- 1Shahid Akbarabadi Clinical Research & Development Unit (ShACRDU), Iran University of Medical Sciences (IUMS), Tehran, Iran
- 2Department of Pediatrics, Ali-Asghar Children’s Hospital, Iran University of Medical Sciences, Tehran, Iran
- 3Biomedical R&D Department, CAPIS Research and Development Co., Mons, Belgium
Background: Screening for critical congenital heart defects should be performed as early as possible and is essential for saving the lives of children and reducing the incidence of undetected adult congenital heart diseases. Heart malformations remain unrecognized at birth in more than 50% of neonates at maternity hospitals. Accurate screening for congenital heart malformations is possible using a certified and internationally patented digital intelligent phonocardiography machine. This study aimed to assess the actual incidence of heart defects in neonates. A pre-evaluation of the incidence of unrecognized severe and critical congenital heart defects at birth in our well-baby nursery was also performed.
Methods: We conducted the Neonates Cardiac Monitoring Research Project (ethics approval number: IR-IUMS-FMD. REC.1398.098) at the Shahid Akbarabadi Maternity Hospital. This study was a retrospective analysis of congenital heart malformations observed after screening 840 neonates. Using a double-blind format, 840 neonates from the well-baby nursery were randomly chosen to undergo routine clinical examinations at birth and digital intelligent phonocardiogram examinations. A pediatric cardiologist performed echocardiography for each neonate classified as having abnormal heart sounds using an intelligent machine or during routine medical examinations. If the pediatric cardiologist requested a follow-up examination, then the neonate was considered to have a congenital heart malformation, and the cumulative incidence was calculated accordingly.
Results: The incidence of heart malformations in our well-baby nursery was 5%. Furthermore, 45% of heart malformations were unrecognized in neonates at birth, including one critical congenital heart defect. The intelligent machine interpreted innocent murmurs as healthy heart sound.
Conclusion: We accurately and cost-effectively screened for congenital heart malformations in all neonates in our hospital using a digital intelligent phonocardiogram. Using an intelligent machine, we successfully identified neonates with CCHD and congenital heart defects that could not be detected using standard medical examinations. The Pouya Heart machine can record and analyze sounds with a spectral power level lower than the minimum level of the human hearing threshold. Furthermore, by redesigning the study, the identification of previously unrecognized heart malformations could increase to 58%.
1. Introduction
Congenital heart malformations are categorized as congenital heart defects (CHD) and critical CHD (CCHD). CHD and CCHD are the leading causes of birth defect-associated illnesses and deaths of infants (1, 2) and account for 10% of all congenital anomalies (3). However, approximately 30% of mortalities attributable to congenital malformations are caused by CCHD and CHD (4). CCHD and severe CHD occur in approximately 3 out of 1,000 neonates (5, 6). Despite antenatal screening processes using fetal echocardiography, more than 50% of heart malformations remain unrecognized in neonates at birth (7, 8). Previous studies have shown that the number of adult patients with CHD is remarkably high and that we are facing a “tsunami” in terms of adult CHD cases (9–11). Consequently, timely screening of the cardiac health of neonates is vital (12, 13).
Because of the limitations of the human auditory system (e.g., masking effect) and the presence of clicks and non-pathological heart sounds, some heart abnormalities may not produce an audible murmur (14, 15); therefore, screening for heart malformations using conventional auscultation is inaccurate. Echocardiography is one of the essential modalities for the diagnosis of congenital heart disease. It must be performed by a pediatric cardiologist for the patient's management; however, the diagnostic time is relatively long. Consequently, performing a Doppler ultrasound examination for every neonate is impractical; therefore, diagnosing all neonates for detecting congenital heart malformations remains debatable (5, 16, 17).
