, of an infectious process. Although placental dysfunction in preeclampsia is accompanied by an inflammatory response with release of cytokines such as tumor necrosis factor-α and interleukin-6 (72, 73), the levels of proinflammatory mediators are lower than those in chorioamnionitis (74, 75).
Subgroup analysis showed that the association between HDP and higher rate of pharmacologic ductal closure was particularly significant for indomethacin. However, meta-regression showed that the difference between the drugs was not statistically significant. Interestingly, Louis et al. reported that the rate of ductal closure induced by prophylactic indomethacin was significantly higher in the offspring of HDP mothers (76). With our present results, we can only speculate on the potential mechanisms responsible for this difference between indomethacin and ibuprofen. A Cochrane meta-analysis concluded that ibuprofen was as effective as indomethacin for PDA closure, whereas the former reduced the risk of necrotizing enterocolitis and transient renal insufficiency, when compared with indomethacin (77). The choice of indomethacin or ibuprofen to treat PDA is highly variable among neonatologists and is often influenced by non-clinical factors such as difficulties with drug supply (10–12). Nevertheless, there are pharmacokinetic, pharmacodynamic and pharmacogenetic differences between ibuprofen and indomethacin that may account for a different response in particular subgroups of preterm infants (78–80).
The third condition in which we have analyzed pharmacological ductal closure is fetal growth restriction. It should be noted that these results were limited because all included studies reported data on SGA and not IUGR. Although the terms SGA and IUGR are often used synonymously, SGA is a statistical definition based on BW, with the 10th percentile as the most frequently used cut-off (81–83). Therefore, the term SGA also encompasses constitutionally small infants without growth restriction (81–83). On the other hand, infants with pathologic growth restriction may have a BW above the 10th percentile (81–83). In addition, prenatal congenital infections may account for a percentage of cases of IUGR and preterm SGA infants are more prone to postnatal infection than their GA-matched appropriately grown controls (84, 85). Therefore, infection/inflammation may also be present in SGA infants.
In a recent meta-analysis, we investigated the association between SGA/IUGR and incidence of PDA. Although we observed a negative association, i.e., the rate of any PDA was lower in the growth-restricted group, this association only involved the subgroup in which SGA was defined using the 10th percentile threshold (27). Moreover, when examining the subgroup of studies that used a definition for growth restriction that went beyond BW for GA (i.e., assessment of fetal growth or presence of abnormal Doppler), meta-analysis could not find a significant association with PDA (27). In addition, we could not find a significant association between SGA/IUGR and the development of hemodynamically significant PDA (27). In the present meta-analysis, we could only find studies focusing on SGA infants, mostly defined by the 10th percentile cutoff. Although very limited by this fact and the low number of studies, our data do not suggest that being SGA affects the rate of pharmacological closure of PDA.
It may be noteworthy that of the two proxies we used to represent placental dysfunction (HDP and SGA), only HDP were significantly associated with pharmacological closure of PDA. First argument that can be used to explain this difference is that not all HDP induce growth retardation and not all SGA infants are smaller because of HDP. In previous meta-analyses, we have shown that the positive association between HDP and SGA is very strong (OR 4.70, 95% CI 3.57–6.17), whereas the associations between chorioamnionitis and HDP (OR 0.11, 95% CI 0.08-0.16) or SGA (OR 0.35, 95% CI 0.24–0.51) were strongly negative (22). Thus, although HDP and SGA are strongly associated, there is a proportion of infants who do not present with both disorders or even who present chorioamnionitis as well. In two previous meta-analyses, we used these same proxies (HDP and SGA) to investigate the association between placental dysfunction and mortality (86) as well as between placental dysfunction and BPD (22). In the second case, the SGA group showed a positive association with BPD, while HDP was not significantly associated with the condition (22). Interestingly, when we analyzed the association between endotype of prematurity and mortality, we observed that the SGA group had a higher risk of mortality (OR 1.68, 95% CI 1.38–2.04) while HDP had a “protective” effect on the mortality of very preterm infants (OR 0.74, 95% CI 0.64–0.86) (86). Meta-analysis also showed a positive mortality odds for chorioamnionitis (OR 1.43, 95% CI 1.25–1.62) (86). Taken together, these data suggest that when HDP-induced placental dysfunction reaches a degree of severity sufficient to affect fetal growth, the risk of worsening prematurity outcome is increased. On the contrary, when HDP is not accompanied by growth retardation, the prognosis is more favorable and even a certain “protective” effect is observed when compared with chorioamnionitis-induced prematurity.
