- 1Department of Paediatrics, Oyster Woman and Child Hospital, Bengaluru, India
- 2Centre for Cellular and Molecular Platforms, National Centre for Biological Sciences, Bengaluru, India
- 3Department of Neonatology, Emergency County Hospital of Bihor, Oradea, Romania
- 4Faculty of Medicine and Pharmacy, University of Oradea, Oradea, Romania
- 5Department of Neonatology, Manipal Hospitals, Bengaluru, India
- 6Department of Paediatric Retina, Narayana Nethralaya Eye Institute, Bengaluru, India
- 7Department of Paediatrics, School for Oncology and Developmental Biology, Maastricht University Medical Center, Maastricht, Netherlands
- 8School of Women’s and Infants’ Health, University of Western Australia, Crawley, WA, Australia
Background: Retinopathy of prematurity (ROP) and abnormal brain development share similar risk factors and mechanisms. There has been contrasting evidence on the association of ROP with adverse neurodevelopmental outcomes.
Objective: We analysed the association between ROP at levels of severity and treatment with all neurodevelopmental outcomes until adolescence.
Data source: We followed PRISMA guidelines and searched Medline and Embase between 1 August 1990 and 31 March 2022.
Study selection and participants: Randomised or quasi-randomised clinical trials and observational studies on preterm infants (<37 weeks) with ROP [type 1 or severe ROP, type 2 or milder ROP, laser or anti-vascular endothelial growth factor (VEGF) treated] were included.
Data extraction and synthesis: We included studies on ROP and any neurocognitive or neuropsychiatric outcomes.
Outcomes: The primary outcomes were as follows: cognitive composite scores evaluated between the ages of 18 and 48 months by the Bayley Scales of Infant and Toddler Development (BSID) or equivalent; neurodevelopmental impairment (NDI; moderate to severe NDI or severe NDI), cerebral palsy, cognitive impairment; and neuropsychiatric or behavioural problems. The secondary outcomes were as follows: motor and language composite scores evaluated between the ages of 18 and 48 months by BSID or equivalent; motor/language impairment; and moderate/severe NDI as defined by the authors.
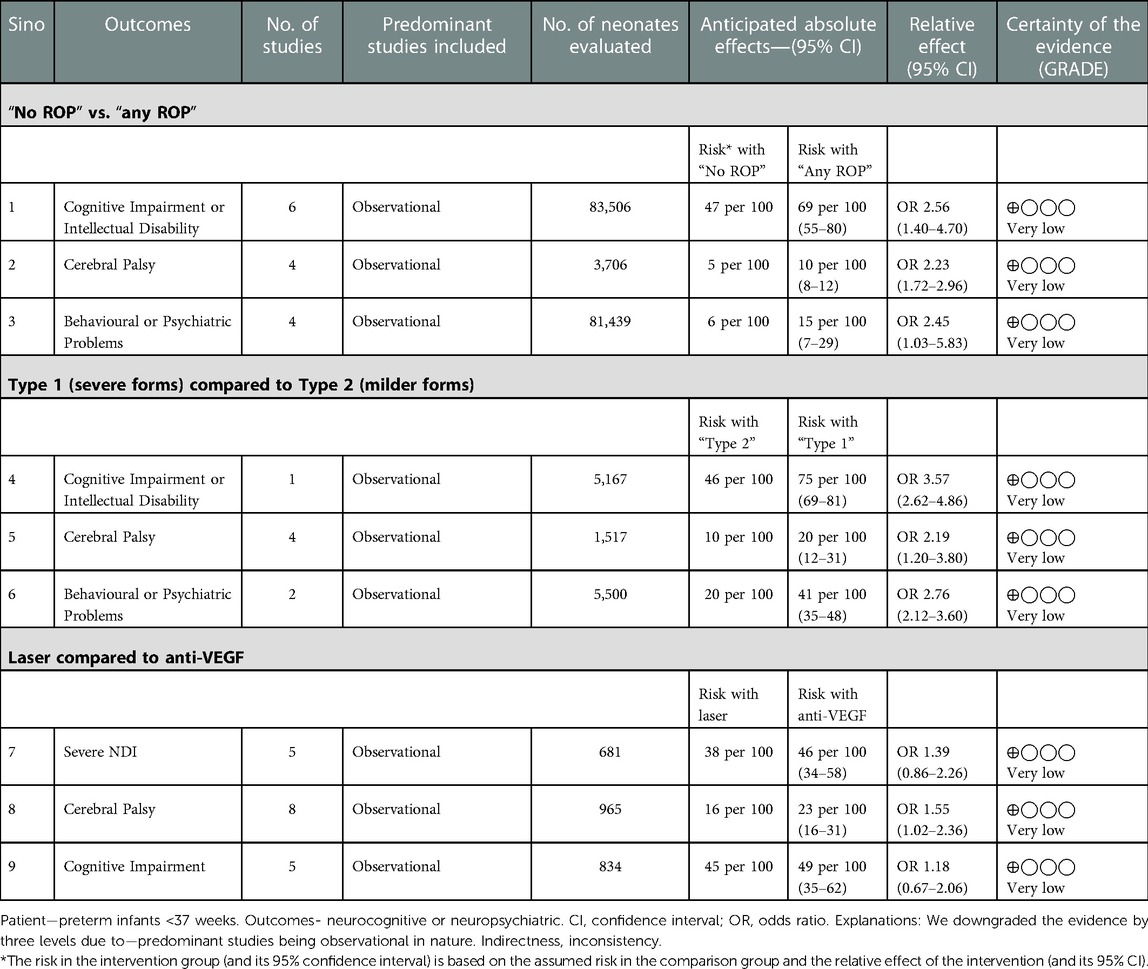
Results: In preterm infants, “any ROP” was associated with an increased risk of cognitive impairment or intellectual disability [n = 83,506; odds ratio (OR): 2.56; 95% CI: 1.40–4.69; p = 0.002], cerebral palsy (n = 3,706; OR: 2.26; 95% CI: 1.72–2.96; p < 0.001), behavioural problems (n = 81,439; OR: 2.45; 95% CI: 1.03–5.83; p = 0.04), or NDI as defined by authors (n = 1,930; OR: 3.83; 95% CI: 1.61–9.12; p = 0.002). Type 1 or severe ROP increased the risk of cerebral palsy (OR: 2.19; 95% CI: 1.23–3.88; p = 0.07), cognitive impairment or intellectual disability (n = 5,167; OR: 3.56; 95% CI: 2.6–4.86; p < 0.001), and behavioural problems (n = 5,500; OR: 2.76; 95% CI: 2.11–3.60; p < 0.001) more than type 2 ROP at 18–24 months. Infants treated with anti-VEGF had higher odds of moderate cognitive impairment than the laser surgery group if adjusted data (gestational age, sex severe intraventricular haemorrhage, bronchopulmonary dysplasia, sepsis, surgical necrotising enterocolitis, and maternal education) were analysed [adjusted OR (aOR): 1.93; 95% CI: 1.23–3.03; p = 0.04], but not for cerebral palsy (aOR: 1.29; 95% CI: 0.65–2.56; p = 0.45). All outcomes were adjudged with a “very low” certainty of evidence.