Screening for CHD in neonates and performing echocardiography examination for positive cases is essential to saving lives and reducing the incidence of undetected pediatric and adult congenital heart diseases. Most heart sounds in neonates are inherent additional sounds that are not indications of any disease; instead, they are merely sounds rooted in the physiology of newborn hearts called innocent murmurs (15). High pulmonary pressure, high heart rates, innocent murmurs, and other factors can lead to inaccuracies when screening for congenital heart malformations in neonates using routine medical examinations, resulting in high positive and negative error rates (2, 18). Occasionally, neonates with healthy hearts are referred to the subspecialty medical departments out of an abundance of caution. The referral of children to pediatric subspecialty cardiovascular centers is a significant concern for parents (19). However, it is vital to screen for severe CHD and CCHD in neonates as early as possible (19). CCHD screening using pulse oximetry can detect approximately 50% of CCHD malformations; however, the other 50% of CCHD (such as coarctation of the aorta) are less likely to be detected by pulse oximetry in neonates (20, 21). Mandated critical CHD screening using pulse oximetry reduces early infant deaths attributable to critical CHD by 33% (22). There are other limitations when using pulse oximetry screening, such as the effect of variations in altitude among geographical locations on the oxygen level and the timing of the procedure; it is recommended that pulse oximetry screening should be performed within 24 h of birth (23).
Based on our internationally patented technology (acoustical model of the heart sound spectrum of children), we developed the first accurate intelligent digital phonocardiogram for screening CHD in children in 2008 (24). The first version required the simultaneous acquisition of electrocardiogram signals to correctly segment the heart sounds of children. However, we have developed a new algorithm for the automatic segmentation of heart sounds of children without using electrocardiogram signals (25). The sensitivity, specificity, and accuracy of an intelligent machine for the detection and discrimination of cardiac murmurs were first published in 2013 (3). The first robust and commercialized intelligent phonocardiogram for automatically screening congenital heart malformations of children (aged 28 days to 12 years) became available for purchase in 2016 (26). After the publication of the report on our internationally patented, acoustical, mathematical model of the heart sound spectrum of children, some medical universities, in conjunction with engineering schools, have developed research versions of the intelligent phonocardiogram and validated its proper functioning (3, 27–31).
The actual incidence of heart malformations (CHD and CCHD) in neonates is subject to social and economic factors (e.g., gestational diabetes in mothers creates the risk of heart malformations in their children). The Doppler ultrasound examination allows accurate screening and diagnosis of CHD in newborns; however, considering the timing of the examinations and that the echo must be done by a pediatric cardiologist, performing this examination for all neonates is impractical, especially in less privileged areas. However, a precise screening for congenital heart malformations in neonates and referring the positive cases to the subspecialist is now possible using a neonatal version of the certified and internationally patented digital intelligent phonocardiography machine known as Pouya Heart.
This study aimed to assess the actual incidence of newborn heart malformations in our well-baby nursery. In addition, our well-baby nursery also performed a pre-evaluation of the incidence of unrecognized severe and critical CHD at birth. This is the first study to evaluate the incidence of heart malformations in neonates using intelligent phonocardiography.
2. Materials and methods
2.1. Study setting
The obstetrics and gynecology departments of our hospital are accredited for the 4-year residency period and are considered the largest specialized centers in the field in Iran. Our hospital is a tertiary referral center for patients requiring gynecological and neonatal services. Furthermore, it has the country's largest and most advanced neonatal intensive care unit, with 60 active beds. Approximately 12 neonates are born daily at our hospital. Each neonate undergoes a routine medical examination, including heart auscultation, which is performed by a neonatologist within the first 24 h of birth and again before discharge. The neonatologist used a Littman Classic II stethoscope (3M, St. Paul, MN). Newborns with possible CCHD or other types of disease requiring special care are transferred to the neonatal intensive care unit, whereas healthy neonates are transferred to the well-baby nursery. Those with possible CHD are also transferred to the well-baby nursery for an echocardiography examination. Echocardiography is also performed for neonates with gestational ages <34 weeks, those with weight <1,500 g, and those whose mothers had gestational diabetes or any other illness associated with the risk of causing heart malformations in their children.