Neonatology is a medical specialty in constant evolution and this is also reflected in the diagnostic and therapeutic approach to PDA. Neonatologists have been debating for decades what is a hemodynamically significant PDA, what are the health consequences of the presence of a ductal shunt for the preterm newborn and when and how PDA should be treated (1, 7, 8). As a result of this debate, there has been a transition from advocating that all PDA should be treated, with an intermediate step in which only targeted group were treated, to a current trend of therapeutic nihilism. That is, the consideration of PDA in very preterm infants as a “physiological” condition whose treatment produces more harm than benefit (1, 7, 8). This may influence our results as the number of infants treated has probably decreased with the passing of time. We have analyzed by meta-regression whether the association between endotype of prematurity and pharmacological closure of the DA has changed over the years. We have found that as cohorts become more contemporary, the response to COX inhibitors in SGA infants tends to decrease. The course of time does not seem to have affected pharmacological ductal closure in infants exposed to chorioamnionitis or HDP. However, it should be noted that our meta-regression analysis is based on few studies. The minimum number of trials per covariate in meta-regression analyses required to minimize the risk of overfitting is unknown but it has been suggested a minimum of 10 studies per examined covariate (87, 88). The meta-regressions presented here include 15–16 studies and are therefore just above the minimum recommended threshold. Therefore, they have mainly an exploratory and hypothesis-generating value.
In conclusion, our data suggest that the pathological condition that triggers prematurity may alter not only the incidence of PDA but also the response to pharmacological treatment. Our meta-analysis further supports the growing evidence that not all preterm infants are identical even if they are of the same gestational age. Therefore, one size fits all is no longer an appropriate approach to perinatal medicine (23, 89–91). Personalized medicine requires an adequate characterization of endotypes and clinical phenotypes so that each infant can receive the therapeutic approach best suited to his or her individual characteristics. Finally, very recent evidence from a randomized controlled trial suggests that expectant management of PDA in extremely preterm infants was non-inferior to ibuprofen treatment with respect to moderate-to severe bronchopulmonary dysplasia, necrotizing enterocolitis, or mortality (92). Further research is warranted to elucidate whether the etiology or endotype of prematurity affects the outcome differently when PDA is managed with a non-pharmacological approach.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
G G-L and M B-L selected studies for inclusion, collected data, contributed to the statistical analysis and interpretation of the results, collaborated in the preparation of the graphs and tables, and reviewed and revised the manuscript. EV conceptualized and designed the study, performed the search, supervised data collection, planned and performed the statistical analysis, and wrote the drafts of the manuscript. All authors contributed to the article and approved the submitted version.
This research was partially funded by FUNDACION CANARIA COLEGIO DE MEDICOS DE LAS PALMAS, Grant No. 26/2021.
We thank Ana Martinez-Olaizola, MSc for her technical and administrative support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1078506/full#supplementary-material.