Conclusion and relevance: Infants with “any ROP” had higher risks of cognitive impairment or intellectual disability, cerebral palsy, and behavioural problems. Anti-VEGF treatment increased the risk of moderate cognitive impairment. These results support the association of ROP and anti-VEGF treatment with adverse neurodevelopmental outcomes.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42022326009.
Introduction
Retinopathy of prematurity (ROP), a neurovascular disease caused by abnormal retinal vascularisation, is a complication after preterm birth that is still the most common cause of blindness in very preterm infants (1, 2). Retinal vascularisation begins around 12 weeks in utero and continues from the centre to the periphery until 44 weeks under the influence of vascular endothelial growth factor (VEGF). The pathogenesis of ROP involves the initial phase of vaso-obliteration of the retinal vasculature due to extrauterine hyperoxia, low levels of insulin-like growth factor 1 (IGF-1), and delayed expression of VEGF receptors. The next phase is characterised by vaso-proliferation secondary to the increased level of local VEGF levels (3–5). ROP is classified by four zones, five stages of severity, and the presence of plus disease, a posterior retinal vascular biomarker often warranting treatment (6, 7). As per the Early Treatment of Retinopathy of Prematurity Randomised (ETROP) trial, the disease is categorised into the following: type 1, defined as zone I, any stage with plus disease or zone I, stage 3 ROP without plus disease or zone II, or stage 2 or 3 ROP with plus disease; and type 2, defined as zone I, stage 1 or 2 without plus disease, or zone II stage 3 without plus disease (8). Several treatment approaches have been developed over time, aiming at the ablation of vessels by cryotherapy or laser photocoagulation to the avascular retina or intravitreal anti-VEGF injection within 48–72 h for type 1 ROP and close monitoring for type 2 ROP (8, 9).
Recent evidence has shown an association of severe ROP with adverse neurodevelopmental outcomes mainly in the cognitive component in preterm infants (10, 11). There are also scanty data on some correlation between ROP and behavioural problems, such as autism spectrum disorders (ASD) in extreme preterm infants attributed to poor brain growth (12, 13). The pathological process involved in ROP and abnormal neurodevelopmental outcomes share a common pathway (14). It could thus be plausible that ROP could be an independent biomarker for adverse neurodevelopmental outcomes (6). The long term follow-up concerning safety and efficacy is not fully understood, especially with the use of anti-VEGF. The epoch of development and treatment of ROP coincides in principle during late pregnancy, when exponential growth of the brain occurs. This growth is only possible with appropriate growth of the microvasculature. We have little information about how development of the aberrant retinal microvasculature in ROP also affects other parts of the brain during this particular phase of exponential brain growth (15–17). This raises the question about the association between ROP, treatment of ROP, and the infant’s neurodevelopmental outcome (18, 19).
We performed a systematic review and a meta-analysis to ask the following three questions: Is there a correlation between ROP and short- or long-term neurodevelopmental outcomes? If so, can we identify a threshold of the severity of ROP disease that is associated with subsequent impaired neurodevelopmental outcome? And third, is there a difference in the association depending on the type of treatment?
Methods
The present systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines (20). In addition, we developed the protocol a priori, which specified the inclusion criteria, the method for evaluating study quality, outcomes, and statistics. The protocol was registered with the international prospective register for systematic reviews, PROSPERO (CRD42022326009).
Search strategy
A systematic literature search was conducted using an appropriate prespecified search strategy in Ovid Medline and Embase between 1 August 1990 and 31 March 2022, using Medical Subject Headings. Details of the search strategy are provided in the Supplementary Material.
Study selection
Randomised or quasi-randomised clinical trials and observational studies that evaluated at least one of the prespecified outcomes were included. Preterm infants (<37 weeks) with any ROP (type 1 or severe ROP, type 2 or milder ROP, laser or anti-VEGF treatment) were included. Preterm infants with genetic syndromes and congenital anomalies were excluded. Preterm infants without ROP were considered to be comparators.
Outcomes
Primary outcomes
(1) Cognitive Composite Scores (CCS) evaluated between 18 and 24 months of age by the Bayley Scale of Infant and Toddler Development (BSID III/IV) or equivalent; between 25 and 48 months if reported.
(2) Neurodevelopmental impairment (NDI) as defined:
(a) Moderate to severe NDI, defined as the presence of one or more of the following: BSID III/IV (cognitive, motor, or language score) <85, cerebral palsy (CP), visual impairment (unilateral or bilateral blindness), or severe to profound hearing impairment (meeting criteria for amplification) evaluated between 18 and 48 months of age.
(b) Severe NDI, defined as the presence of one or more: BSID III/IV (cognitive, motor, or language score) <70, CP with a Gross Motor Functional Classification Scale (GMFCS) level ≥3, blindness (bilateral blindness with or without some functional vision in one or both eyes), or severe to profound hearing impairment (requiring cochlear implants in one/both ears or permanent hearing loss that prevented the understanding of instructions) evaluated at 18–48 months of age.
(3) CP (any type) evaluated clinically between 18 and 48 months of age.
(4) Cognitive impairment (6 months to 21 years): moderate (BSID-III < 85) or severe (BSID-III < 70) or defined by any comparable validated tool.
(5) Neuropsychiatric or behavioural problems (attention deficit hyperactivity disorder, ASDs, or others) evaluated by any validated tool.