2.2. Study design and population
This was a retrospective analysis of 840 neonates. Neonates were randomly selected from the well-baby nursery to undergo intelligent phonocardiogram screening in a double-blinded manner. Informed consent was obtained from their legal guardians according to the study protocol. The local ethics committee of the Shahid Akbarabadi Clinical Research and Development Unit approved the study (approval number: IR.IUMS.FMD.REC.1398.098), which was conducted according to the guidelines of the World Medical Association and Declaration of Helsinki.
A pediatric cardiologist performed echocardiography to obtain a diagnosis for each neonate classified as having abnormal heart sounds by the intelligent machine. The dataset included the following: pulse rate and strength; neonatologist auscultation results; results of pulse oximetry testing of four limbs; family medical history; intelligent phonocardiography examination results; echocardiography diagnosis results.
2.3. Intelligent phonocardiography machine
The intelligent phonocardiogram comprises an electronic stethoscope, a medical computer cart, and a medical monitor (25). It is controlled by an operator who must have a bachelor's degree in nursing or midwifery and has undergone 60 h of training on the use of the machine. The technician records 10 s of heart sounds from two different areas of one of the four thoracic sites of the newborn using the digital stethoscope of the intelligent machine. The results appear on the screen as normal or abnormal in real-time and on a printout. The heart sounds are recorded in a room with reduced background noise; there is no time limitation when performing the intelligent machine examination. Two consecutive examinations require an average of approximately 6 min. The machine considers the heart sounds healthy if the diagnostic results at both thoracic sites are normal. In addition, the intelligent machine automatically interprets innocent murmurs as healthy sounds (3, 24, 25).
2.4. Neonatal version of the intelligent phonocardiography machine: Pouya Heart
The intelligent phonocardiogram, Pouya Heart, comprises passive technology for the accurate screening of CHD and CCHD in neonates. It is based on our internationally patented technology (European patent number: 2249707; United States patent number: 8,649,854; Indian patent number: 333694; Iranian patent number: 139/02/25-69899; Russian patent number: 2512794; Chinese patent number: 1045312; Patent Cooperation Treaty number: EP2009/051410). The operating principle of the Pouya Heart machine is based on the spectral analysis of the heart sounds of neonates on an acoustical, mathematical model of the heart sound spectrum of neonates in conjunction with advanced artificial intelligence and machine-learning techniques (24). The intelligent machine classifies heart sounds into normal or abnormal in real-time. The minimum hearing level of the human ear differs depending on the frequency. Therefore, the Pouya Heart machine can record and analyze sounds with a spectral power level lower than the minimum level of the human hearing threshold.
The Pouya Heart machine is an intelligent heart sound spectrometer for neonates. A prism is an optical spectrometer that separates photons according to their frequencies and produces red, green, and blue lights. Any lesions, weaknesses, or stiffness in the heart, organs, or blood circulation have a spectral influence on the heart sound spectrum of neonates. When using auscultation for the heart of the neonate, a patent foramen ovale (PFO) is practically inaudible; however, the incidence of a small PFO is relatively high for newborns (32, 33). A PFO influences the heart sound spectrum; therefore, the machine can be set to detect the PFO by spectrally analyzing the heart sounds. The Pouya Heart machine has received approval and certifications from the Iranian Society of Neonatology and the Ministry of Health and Medical Education.