1. Hundscheid T, Onland W, Van Overmeire B, Dijk P, van Kaam AH, Dijkman KP, et al. Early treatment versus expectative management of patent ductus arteriosus in preterm infants: a multicentre, randomised, non-inferiority trial in Europe (BeNeDuctus trial). BMC Pediatr. (2018) 18(1):262. doi: 10.1186/s12887-018-1215-7
2. Reese J, Laughon MM. The patent ductus arteriosus problem: infants who still need treatment. J Pediatr. (2015) 167(5):954–6. doi: 10.1016/j.jpeds.2015.08.023
3. Sallmon H, Koehne P, Hansmann G. Recent advances in the treatment of preterm newborn infants with patent ductus arteriosus. Clin Perinatol. (2016) 43(1):113–29. doi: 10.1016/j.clp.2015.11.008
4. Villamor E, Moreno L, Mohammed R, Pérez-Vizcaíno F, Cogolludo A. Reactive oxygen species as mediators of oxygen signaling during fetal-to-neonatal circulatory transition. Free Radic Biol Med. (2019) 142:82–96. doi: 10.1016/j.freeradbiomed.2019.04.008
5. Stoller JZ, DeMauro SB, Dagle JM, Reese J. Current perspectives on pathobiology of the ductus arteriosus. J Clin Exp Cardiolog. (2012) 8(1):S8–001. doi: 10.4172/2155-9880.S8-001
6. Gillam-Krakauer M, Reese J. Diagnosis and management of patent ductus arteriosus. Neoreviews. (2018) 19(7):e394–402. doi: 10.1542/neo.19-7-e394
7. de Waal K, Prasad R, Kluckow M. Patent ductus arteriosus management and the drift towards therapeutic nihilism–what is the evidence? Seminars Semin Fetal Neonatal Med. (2021) 26:101219. doi: 10.1016/j.siny.2021.101219
8. El-Khuffash A, Rios DR, McNamara PJ. Toward a rational approach to patent ductus arteriosus trials: selecting the population of interest. J Pediatr. (2021) 233:11–3. doi: 10.1016/j.jpeds.2021.01.012
9. Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki AA, et al. Association of placebo, indomethacin, ibuprofen, and Acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. Jama. (2018) 319(12):1221–38. doi: 10.1001/jama.2018.1896
10. Guimarães H, Rocha G, Tomé T, Anatolitou F, Sarafidis K, Fanos V. Non-steroid anti-inflammatory drugs in the treatment of patent ductus arteriosus in European newborns. J Matern Fetal Neonatal Med. (2009) 22(sup3):77–80. doi: 10.1080/14767050903198314
11. Slaughter JL, Reagan PB, Bapat RV, Newman TB, Klebanoff MA. Nonsteroidal anti-inflammatory administration and patent ductus arteriosus ligation, a survey of practice preferences at US children’s hospitals. Eur J Pediatr. (2016) 175(6):775–83. doi: 10.1007/s00431-016-2705-y
12. Slaughter JL, Reagan PB, Newman TB, Klebanoff MA. Comparative effectiveness of nonsteroidal anti-inflammatory drug treatment vs no treatment for patent ductus arteriosus in preterm infants. JAMA Pediatr. (2017) 171(3):e164354-e. doi: 10.1001/jamapediatrics.2016.4354
13. Sallmon H, Weber SC, Dirks J, Schiffer T, Klippstein T, Stein A, et al. Association between platelet counts before and during pharmacological therapy for patent ductus arteriosus and treatment failure in preterm infants. Front Pediatr. (2018) 6:41. doi: 10.3389/fped.2018.00041
14. Weichert J, Hartge DR, Axt-Fliedner R. The fetal ductus arteriosus and its abnormalities—a review. Congenit Heart Dis. (2010) 5(5):398–408. doi: 10.1111/j.1747-0803.2010.00424.x
15. Liu C, Zhu X, Li D, Shi Y. Related factors of patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. Front Pediatr. (2021) 8:605879. doi: 10.3389/fped.2020.605879