Secondary outcome(s)
(1) Motor and language composite scores evaluated between 18 and 48 months of age by BSID-III/IV or any validated tool.
(2) Motor impairment evaluated between 18 and 48 months of age: moderate (BSID-III < 85) or severe (BSID-III < 70) or defined by any comparable validated tool.
(3) Language impairment evaluated between 18 and 48 months of age: moderate (BSID-III < 85) or severe (BSID-III < 70) or defined by any comparable validated tool.
(4) Motor function evaluated above 4 years of age using any validated tool.
(5) Moderate or severe NDI as defined by the authors.
Data extraction (selection and coding)
Two authors (SD and PG) searched the databases per a predefined search strategy. The final articles were compiled and transferred to Rayyan software (www.rayyan.ai) and the duplicates were removed. Title and abstract screening and full-text screening of articles were done independently by SD/BK. Any discrepancy was resolved by discussion with all the authors. All authors agreed with the final list of articles. The trial authors were contacted by email correspondence to request missing data if needed. The discrepancies were resolved by discussion and consensus with authors BK/NN/AV.
Assessment of methodological quality
All included studies were assessed for methodological quality. The risk of bias was assessed using elements of the Cochrane Collaboration tool for randomised studies (21). For observational studies, the risk of bias for included studies was assessed using a modified Newcastle-Ottawa Scale (NOS) (22) and the following domains were evaluated: selection; comparability; and outcome. A priori, a score of >7/9 was deemed low risk, a score of 4–6/9 was deemed a moderate risk, and a score of ≤3/9 was deemed a high risk of bias. Two authors (SD and PG) performed the risk of bias independently; conflicts were resolved after discussion and consensus with other authors (BK and NN). Similarly, two authors (SD and PG) assessed the certainty of evidence (confidence in the estimate of effect) for each outcome based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework (23). Any discrepancy arising out of subjective assessments was resolved by discussion and consensus.