3. Results
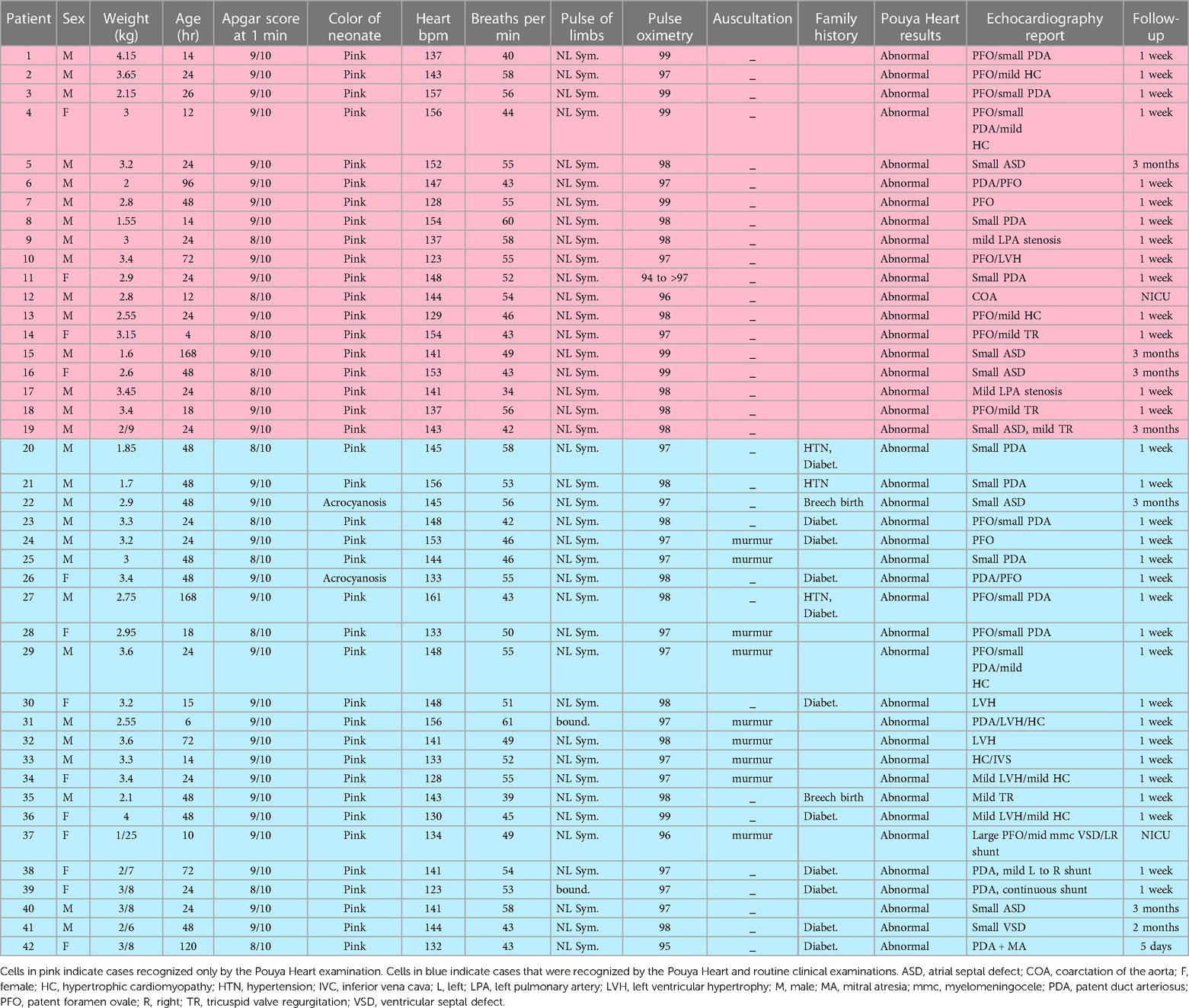
Screening using Pouya Heart was performed for 840 neonates with a median age of 30.85 h and a median weight of 3.12 kg to detect congenital heart malformations. This resulted in detecting abnormal heart sounds in 43 neonates, including one CCHD, one severe CHD, and one false-positive error. The screening and diagnosis results of the 42 neonates with congenital heart malformations identified by echocardiography are shown in Table 1.
Based on the routine medical examination results, 45% of the abnormalities observed in 42 neonates, including one CCHD, were unrecognized at birth (Table 1). The incidence of congenital heart malformations in our well-baby nursery was 5%. Routine medical examination was unable to recognize one case of CCHD at birth (Table 1). The routine medical examination results of this neonate with a CCHD included pulse oximetry of 96%, normal auscultation results, and no family history of abnormalities. Screening using Pouya Heart requires approximately 6 min and is performed by a nurse or midwife.