16. Mitra S, Wahab MGA. Indomethacin dose-interruption and maternal chorioamnionitis are risk factors for indomethacin treatment failure in preterm infants with patent ductus arteriosus. J Clin Neonatol. (2015) 4(4):250. doi: 10.4103/2249-4847.165688
17. Kim ES, Kim E-K, Choi CW, Kim H-S, Kim BI, Choi J-H, et al. Intrauterine inflammation as a risk factor for persistent ductus arteriosus patency after cyclooxygenase inhibition in extremely low birth weight infants. J Pediatr. (2010) 157(5):745–50. e1. doi: 10.1016/j.jpeds.2010.05.020
18. Ahamed M, Verma P, Lee S, Vega M, Wang D, Kim M, et al. Predictors of successful closure of patent ductus arteriosus with indomethacin. J Perinatol. (2015) 35(9):729–34. doi: 10.1038/jp.2015.33
19. Itabashi K, Ohno T, Nishida H. Indomethacin responsiveness of patent ductus arteriosus and renal abnormalities in preterm infants treated with indomethacin. J Pediatr. (2003) 143(2):203–7. doi: 10.1067/S0022-3476(03)00303-2
20. Mirea L, Sankaran K, Seshia M, Ohlsson A, Allen AC, Aziz K, et al. Treatment of patent ductus arteriosus and neonatal mortality/morbidities: adjustment for treatment selection bias. J Pediatr. (2012) 161(4):689–94. e1. doi: 10.1016/j.jpeds.2012.05.007
21. McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. (2008) 168(9):980–9. doi: 10.1093/aje/kwn202
22. Pierro M, Villamor-Martinez E, van Westering-Kroon E, Alvarez-Fuente M, Abman SH, Villamor E. Association of the dysfunctional placentation endotype of prematurity with bronchopulmonary dysplasia: a systematic review, meta-analysis and meta-regression. Thorax. (2022) 77:268–75. doi: 10.1136/thoraxjnl-2020-216485
23. Pierro M, Van Mechelen K, van Westering-Kroon E, Villamor-Martínez E, Villamor E. Endotypes of prematurity and phenotypes of bronchopulmonary dysplasia: toward personalized neonatology. J Pers Med. (2022) 12(5):687. doi: 10.3390/jpm12050687
24. Parsons A, Netsanet A, Seedorf GJ, Abman SH, Taglauer ES. Understanding the role of placental pathophysiology in the development of bronchopulmonary dysplasia (BPD). Am J Physiol. (2022) 323(6):L651–L8. doi: 10.1152/ajplung.00204.2022
25. Villamor-Martinez E, Álvarez-Fuente M, Ghazi AM, Degraeuwe P, Zimmermann LJ, Kramer BW, et al. Association of chorioamnionitis with bronchopulmonary dysplasia among preterm infants: a systematic review, meta-analysis, and meta-regression. JAMA Netw Open. (2019) 2(11):e1914611-e. doi: 10.1001/jamanetworkopen.2019.14611
26. Behbodi E, Villamor-Martínez E, Degraeuwe PL, Villamor E. Chorioamnionitis appears not to be a risk factor for patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. Sci Rep. (2016) 6(1):1–10. doi: 10.1038/srep37967
27. Villamor-Martinez E, Kilani MA, Degraeuwe PL, Clyman RI, Villamor E. Intrauterine growth restriction and patent ductus arteriosus in very and extremely preterm infants: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2019) 10:58. doi: 10.3389/fendo.2019.00058
28. Villamor-Martinez E, Cavallaro G, Raffaeli G, Mohammed Rahim OM, Gulden S, Ghazi AM, et al. Chorioamnionitis as a risk factor for retinopathy of prematurity: an updated systematic review and meta-analysis. PloS one. (2018) 13(10):e0205838. doi: 10.1371/journal.pone.0205838
29. Villamor-Martinez E, Fumagalli M, Mohammed Rahim O, Passera S, Cavallaro G, Degraeuwe P, et al. Chorioamnionitis is a risk factor for intraventricular hemorrhage in preterm infants: a systematic review and meta-analysis. Front Physiol. (2018) 9:1253. doi: 10.3389/fphys.2018.01253