Data synthesis and statistical analysis
All the studies were combined and analysed using Comprehensive Meta-Analysis version 3.0 software (Biostat Inc., Boston, MA, USA). For continuous outcomes, the mean difference with 95% CI was calculated and for dichotomous outcomes, the odds ratio (OR) with 95% CI was calculated from the data provided in the studies. Adjusted ORs (aORs) for potential confounders were extracted from the studies reporting these data. Studies reporting continuous variables as median and range or interquartile range were converted to mean and SD using the published calculator (24). A random effects model was used to calculate the summary statistics owing to anticipated heterogeneity. For some variables, such as gestational age (GA), a fixed outcome model was used. Statistical heterogeneity was assessed by using the Cochran Q statistic and the I2 statistic, which is derived from the Q statistic and describes the proportion of total variation that is due to heterogeneity beyond chance. We used the Egger regression test and funnel plots to assess publication bias. GA as a potential source of variability between the groups was identified a priori and was included for meta-regression (25).
Results
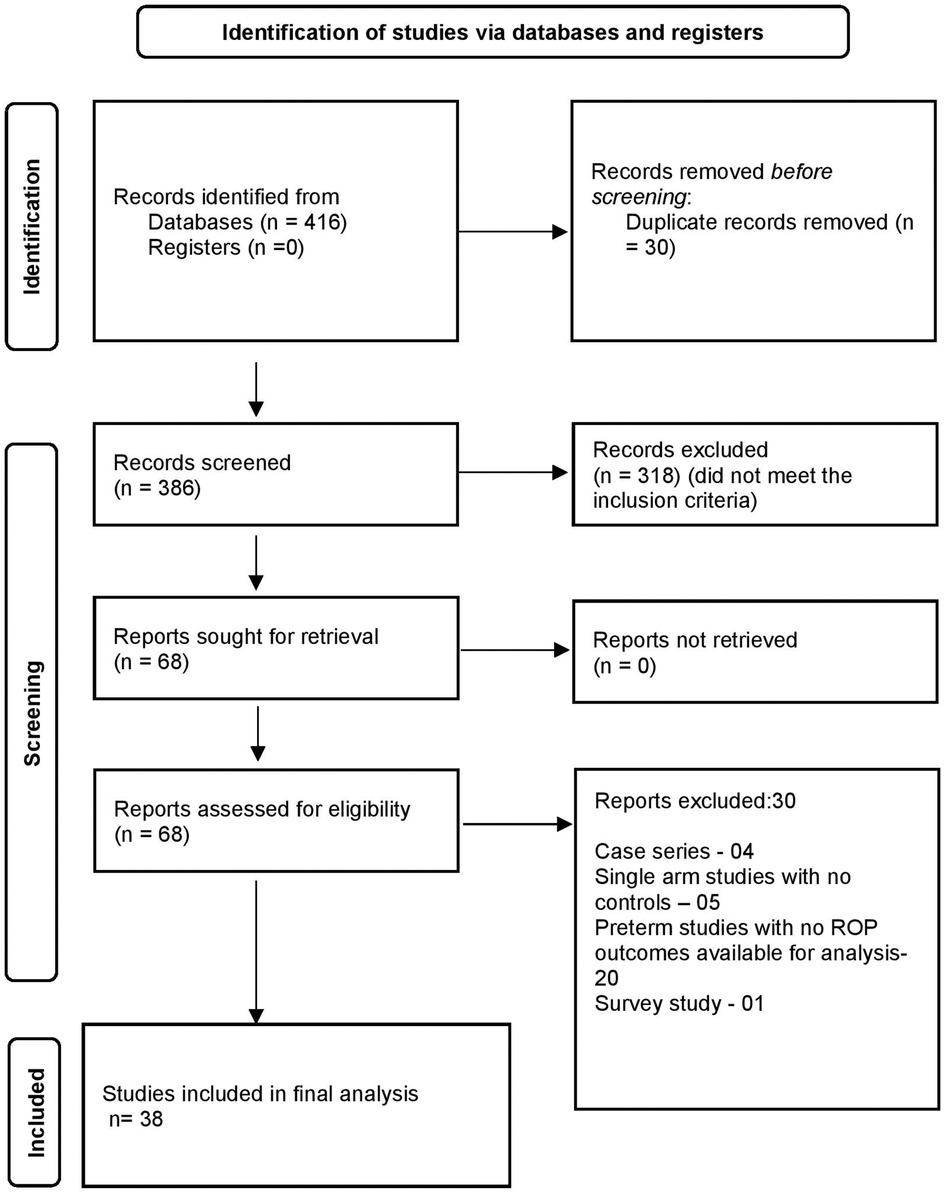
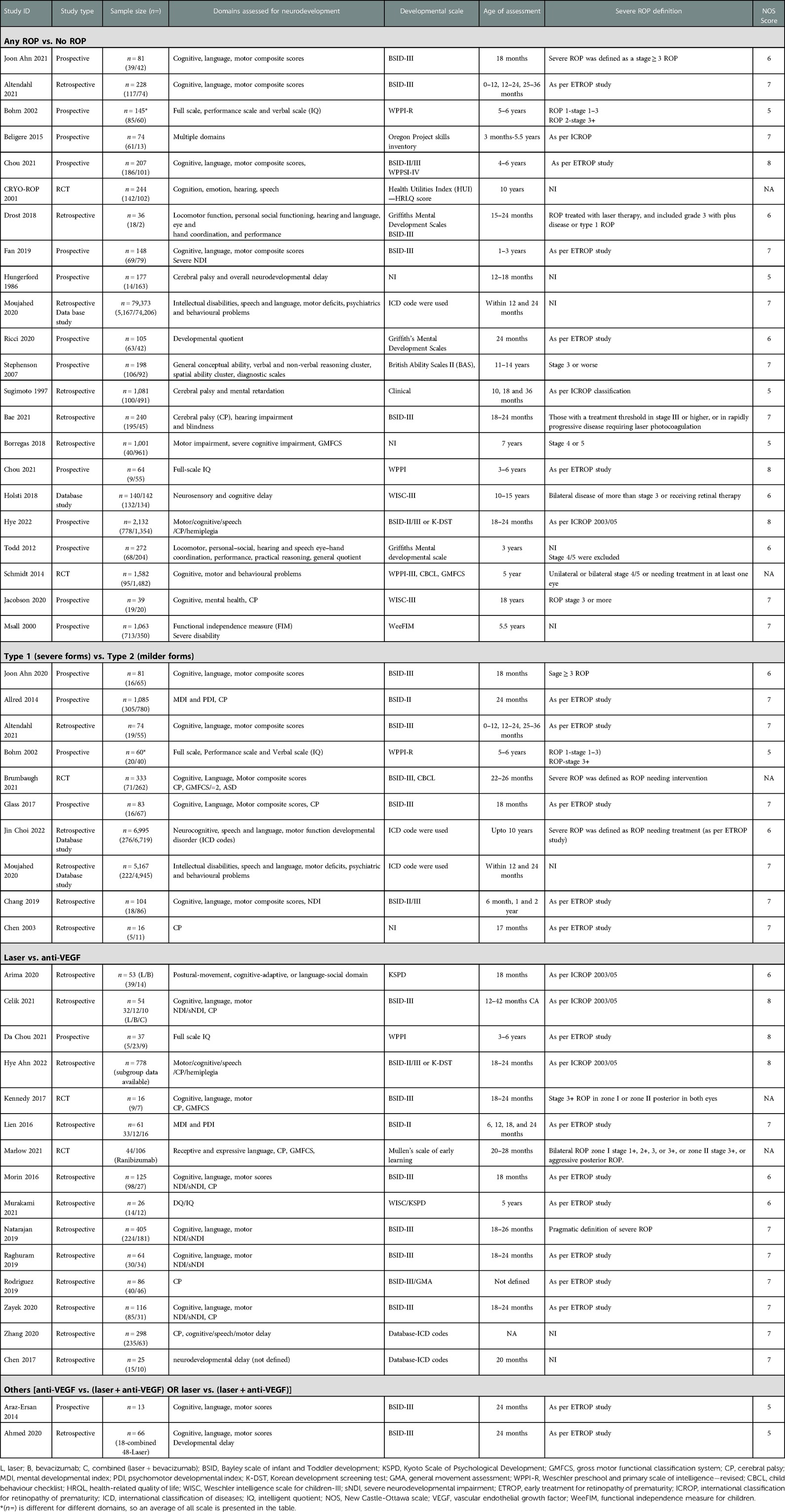
A total of 416 articles were identified through all databases, of which 386 articles underwent title and abstract screening. There were 68 full-text articles assessed for eligibility. Finally, 38 articles were deemed eligible for inclusion in the analysis. The selection process of articles and final inclusion as per PRISMA guidelines (20) is provided in Figure 1.
(a) Any ROP vs No ROP
A total of 22 studies (10, 11, 26–45) reported on neurodevelopmental outcomes (includes all outcomes) between “no ROP” and “any ROP.”