The sensitivity, which indicated the precision of the false-negative results, was 100%, 97.1%, and 94.8% for the intelligent phonocardiogram examination, routine medical examination, and auscultation, respectively (Table 2). The intelligent machine examinations were performed at 1–192 h after birth, and Doppler echocardiography was performed at 2–72 h after screening using Pouya Heart.
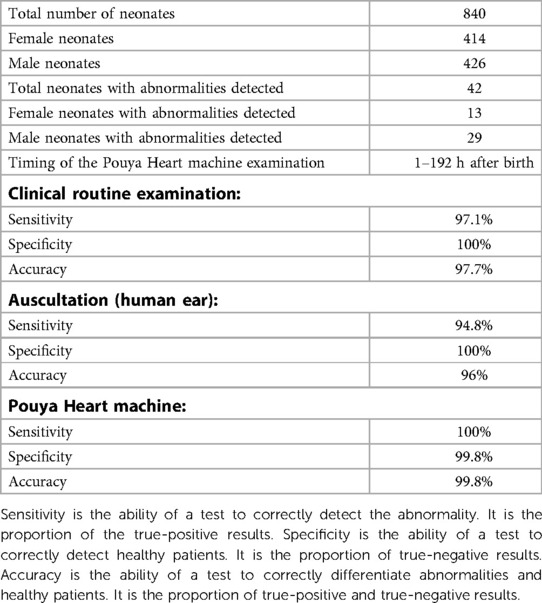
Table 2. Data of the 42 patients and the sensitivity, specificity, and accuracy of the pouya heat machine, clinical routine examination, and auscultation.
4. Discussion
The intelligent phonocardiogram detected heart malformations in 43 neonates. Among these, 42 neonates had heart defects confirmed by echocardiography. The pediatric cardiologist requested follow-up examinations for all 42 neonates. However, the pediatric cardiologist performed echocardiography 48 h after auscultation using Pouya Heart. Consequently, it is possible that Pouya Heart detected trivial heart defects that self-recovered after 48 h, self-recovery of trivial CHD after 48 h is possible. The complete screening process using Pouya Heart required approximately 6 min; a trained technician operated the machine. The main cost of this study was attributable to the screening of 840 neonates with Pouya Heart and echocardiography to diagnose malformations in 43 neonates. The only alternative method of obtaining such results is the performance of 840 echocardiography examinations by a pediatric cardiologist; however, this is not practical.
This study had some limitations. First, screening for CHD and CCHD in newborns using Pouya Heart is novel; consequently, some families did not allow their newborns to undergo the recommended echocardiography examinations. Second, because of the study design, we had to replace neonates who did not undergo the echocardiography examination with randomly chosen neonates from the well-baby nursery. Eleven neonates with abnormal heart sound detected by the Pouya Heart did not undergo echocardiography; consequently, they were replaced with other neonates from the well-baby nursery. Therefore, the number of neonates with congenital heart malformations who were not detected by the routine medical examination could have increased to 58% if those 11 neonates had undergone echocardiography or if other newborns from the well-baby nursery were unavailable as replacements or if we replaced those 11 neonates with those with abnormal heart sounds recognized by the Pouya Heart (total neonates examined was 855). Third, some neonates for whom an echocardiography follow-up was recommended were referred to other subspecialist centers; some might not follow those recommendations.
Consequently, it was impossible to include the follow-up results evaluation in this study, although it would be very informative to investigate the echocardiography follow-up evaluations. Forth, screening using Pouya Heart is based on an analysis of heart sounds (3, 26). Therefore, it is important to reduce the interference of background noise. Fifth, functional murmurs have a specific spectrum and are considered healthy by Pouya Heart. Any murmur is considered functional if the murmur has the same specific spectral effect as that of the functional murmurs. However, considering the size of the data in this study and the complexities of the different congenital defects, such as the branch pulmonary artery systolic murmur that can be construed as normal if it is heard in a preterm newborn in the first few days or weeks of life, but it can also be abnormal, for example, in a neonate with Alagille syndrome, Pouya Heart may lead to some false positive. Therefore, it is important to examine such complex cases in different periods interdependently with the Pouya Heart and set the spectral pattern accordingly. The adult CHD rate increased by 5% annually (9). Using both Pouya Heart and routine medical examinations can result in the timely detection of congenital heart malformations in neonates and can considerably reduce the number of adults with CHD.