30. Villamor-Martinez E, Lubach GA, Rahim OM, Degraeuwe P, Zimmermann LJ, Kramer BW, et al. Association of histological and clinical chorioamnionitis with neonatal sepsis among preterm infants: a systematic review, meta-analysis, and meta-regression. Front Immunol. (2020) 11:972. doi: 10.3389/fimmu.2020.00972
31. Park HW, Choi Y-S, Kim KS, Kim S-N. Chorioamnionitis and patent ductus arteriosus: a systematic review and meta-analysis. PloS one. (2015) 10(9):e0138114. doi: 10.1371/journal.pone.0138114
32. Simon SR, Van Zogchel L, Bas-Suárez MP, Cavallaro G, Clyman RI, Villamor E. Platelet counts and patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. Neonatology. (2015) 108(2):143–51. doi: 10.1159/000431281
33. González-Luis G, Ghirardello S, Bas-Suárez P, Cavallaro G, Mosca F, Clyman RI, et al. Platelet counts and patent ductus arteriosus in preterm infants: an updated systematic review and meta-analysis. Front Pediatr. (2021) 965. doi: 10.3389/fped.2020.613766
34. Borges-Lujan M, Gonzalez-Luis GE, Roosen T, Huizing MJ, Villamor E. Sex differences in patent ductus arteriosus incidence and response to pharmacological treatment in preterm infants: a systematic review, meta-analysis and meta-regression. J Pers Med. (2022) 12(7):1143. doi: 10.3390/jpm12071143
35. Borenstein M, Higgins J. Meta-analysis and subgroups. Prev Sci. (2013) 14(2):134–43. doi: 10.1007/s11121-013-0377-7
36. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
37. Boo NY, Mohd-Amin I, Bilkis A, Yong-Junina F. Predictors of failed closure of patent ductus arteriosus with indomethacin. Singapore Med J. (2006) 47(9):763.16924357
38. Brooks J, Travadi J, Patole S, Doherty D, Simmer K. Is surgical ligation of patent ductus arteriosus necessary? The western Australian experience of conservative management. Arch Dis Child. (2005) 90(3):F235–FF9. doi: 10.1136/adc.2004.057638
39. Dix L, Molenschot M, Breur J, de Vries W, Vijlbrief D, Groenendaal F, et al. Cerebral oxygenation and echocardiographic parameters in preterm neonates with a patent ductus arteriosus: an observational study. Arch Dis Child. (2016) 101(6):F520–F6. doi: 10.1136/archdischild-2015-309192
40. Engeseth MS, Engan M, Clemm H, Vollsæter M, Nilsen RM, Markestad T, et al. Voice and exercise related respiratory symptoms in extremely preterm born children after neonatal patent ductus arteriosus. Front Pediatr. (2020) 8:150. doi: 10.3389/fped.2020.00150
41. Godambe S, Newby B, Shah V, Shah PS. Effect of indomethacin on closure of ductus arteriosus in very-low-birthweight neonates 1. Acta Pædiatrica. (2006) 95(11):1389–93. doi: 10.1080/08035250600615150
42. Hsu J-H, Yang S-N, Chen H-L, Tseng H-I, Dai Z-K, Wu J-R. B-type natriuretic peptide predicts responses to indomethacin in premature neonates with patent ductus arteriosus. J Pediatr. (2010) 157(1):79–84. doi: 10.1016/j.jpeds.2009.12.045
43. Louis D, Wong C, Ye XY, McNamara PJ, Jain A. Factors associated with non-response to second course indomethacin for PDA treatment in preterm neonates. J Matern Fetal Neonatal Med. (2018) 31(11):1407–11. doi: 10.1080/14767058.2017.1317736
44. Madan JC, Kendrick D, Hagadorn JI III, Frantz ID, Health NIoC, Network HDNR. Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics. (2009) 123(2):674. doi: 10.1542/peds.2007-2781
45. Mydam J, Rastogi A, Naheed ZJ. Base excess and hematocrit predict response to indomethacin in very low birth weight infants with patent ductus arteriosus. Ital J Pediatr. (2019) 45(1):1–9. doi: 10.1186/s13052-019-0706-y