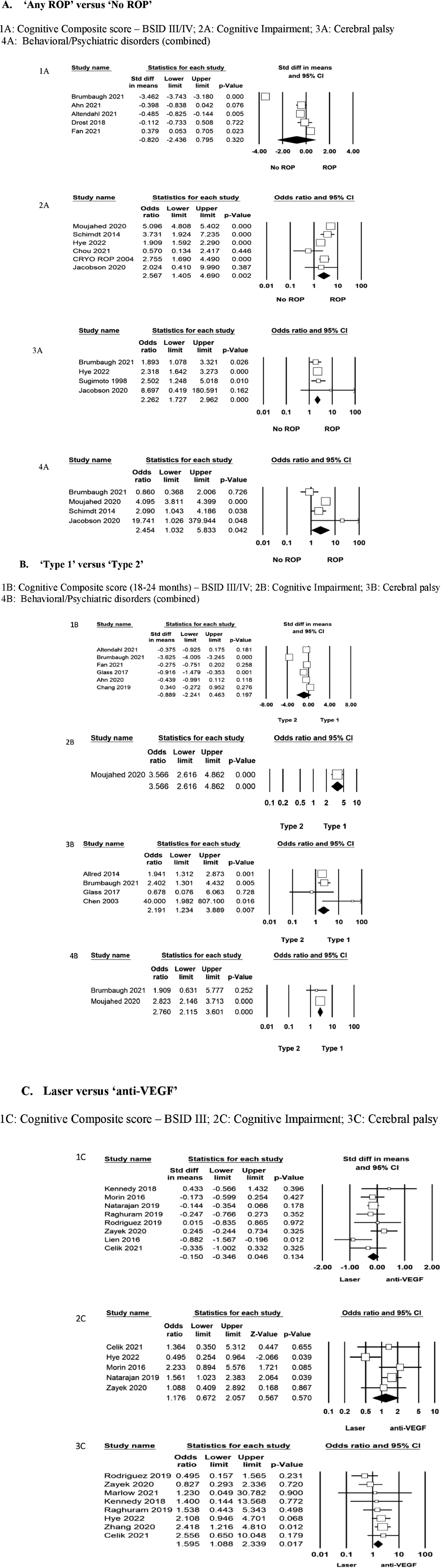
The primary outcome of CCS on BSID-III/IV was reported in five studies (n = 922) at 18–24 months (26, 28, 34, 35, 44). The standard mean difference (SMD) was not different between the “no ROP” and “any ROP” groups (SMD: −0.820 to −2.43; p = 0.32; I2=98%). Data were inadequate to pool for the meta-analysis for moderate or severe NDI. Cognitive impairment/intellectual disability as defined by the author using different scales was reported in six studies (10, 30, 33, 37, 41, 43). A total of 83,506 infants were included in the analysis, which showed significantly increased odds in the “any ROP” group (OR: 2.56; 95% CI: 1.40–4.69; p = 0.002; I2=95%). CP was reported in four studies (38, 41, 43, 44) (n = 3,706), which showed increased odds of CP (OR: 2.26; 95% CI: 1.72–2.96; p < 0.001; I2=0%) in the “any ROP” group. Behavioural or neuropsychiatric problems as defined by the authors were reported in four studies (n = 81,439) (10, 37, 41, 44). Two studies used the Child Behaviour Check List (10, 44) and one study (37) used the International Classification of Disease codes (ICD), whereas another study (41) used the Swedish questionnaire to define the problem between the ages of 2 and 18 years. There was a statistically significant difference with increased odds in the “any ROP” group (OR: 2.45; 95% CI: 1.03–5.83; p = 0.04; I2=83%) (Figure 2 (1A, 2A, 3A, 4A)).
Secondary outcomes of language and motor composite score (BSID-III/IV) were reported in four studies (n = 877) (26, 28, 35, 44) at 18–24 months. The SMD favoured the “No ROP” group for both domains (SMD: −0.73 to −0.15; p = 0.002; I2=70% and SMD: −0.46 to −0.11; p = 0.001; I2=28%, respectively). NDI as defined by authors was reported in five studies (n = 1,930) (29, 31, 36, 42, 45). The age at which NDI was defined varied from 3 months to 7 years of life. “Any ROP” increased the odds of NDI significantly (OR: 3.83; 95% CI: 1.61–9.12; p = 0.002; I2=72%) (Supplementary Figures).
(b) Type 1 vs Type 2
A total of 11 studies (26–28, 34, 35, 44, 46–50) reported data between mild and severe forms of ROP. Six studies (28, 35, 43, 44, 47, 49) (n = 689) reported on the primary outcome of CCS measured by BSID-III/IV between the ages of 18 and 24 months. The results were not statistically significant between the groups (SMD: 0.88 to −2.24; p = 0.19; I2=97%). Four studies (n = 1,517) reported CP (44, 46–48). Type 1 or severe ROP increased the risk of CP (OR: 2.19; 95% CI: 1.23–3.88; p = 0.07; I2=40%) twofold compared to type 2 ROP at 18–24 months. Cognitive impairment or intellectual disability was reported in one study (n = 5,167) (37). The odds for cognitive impairment were increased in type 1 ROP (OR: 3.56; 95% CI: 2.6–4.86; p < 0.001). Behavioural or neuropsychiatric problems were favouring type 2 ROP significantly (two studies, n = 5,500; OR: 2.76; 95% CI: 2.11–3.60; p < 0.001; I2=0%) (37, 44). Moujahed et al. (37) compared treated vs. not treated, which for study purposes we used as type 1 and type 2 for analysis. Cognitive impairment (BSID III < 85) or studies of moderate to severe NDI or severe NDI were not enough to pool for the analysis (Figure 2 (1B, 2B, 3B, 4B)).
Secondary outcomes from six studies (n = 687) of motor (SMD: −2.46 to 0.49; p = 0.19; I2=98%) and language composite score (SMD: −1.90 to 0.60; p = 0.31; I2=98%) were not different between the two groups (26, 28, 35, 44, 47, 49). For other outcomes, the number of studies was insufficient to pool for meta-analysis (Supplementary Figures).
(c) Anti VEGF vs Laser
A total of 15 studies (30, 43, 51–63) reported the outcomes for anti-VEGF vs. laser (Bevacizumab 14 studies, Ranibizumab 1 study) and were included in the analysis. The primary outcome of CCS measured by BSID-III/IV or any other validated tool between 18 and 24 months was reported by nine studies (n = 803) (51–54, 56, 58, 60, 62, 63). One study used a different scale for assessment (51). The analysis was performed separately for different scales using the BSID-II/III, Kyoto Scale of Psychological Development (KSPD), and combined (Supplementary Material). There was no heterogeneity between studies (eight studies, I2=0%). The pooled size of the effect estimate was not significant (SMD: −0.34 to 0.04; p = 0.13; I2=0%). CP was reported in eight studies (n = 965) (43, 52–57, 60). There was statistical significance noted with the anti-VEGF group having higher odds of CP than laser surgery both in the random effects (OR: 1.55; 95% CI: 1.02–2.36; p = 0.04; I2=11%) and the fixed effect models (OR: 1.59; 95% CI: 1.08–2.33; p = 0.01). Cognitive impairment (BSID III/IV score <85 or any validated scale) was not different between the two groups (five studies, n = 834, OR: 1.17; 95% CI: 0.67–2.05; I2=60%) (43, 52, 53, 58, 63) (Figure 2 (1C, 2C, 3C)).