In summary, we introduced a novel, practical, and cost-effective screening method for CHD and CCHD in newborns that can be performed immediately after birth. As a result, Pouya Heart detected heart malformations, including CCHD and CHD, that were unrecognized at birth by routine medical examinations at our hospital. Moreover, Pouya Heart interpreted innocent murmurs as healthy heart sounds.
Timely screening of the cardiac health of newborns is vital. Screening for CCHD should be performed as early as possible. Furthermore, screening for CHD in neonates is essential to saving lives and reducing the incidence of undetected pediatric and adult congenital heart diseases. The Pouya Heart machine can detect more congenital heart abnormalities in neonates than routine clinical examinations because of the high pressure on the right side of the heart. With many defects and anomalies, a clear murmur is sometimes not heard because of the high pressure. This critical issue emphasizes the need to use the Pouya Heart machine for screening CHD in neonates. Screening for heart malformations in newborns using Pouya Heart will significantly improve pediatric heart healthcare and eliminate unrecognized adult CHD. This intelligent machine should be tested at various hospitals in different countries to validate its accuracy across populations.
Echocardiography examination, which must be performed by a pediatric cardiologist, is essential for simultaneous screening, diagnosis, and patient's management. The Pouya Heart machine is a screening tool for congenital heart malformations. Therefore, we can conclude that the cost of conducting 43 echocardiography examinations and 840 intelligent phonocardiogram examinations to that of conducting 840 echocardiography examinations was highly cost-effective.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The local ethics committee of the Shahid Akbarabadi Clinical Research & Development Unit approved the study; approval number: IR.IUMS.FMD.REC.1398.098. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
AB is the principal author of the study and contributed to the design of the study, performed data verification and analyses, and drafted the first version of the manuscript. MK contributed to the study's design and drafted the first version of the manuscript. MV performed data collection, verification, and analyses and drafted the first version of the manuscript. AS is the principal inventor of the Pouya Heart, performed technical supervision, and drafted the first version of the manuscript. All authors provided substantial conceptual and intellectual contributions and approved the final version of the manuscript.
Funding
This study was funded by the Shahid Akbarabadi Clinical R&D Unit, Iran University of Medical Sciences (ethics approval number: IR-IUMS-FMD. REC.1398.098). This study received funding for the publication fee from CAPIS. However, the funder was not involved in the study design, collection, analysis, interpretation of data, writing of this article, or decision to submit it for publication. All authors declare no other competing interests.
Acknowledgments
The authors would like to thank the Shahid Akbarabadi Clinical Research & Development Unit and the Iran University of Medical Sciences for their intensive and elaborate research work during the trial and close cooperation throughout the study. In addition, the authors thank the CAPIS research and development company based in Belgium (www.capis.be) for their technical supervision. The authors also thank Research Square for preprinting the manuscript (https://doi.org/10.21203/rs.3.rs-403721/v2). Finally, the authors thank Editage (www.editage.com) for English language editing.