46. Rooney SR, Shelton EL, Aka I, Shaffer CM, Clyman RI, Dagle JM, et al. CYP2C9* 2 is associated with indomethacin treatment failure for patent ductus arteriosus. Pharmacogenomics. (2019) 20(13):939–46. doi: 10.2217/pgs-2019-0079
47. Shah NA, Hills NK, Waleh N, McCurnin D, Seidner S, Chemtob S, et al. Relationship between circulating platelet counts and ductus arteriosus patency after indomethacin treatment. J Pediatr. (2011) 158(6):919–23. e1-2. doi: 10.1016/j.jpeds.2010.11.018
48. Uchiyama A, Nagasawa H, Yamamoto Y, Tatebayashi K, Suzuki H, Yamada K, et al. Clinical aspects of very-low-birthweight infants showing reopening of ductus arteriosus. Pediatr Int. (2011) 53(3):322–7. doi: 10.1111/j.1442-200X.2010.03251.x
49. Weiss H, Cooper B, Brook M, Schlueter M, Clyman R. Factors determining reopening of the ductus arteriosus after successful clinical closure with indomethacin. J Pediatr. (1995) 127(3):466–71. doi: 10.1016/S0022-3476(95)70084-6
50. Yang C-Z, Lee J. Factors affecting successful closure of hemodynamically significant patent ductus arteriosus with indomethacin in extremely low birth weight infants. World J Pediatr. (2008) 4(2):91–6. doi: 10.1007/s12519-008-0017-7
51. Dani C, Bertini G, Corsini I, Elia S, Vangi V, Pratesi S, et al. The fate of ductus arteriosus in infants at 23–27 weeks of gestation: from spontaneous closure to ibuprofen resistance. Acta Paediatr. (2008) 97(9):1176–80. doi: 10.1111/j.1651-2227.2008.00871.x
52. Hsu K-H, Wu T-W, Wu I-H, Lai M-Y, Hsu S-Y, Huang H-W, et al. Baseline cardiac output and its alterations during ibuprofen treatment for patent ductus arteriosus in preterm infants. BMC Pediatr. (2019) 19(1):1–8. doi: 10.1186/s12887-018-1376-4
53. Oh SH, Lee BS, Jung E, Oh MY, Do H-J, Kim EA-R, et al. Plasma B-type natriuretic peptide cannot predict treatment response to ibuprofen in preterm infants with patent ductus arteriosus. Sci Rep. (2020) 10(1):1–7. doi: 10.1038/s41598-019-56847-4
54. Pees C, Walch E, Obladen M, Koehne P. Echocardiography predicts closure of patent ductus arteriosus in response to ibuprofen in infants less than 28 week gestational age. Early Hum Dev. (2010) 86(8):503–8. doi: 10.1016/j.earlhumdev.2010.06.012
55. Seon H-S, Lee J-B, Kim I-U, Kim S-H, Lee J-H, Kim D-H, et al. Association with ductus arteriosus closure by ibuprofen and intrauterine inflammation in very low birth weight infants. Korean J Perinatol. (2013) 24(3):158–67. doi: 10.14734/kjp.2013.24.3.158
56. Bas-Suárez MP, González-Luis GE, Saavedra P, Villamor E. Platelet counts in the first seven days of life and patent ductus arteriosus in preterm very low-birth-weight infants. Neonatology. (2014) 106(3):188–94. doi: 10.1159/000362432
57. Bravo Laguna MC. Evaluación del tratamiento farmacológico convencional para el cierre del ductus arterioso persistencte en el recién nacido pretérmino: impacto de nuevas líneas terapéuticas. 2011. https://dialnet.unirioja.es/servlet/dctes?codigo = 33240
58. Härkin P, Marttila R, Pokka T, Saarela T, Hallman M. Morbidities associated with patent ductus arteriosus in preterm infants. Nationwide cohort study. J Matern Fetal Neonatal Med. (2018) 31(19):2576–83. doi: 10.1080/14767058.2017.1347921
59. Lee JA, Sohn JA, Oh S, Choi BM. Perinatal risk factors of symptomatic preterm patent ductus arteriosus and secondary ligation. Pediatr Neonatology. (2020) 61(4):439–46. doi: 10.1016/j.pedneo.2020.03.016