The secondary outcome of language composite score evaluated by BSID III/IV or any other validated scale was not different between the two groups (SMD: −0.22 to 0.08; p = 0.35; I2=0%) from eight studies (n = 748) (one study used KSPD, analysed separately) (52–54, 56, 58, 60, 62, 63). Moderate (BSID-III/IV < 85) (two studies, p = 0.66; I2=39%) or severe language impairment (BSID-III/IV < 70) (two studies, p = 0.77; I2=39%), as defined, was not different between the two groups. The motor composite score was not significantly different (nine studies, n = 792, SMD: −0.43 to 0.03; p = 0.08; I2=40) between the two groups from eight studies that used the BSID for assessment were analysed separately and there was no difference in outcome either (Supplementary Material). Moderate (BSID-III/IV < 85) (four studies, OR: 1.26; 95% CI: 0.91–1.75; p = 0.14; I2=0%) or severe motor impairment (BSID-III < 70) (two studies, OR: 1.10; 95% CI: 0.71–1.68; p = 0.66; I2=0%) were not different between the two groups. Five studies reported on moderate or severe NDI (n = 316, OR: 1.34; 95% CI: 0.77–2.32; I2=0%) and severe NDI (n = 681, OR: 1.39; 95% CI: 0.85–2.2; I2=39%), as defined; the results were not different between the groups (52, 53, 58, 60, 63). We tested whether the combined effect of “anti-VEGF plus laser” was less favourable for neurodevelopmental outcomes compared to “anti-VEGF.” Studies were not adequate for any conceivable conclusion or analysis (30, 52, 62). We analysed adverse outcomes (any) vs. no adverse outcomes due to the paucity of data for various outcomes to be combined. There was no difference observed between the two groups (Supplementary Material). A summary of all included studies is provided in Table 1.
Meta-regression and adjusted analysis
The outcomes of CP and cognitive, language, and motor composite scores were adjusted by meta-regression with the GA as the confounding factor. GA did not account for the differences noted between the groups (Supplementary Material).
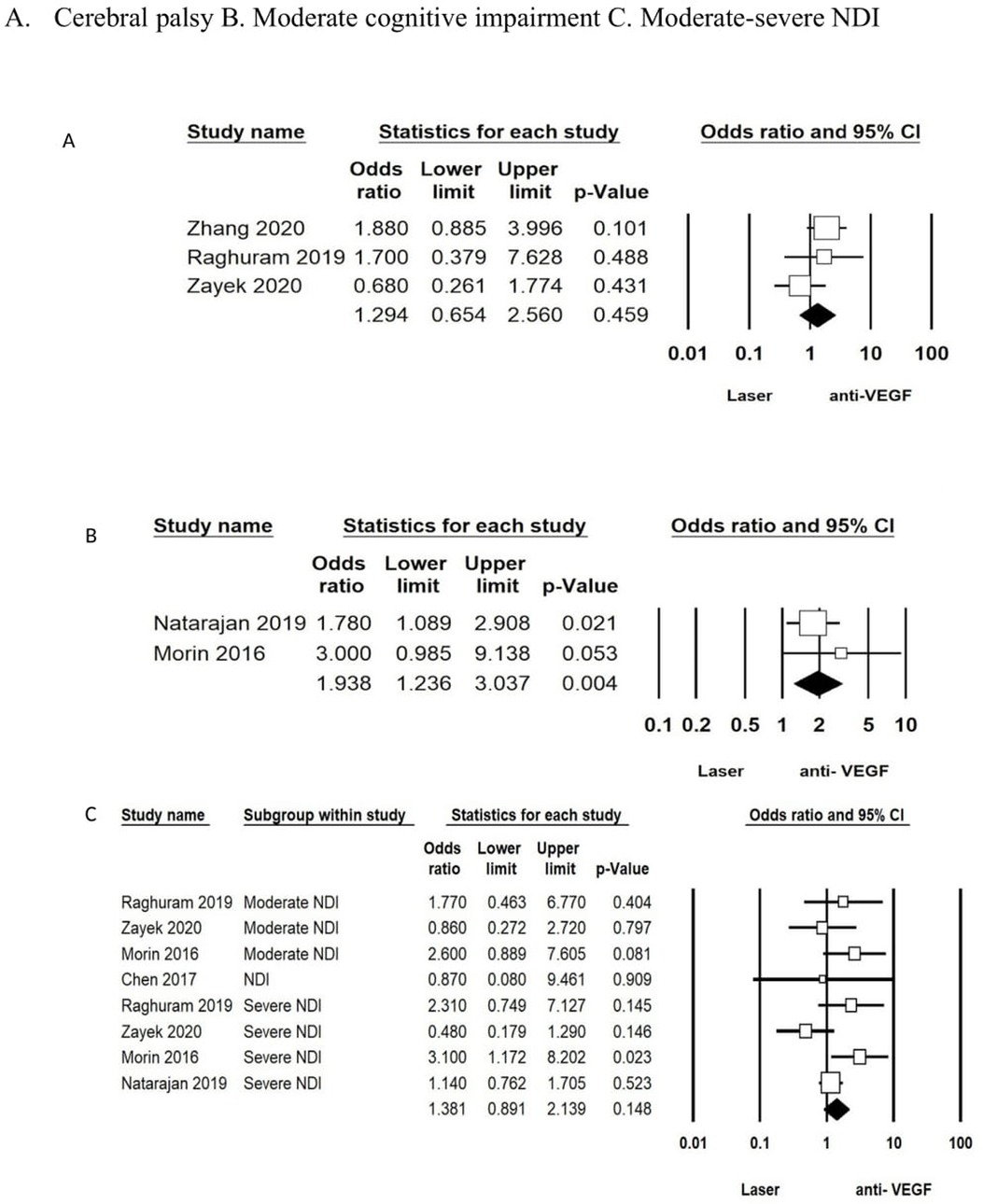
The analysis for the studies that adjusted for comorbidities (IVH, white matter injury, surgical NEC, BPD), GA, and sex was conducted using the aORs. Two studies (58, 63) reported on aOR in the laser vs. anti-VEGF group and showed an increased risk for moderate cognitive impairment in the anti-VEGF group (aOR: 1.93; 95% CI: 1.23–3.03; p = 0.04). There was no difference for CP after adjusting for confounding variables (aOR: 1.29; 95% CI: 0.65–2.56; p = 0.45) between the two groups reported in three studies (53, 55, 60). The combined outcome of moderate or severe NDI or NDI as defined by the authors from five studies (53, 58, 60, 61, 63) was also not different between the groups (aOR: 1.38; 95% CI: 0.89–2.13; p = 0.14) (Figure 3 (A, B, C)).