Conflict of interest
AAS was employed by the CAPIS research and development company based in Belgium. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. (2013) 131:e1502–8. doi: 10.1542/peds.2012-3435
2. Bairoliya N, Fink G. Causes of death and infant mortality rates among full-term births in the United States between 2010 and 2012: an observational study. PLOS Med. (2018) 15:e1002531. doi: 10.1371/journal.pmed.1002531
3. Kocharian A, Sepehri AA, Janani A, Malakan-Rad E. Efficiency, sensitivity and specificity of automated auscultation diagnosis device for detection and discrimination of cardiac murmurs in children. Iran J Pediatr. (2013) 23:445–50. PMID: 24427499, PMCID: PMC3883375
4. Tavel ME. Cardiac auscultation. A glorious past–but does it have a future? Circulation. (1996) 93:1250–3. doi: 10.1161/01.CIR.93.6.1250
5. Wren C, Richmond S, Donaldson L. Presentation of congenital heart disease in infancy: implications for routine examination. Arch Dis Child Fetal Neonatal Ed. (1999) 80:F49–53. doi: 10.1136/fn.80.1.f49
6. Suard C, Flori A, Paoli F, Loundou A, Fouilloux V, Sigaudy S, et al. Accuracy of prenatal screening for congenital heart disease in population: a retrospective study in southern France. PLoS One. (2020) 15:e0239476. doi: 10.137/journal.pone.0239476
7. Yoon SA, Hong WH, Cho HJ. Congenital heart disease diagnosed with echocardiogram in newborns with asymptomatic cardiac murmurs: a systematic review. BMC Pediatr. (2020) 20:322. doi: 10.1186/s12887-020-02212-8
8. Van Velzen CL, Clur SA, Rijlaarsdam MEB, Bax CJ, Pajkrt E, Heymans MW, et al. Prenatal detection of congenital heart disease–results of a national screening programme. BJOG. (2016) 123:400–7. doi: 10.1111/1471-0528.13274
9. Brida M, Gatzoulis MA. Adult congenital heart disease: past, present and future. Acta Paediatr. (2019) 108:1757–64. doi: 10.1111/apa.14921
10. Downing KF, Oster ME, Klewer SE, Rose CE, Nembhard WN, Andrews JG, et al. Disability among young adults with congenital heart defects: congenital heart survey to recognize outcomes, needs, and well-being 2016-2019. J Am Heart Assoc. (2021) 10:e022440. doi: 10.1161/JAHA.121.022440
11. Brida M, Šimkova I, Jovović L, Prokšelj K, Antonová P, Balint HO, et al. European Society of cardiology working group on adult congenital heart disease and study group for adult congenital heart care in central and south eastern European countries consensus paper: current status, provision gaps and investment required. Eur J Heart Fail. (2021) 23:445–53. doi: 10.1002/ejhf.2040
12. Massin MM, Dessy H. Delayed recognition of congenital heart disease. Postgrad Med J. (2006) 82:468–70. doi: 10.1136/pgmj.2005.044495
13. Moons P, Sluysmans T, De Wolf D, Massin M, Suys B, Benatar A, et al. Congenital heart disease in 111 225 births in Belgium: birth prevalence, treatment and survival in the 21st century. Acta Paediatr. (2009) 98:472–7. doi: 10.1111/j.1651-2227.2008.01152.x
14. Harold JG. Cardiology patient page. Screening for critical congenital heart disease in newborns. Circulation. (2014) 130:e79–81. doi: 10.1161/CIRCULATIONAHA.113.008522
16. Kondo M, Ohishi A, Baba T, Fujita T, Iijima S. Can echocardiographic screening in the early days of life detect critical congenital heart disease among apparently healthy newborns? BMC Pediatr. (2018) 18:359. doi: 10.1186/s12887-018-1344-z
18. Zhao QM, Niu C, Liu F, Wu L, Ma XJ, Huang GY. Accuracy of cardiac auscultation in detection of neonatal congenital heart disease by general paediatricians. Cardiol Young. (2019) 29:679–83. doi: 10.1017/S1047951119000799
19. Kostopoulou E, Dimitriou G, Karatza A. Cardiac murmurs in children: a challenge for the primary care physician. Curr Pediatr Rev. (2019) 15:131–8. doi: 10.2174/1573396315666190321105536