60. Sadeck LS, Leone CR, Procianoy RS, Guinsburg R, Marba S, Martinez FE, et al. Effects of therapeutic approach on the neonatal evolution of very low birth weight infants with patent ductus arteriosus. J Pediatr (Rio J). (2014) 90(6):616–23. doi: 10.1016/j.jped.2014.04.010
61. Kajimura I, Akaike T, Minamisawa S. Lipopolysaccharide delays closure of the rat ductus arteriosus by induction of inducible nitric oxide synthase but not prostaglandin E2. Circ J. (2016) 80(3):703–11. doi: 10.1253/circj.CJ-15-1053
62. Green CA, Westreich D, Laughon MM, Stamilio DM, Strauss RA, Reese J, et al. Association of chorioamnionitis and patent ductus arteriosus in a national US cohort. J Perinatol. (2021) 41(1):119–25. doi: 10.1038/s41372-020-00866-x
63. Hu Y, Jin H, Jiang Y, Du J. Prediction of therapeutic response to cyclooxygenase inhibitors in preterm infants with patent ductus arteriosus. Pediatr Cardiol. (2018) 39(4):647–52. doi: 10.1007/s00246-018-1831-x
64. Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr. (1996) 128(4):470–8. doi: 10.1016/S0022-3476(96)70356-6
65. Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med. (2012) 17(1):12–9. doi: 10.1016/j.siny.2011.09.001
66. Torricelli M, Voltolini C, Toti P, Vellucci FL, Conti N, Cannoni A, et al. Histologic chorioamnionitis: different histologic features at different gestational ages. J Matern Fetal Neonatal Med. (2014) 27(9):910–3. doi: 10.3109/14767058.2013.846313
67. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. (2015) 213(4):S29–52. doi: 10.1016/j.ajog.2015.08.040
68. Shah D, Shenai J, Vaughn W. Neonatal outcome of premature infants of mothers with preeclampsia. J Perinatol. (1995) 15(4):264–7.8558332
69. Huang H-C, Yang H-I, Chou H-C, Chen C-Y, Hsieh W-S, Tsou K-I, et al. Preeclampsia and retinopathy of prematurity in very-low-birth-weight infants: a population-based study. PloS one. (2015) 10(11):e0143248.26588850
70. Yen T-A, Yang H-I, Hsieh W-S, Chou H-C, Chen C-Y, Tsou K-I, et al. Preeclampsia and the risk of bronchopulmonary dysplasia in VLBW infants: a population based study. PloS one. (2013) 8(9):e75168. doi: 10.1371/journal.pone.0075168
71. Wood EM, Hornaday KK, Slater DM. Prostaglandins in biofluids in pregnancy and labour: a systematic review. PloS one. (2021) 16(11):e0260115. doi: 10.1371/journal.pone.0260115
72. LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep. (2007) 9(6):480–5. doi: 10.1007/s11906-007-0088-1
73. Alston MC, Redman LM, Sones JL. An overview of obesity, cholesterol, and systemic inflammation in preeclampsia. Nutrients. (2022) 14(10):2087. doi: 10.3390/nu14102087
74. Stallmach T, Hebisch G, Joller H, Kolditz P, Engelmann M. Expression pattern of cytokines in the different compartments of the feto-maternal unit under various conditions. Reprod Fertil Dev. (1995) 7(6):1573–80. doi: 10.1071/RD9951573
75. Peltier MR. Immunology of term and preterm labor. Reprod Biol Endocrinol. (2003) 1(1):1–11. doi: 10.1186/1477-7827-1-122
76. Louis D, ElSayed YN, Ojah C, Alvaro R, Shah PS, Dunn M, et al. Predictors of PDA treatment in preterm neonates who had received prophylactic indomethacin. Am J Perinatol. (2018) 35(05):509–14. doi: 10.1055/s-0037-1608792
77. Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. (2018) 9. doi: 10.1002/14651858.CD003481.pub7
78. Smith CJ, Ryckman KK, Bahr TM, Dagle JM. Polymorphisms in CYP2C9 are associated with response to indomethacin among neonates with patent ductus arteriosus. Pediatr Res. (2017) 82(5):776–80. doi: 10.1038/pr.2017.145