Risk of bias assessment and certainty of evidence
The risk of bias assessment was performed as per the ROB.2 tool (21) for randomised controlled trials and NOS for observational studies (22). Most of the studies were of fair or good quality. Three randomised controlled trials (10, 44, 57) were considered to be with a “low risk” of bias and two studies (33, 54) with a “high risk” of bias (Supplementary Material). The certainty of evidence was graded as “very low” for all the outcomes (Table 2).
Publication bias
Neither visual inspection of funnel plots nor the Egger test suggested publication or selection bias for the outcome of CP. The number of studies was insufficient for other outcomes to evaluate publication bias (Supplementary Material).
Discussion
We reported the first systematic review and meta-analysis on ROP and the impact of grading and various treatment on short- and long-term neurodevelopmental and neuropsychiatric outcomes from 3 months to 18 years of age.
We found that “any ROP” in preterm infants increased the risk of cognitive impairment or intellectual disability, CP, neuropsychiatric issues, and NDI (as defined by authors) significantly compared to the “No ROP” group. Type 1 or “severe forms” of ROP (stage ≥3) increased the risk of CP and neuropsychiatric disorders significantly compared to infants with type 2 ROP or milder forms (stage <3). With regard to the modality of treatment, anti-VEGF increased the risk of CP significantly with no effect on cognitive, language, or motor impairment on unadjusted analysis. However, the significance was lost on adjusting for confounding factors, such as GA, sex sepsis, white matter injury, postnatal steroids, red blood cell transfusion, thrombocytopenia, and total parenteral nutrition. Unfortunately, these risk factors were reported in only three studies (53, 55, 60). The association of a higher risk for moderate cognitive impairment (BSID <85) after anti-VEGF treatment was present with the use of adjusted data (GA, sex, severe IVH or white matter injury, BPD, surgical NEC, sepsis, maternal education, and SNAP II score), which were reported in only two studies (58, 63).
The concern about the detrimental effects of anti-VEGF antibodies on the developing brain has been reported in previous studies (53–56, 58, 59, 60, 63). The blood concentrations of anti-VEGF can be detected for up to 2 months (64–66). Anti-VEGF acts by the destruction of astrocytes leading to reduced brain volume from animal studies (14). A previous review had found significantly lower cognitive scores in infants treated with anti-VEGF (67). The number of studies included in the meta-analysis was lower and the pooling of data from studies that used different developmental scales for assessment may have contributed to the statistical significance (51) compared to the present review. A more recent meta-analysis (68) found no difference in outcomes between the anti-VEGF treated group compared to the laser or no treatment groups. The mere association of CP with anti-VEGF therapy in the included studies where adjusted analysis was not possible could be due to the infants who received anti-VEGF being sicker, smaller, or with other comorbidities such as IVH or impaired microvascular development (BDP or IUGR). Few authors have adjusted for confounding factors such as GA and sickness level of the infants. However, major factors, such as the need for major respiratory support, NEC, and IVH, which are independent risk factors for neurodevelopmental impairment, were not consistently adjusted across all included studies. This subtle yet significant association of anti-VEGF with poor neurodevelopmental outcomes should warrant large prospective and adequately powered trials to assess its impact on the developing brain during this critical period. Therefore, we think that rigorous indication for anti-VEGF antibody treatment is mandatory until additional clinical data become available. While the indications for anti-VEGF for the treatment of ROP continue to be deliberated across the world, its popularity appears to have increased over the last two decades owing to its apparent “ease” compared to laser treatment and possibly reduced refractive error. Our data analysis raises the legitimate concern that this apparent “ease” comes with relevant side effects on the developing brain. In light of our findings, it must serve to up the ante, counsel the parents more thoroughly, and follow up with these infants more closely. The risk and benefits of the drug, and a serious consideration to rule out alternative laser therapy, must be declared to the parents until more evidence or stronger associations are found to prove the contrary.
The comparison of “anti-VEGF plus laser” vs. “anti-VEGF” was limited. Our data imply that additional laser treatment did not increase the risk for adverse neurodevelopmental outcomes by anti-VEGF treatment per se. This fact will be important in assessing any risk and benefit when the persistent peripheral avascular retina, recurrence of ROP, and incomplete regression are encountered, and may suggest that laser rather than a second anti-VEGF injection may be systemically safer.
The association between ROP and neurodevelopmental outcomes has been reported for the past two decades in previous studies (36, 38, 41). ROP has been associated with reduced head circumference, cerebellar volume, and unmyelinated white matter volume in previous studies (70, 71). The CAP trial has shown that severe ROP increases the risk of poor cognitive and motor outcomes by three- to fourfold (10). A large database study involving 79,373 infants showed that infants needing treatment for ROP are at increased risk for intellectual disabilities, psychiatric and behavioural disorders, speech and language impairment, and ASDs (12, 13, 37). However, several other studies have found no association between ROP and adverse neurodevelopmental outcomes, and any deviations of development are attributed to prematurity as such but not with ROP (26, 28, 32, 40). Although contrasting evidence, the consistent association of ROP and poor neurodevelopmental outcomes could not be merely incidental and its role in causation needs to be further explored. The plausible explanation for the causal role is attributed to the elevated inflammatory markers (46, 72–75), deficiency of insulin-like growth factor (IGF-1) (76–78), hyperoxia or fluctuating oxygen levels (79, 80) in both ROP and brain injury, and combinations thereof.
Strengths and limitations
The strength of this meta-analysis was that we used a broad comprehensive search strategy to include articles with all possible neurodevelopmental outcomes from infancy until adulthood. The included studies in the present review measured different outcomes at different time points using various developmental scales. Most studies used the definition of “severe ROP” as per the ETROP trial; however, some studies used more pragmatic definitions, such as stage ≥3 or those needing treatment. Few studies reported outcomes using ICD codes retrospectively. This heterogeneity is inherent to the development of clinical care over a period of time.
The majority of the studies were observational and heterogenous, and the non-uniformity of definitions used to define developmental disorder(s) and various developmental scales to measure the outcome adds to the limitations of the study. The analysis of different treatments on neurodevelopmental outcomes using adjusted data was also a strength in helping to define subsequent clinical questions on the indication of anti-VEGF treatment.