20. Chang RK, Gurvitz M, Rodriguez S. Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med. (2008) 162:969–74. doi: 10.1001/archpedi.162.10.969
21. Abouk R, Grosse SD, Ailes EC, Oster ME. Association of US state implementation of newborn screening policies for critical congenital heart disease with early infant cardiac deaths. J Am Med Assoc. (2017) 318:2111–8. doi: 10.1001/jama.2017.17627
22. Ailes EC, Gilboa SM, Honein MA, Oster ME. Estimated number of infants detected and missed by critical congenital heart defect screening. Pediatrics. (2015) 135:1000–8. doi: 10.1542/peds.2014-3662
23. Liu X, Xu W, Yu J, Shu Q. Screening for congenital heart defects: diversified strategies in current China. World J Pediatr Surg. (2019) 2:e000051. doi: 10.1136/wjps-2019-000051
24. Sepehri AA, Hancq J, Dutoit T, Gharehbaghi A, Kocharian A, Kiani A. Computerized screening of children congenital heart diseases. Comput Methods Programs Biomed. (2008) 92:186–92. doi: 10.1016/j.cmpb.2008.06.015
25. Sepehri AA, Gharehbaghi A, Dutoit T, Kocharian A, Kiani A. A novel method for pediatric heart sound segmentation without using the ECG. Comput Methods Programs Biomed. (2010) 99:43–8. doi: 10.1016/j.cmpb.2009.10.006
26. Sepehri AA, Kocharian A, Janani A, Gharehbaghi A. An intelligent phonocardiography for automated screening of pediatric heart diseases. J Med Syst. (2016) 40:16. doi: 10.1007/s10916-015-0359-3
27. Wang J, You T, Yi K, Gong Y, Xie Q, Qu F, et al. Intelligent diagnosis of heart murmurs in children with congenital heart disease. J Healthc Eng. (2020) 2020:9640821. doi: 10.1155/2020/9640821
28. Lv J, Dong B, Lei H, Shi G, Wang H, Zhu F, et al. Artificial intelligence-assisted auscultation in detecting congenital heart disease. Eur Heart J Digit Health. (2021) 2:119–24. doi: 10.1093/ehjdh/ztaa017
29. Thompson WR, Reinisch AJ, Unterberger MJ, Schriefl AJ. Artificial intelligence-assisted auscultation of heart murmurs: validation by virtual clinical trial. Pediatr Cardiol. (2019) 40:623–9. doi: 10.1007/s00246-018-2036-z
30. Lai LSW, Redington AN, Reinisch AJ, Unterberger MJ, Schriefl AJ. Computerized automatic diagnosis of innocent and pathologic murmurs in pediatrics: a pilot study. Congenit Heart Dis. (2016) 11:386–95. doi: 10.1111/chd.12328
31. Elgendi M, Bobhate P, Jain S, Guo L, Rutledge J, Coe Y, et al. Spectral analysis of the heart sounds in children with and without pulmonary artery hypertension. Int J Cardiol. (2014) 173:92–9. doi: 10.1016/j.ijcard.2014.02.025
32. Das BB. Patent foramen ovale in fetal life, infancy and childhood. Med Sci. (2020) 8:25. doi: 10.3390/medsci8030025
Keywords: congenital heart malformations, congenital heart diseases, critical congenital heart defects, innocent murmurs, intelligent phonocardiogram, intelligent phonocardiography, Pouya Heart
Citation: Bordbar A, Kashaki M, Vafapour M and Sepehri AA (2023) Determining the incidence of heart malformations in neonates: A novel and clinically approved solution. Front. Pediatr. 11:1058947. doi: 10.3389/fped.2023.1058947
Received: 30 September 2022; Accepted: 27 February 2023;
Published: 15 March 2023.
Edited by:
Theodor Tirilomis, University of Göttingen, GermanyReviewed by:
Fatima Ali, Aga Khan University, PakistanShreepal Jain, Bai Jerbai Wadia Hospital for Children, India
Elaheh Malakan Rad, Tehran University of Medical Sciences, Iran
© 2023 Bordbar, Kashaki, Vafapour and Sepehri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir A. Sepehri c2VwZWhyaUBjYXBpcy5iZQ==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Abbreviations CHD, congenital heart disease; CCHD, critical congenital heart disease.
 Arash Bordbar1
Arash Bordbar1 Amir A. Sepehri
Amir A. Sepehri