79. Lewis TR, Shelton EL, Van Driest SL, Kannankeril PJ, Reese J. Genetics of the patent ductus arteriosus (PDA) and pharmacogenetics of PDA treatment. Semin Fetal Neonatal Med. (2018) 23(4):232–8. doi: 10.1016/j.siny.2018.02.006
80. Sallmon H, Akanbi S, Weber SC, Gratopp A, Rheinländer C, Koehne P. Ibuprofen and indomethacin differentially regulate vascular endothelial growth factor and its receptors in ductus arteriosus endothelial cells. Cardiol Young. (2018) 28(3):432–7. doi: 10.1017/S1047951117002311
81. Aucott SW, Donohue PK, Northington FJ. Increased morbidity in severe early intrauterine growth restriction. J Perinatol. (2004) 24(7):435–40. doi: 10.1038/sj.jp.7211116
82. Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol. (2011) 204(4):288–300. doi: 10.1016/j.ajog.2010.08.055
83. Gordijn S, Beune I, Thilaganathan B, Papageorghiou A, Baschat A, Baker P, et al. Consensus definition of fetal growth restriction: a delphi procedure. Ultrasound Obstet Gynecol. (2016) 48(3):333–9. doi: 10.1002/uog.15884
84. Hendrix N, Berghella V. Non-placental causes of intrauterine growth restriction. Semin Perinatol. (2008) 32(3):161–5. doi: 10.1053/j.semperi.2008.02.004
85. Longo S, Borghesi A, Tzialla C, Stronati M. IUGR And infections. Early Hum Dev. (2014) 90:S42–S4. doi: 10.1016/S0378-3782(14)70014-3
86. Hundscheid T, Villamor-Martínez E, Villamor E. Association between endotype of prematurity and mortality: a systematic review, meta-analysis and meta-regression. medRxiv. (2023). doi: 10.1101/2023.01.21.23284854
87. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Hoboken, NJ, USA: John Wiley & Sons (2019).
88. Geissbühler M, Hincapié CA, Aghlmandi S, Zwahlen M, Jüni P, da Costa BR.. most published meta-regression analyses based on aggregate data suffer from methodological pitfalls: a meta-epidemiological study. BMC Med Res Methodol. (2021) 21(1):1–9. doi: 10.1186/s12874-021-01310-0
89. De Luca D, Autilio C, Pezza L, Shankar-Aguilera S, Tingay DG, Carnielli VP. Personalized medicine for the management of RDS in preterm neonates. Neonatology. (2021) 118(2):127–38. doi: 10.1159/000513783
90. Fanni D, Ambu R, Gerosa C, Nemolato S, Castagnola M, Van Eyken P, et al. Cytochrome P450 genetic polymorphism in neonatal drug metabolism: Role and practical consequences towards a new drug culture in neonatology. London, England: SAGE Publications Sage UK (2014). 5–13.
91. Allegaert K, Cosaert K, van den Anker N. Neonatal formulations: the need for a tailored, knowledge driven approach. Curr Pharm Des. (2015) 21(39):5674–9. doi: 10.2174/1381612821666150901110207
Keywords: ductus arteriosus, chorioamnionitis, hypertensive disorders of pregnancy, preeclampsia, small for gestational age, endotypes, indomethacin, ibuprofen
Citation: Gonzalez-Luis Gema E, Borges-Lujan M and Villamor E (2023) Association between endotypes of prematurity and pharmacological closure of patent ductus arteriosus: A systematic review and meta-analysis. Front. Pediatr. 11:1078506. doi: 10.3389/fped.2023.1078506
Received: 24 October 2022; Accepted: 10 February 2023;
Published: 3 March 2023.
Edited by:
Mikko Hallman, University of Oulu, FinlandReviewed by:
Ömer Erdeve, Ankara University, Türkiye© 2023 Gonzalez-Luis, Borges-Lujan and Villamor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eduardo Villamor ZS52aWxsYW1vckBtdW1jLm5s
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.