Conclusion
Our data support the hypothesis that ROP in preterm infants may be an independent indicator for impaired microvascular development in the brain resulting in poor neurodevelopment outcomes. Clinical data analyses and trials need to address the question of the long-term safety of anti-VEGF treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: SD, BK, KN, PG, and AV. Data curation: SD, PT, and DM. Methodology: SD, BK, KN, and PG. Data analysis: SD, PT, DM, RG, and BK. Project administration: BK and SD. Supervision: BK and SD. Writing – original draft: SD and BK. Writing – reviewing and editing: SD, BK, KN, AV, PT, DM, and RG. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1055813/full#supplementary-material.
Abbreviations
ROP, retinopathy of prematurity; VEGF, vascular endothelial growth factor; BSID, Bayley Scales of Infant and Toddler Development; KSPD, Kyoto Scale of Psychological Development; GMFCS, Gross Motor Functional Classification scale; ASD, autism spectrum disorder; ADHD, attention deficit hyperactivity disorder; CP, cerebral palsy; SMD, standard mean difference; OR, odds ratio; CI, confidence interval; SD, standard deviation; GA, gestational age; IVH, intraventricular haemorrhage; NEC, necrotising enterocolitis; BPD, bronchopulmonary dysplasia; SNAP, Score for Neonatal Acute Physiology; CAP, caffeine in apnoea of prematurity; ETROP, early treatment for retinopathy of prematurity; CBCL, child behaviour checklist; IUGR, intrauterine growth restriction
References
1. Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. (2018) 63:618–37. doi: 10.1016/j.survophthal.2018.04.002
2. Blencowe H, Lee ACC, Cousens S, Bahalim A, Narwal R, Zhong N, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res. (2013) 74(Suppl 1):17–34. doi: 10.1038/PR.2013.204
3. Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. (1995) 15:4738–47. doi: 10.1523/JNEUROSCI.15-07-04738.1995
4. Kermorvant-Duchemin E, Sapieha P, Sirinyan M, Beauchamp M, Checchin D, Hardy P, et al. Understanding ischemic retinopathies: emerging concepts from oxygen-induced retinopathy. Doc Ophthalmol. (2010) 120:51–60. doi: 10.1007/S10633-009-9201-X
5. Hasegawa T, McLeod DS, Prow T, Merges C, Grebe R, Lutty GA. Vascular precursors in developing human retina. Invest Ophthalmol Vis Sci. (2008) 49:2178–92. doi: 10.1167/IOVS.07-0632
6. Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Chan RVP, Berrocal A, et al. International classification of retinopathy of prematurity, third edition. Ophthalmology. (2021) 128:E51–68. doi: 10.1016/j.ophtha.2021.05.031
7. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. (2005) 123:991. doi: 10.1001/archopht.123.7.991
8. Good WV. Early treatment for retinopathy of prematurity cooperative group. Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. (2004) 102:233–48; discussion 248–50. PMID: 15747762; PMCID: PMC1280104
9. Palmer EA. Results of U.S. randomized clinical trial of cryotherapy for ROP (CRYO-ROP). Doc Ophthalmol. (1990) 74:245–51. doi: 10.1007/BF02482615
10. Schmidt B, Davis PG, Asztalos EV, Solimano A, Roberts RS. Association between severe retinopathy of prematurity and nonvisual disabilities at age 5 years. J Am Med Assoc. (2014) 311:523. doi: 10.1001/jama.2013.282153
11. Holsti A, Serenius F, Farooqi A. Impact of major neonatal morbidities on adolescents born at 23–25 weeks of gestation. Acta Paediatr Int Acta Paediatrica. (2018) 107:1893–901. doi: 10.1111/apa.14445
12. Eklöf E, Mårtensson GE, Ådén U, Padilla N. Reduced structural brain asymmetry during neonatal life is potentially related to autism spectrum disorders in children born extremely preterm. Autism Res. (2019) 12:1334–43. doi: 10.1002/aur.2169
13. Padilla N, Eklöf E, Mårtensson GE, Bölte S, Lagercrantz H, Ådén U. Poor brain growth in extremely preterm neonates long before the onset of autism spectrum disorder symptoms. Cereb Cortex. (2015) 27:bhv300. doi: 10.1093/cercor/bhv300
14. Rattner A, Williams J, Nathans J. Roles of HIFs and VEGF in angiogenesis in the retina and brain. J Clin Invest. (2019) 129:3807–20. doi: 10.1172/JCI126655
15. Nelson CA. Hazards to early development: the biological embedding of early life adversity. Neuron. (2017) 96:262–6. doi: 10.1016/J.NEURON.2017.09.027
16. Gantenbein KV, Kanaka-Gantenbein C. Highlighting the trajectory from intrauterine growth restriction to future obesity. Front Endocrinol. (2022) 13:1245–52. doi: 10.3389/FENDO.2022.1041718
17. Silva CCV, el Marroun H, Sammallahti S, Vernooij MW, Muetzel RL, Santos S, et al. Patterns of fetal and infant growth and brain morphology at age 10 years. JAMA Netw Open. (2021) 4:e2138214. doi: 10.1001/JAMANETWORKOPEN.2021.38214
18. Tan H, Blasco P, Lewis T, Ostmo S, Chiang MF, Campbell JP. Neurodevelopmental outcomes in preterm infants with retinopathy of prematurity. Surv Ophthalmol. (2021) 66:877–91. doi: 10.1016/j.survophthal.2021.02.012
19. Morken TS, Dammann O, Skranes J, Austeng D. Retinopathy of prematurity, visual and neurodevelopmental outcome, and imaging of the central nervous system. Semin Perinatol. (2019) 43:381–9. doi: 10.1053/j.semperi.2019.05.012
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:89. doi: 10.1136/bmj.n71
21. Higgins JPT, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions version 6.2. Cochrane (2021).
22. Wells G, Shea B, O’Connell D, Peterson D, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2012).
23. Schunemann HBJ, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. Cochrane (2015).
24. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
25. Meta-regression. In Introduction to meta-analysis. Chichester, UK: John Wiley & Sons, Ltd. 187–203.
26. Ahn SJ, Lee JY, Lee JY, Lee YJ, Lee JM, Lee BR, et al. Brain white matter maturation and early developmental outcomes in preterm infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci. (2021) 62:2. doi: 10.1167/IOVS.62.2.2
27. Böhm B, Katz-Salamon M, Smedler A-C, Lagercrantz H, Forssberg H. Developmental risks and protective factors for influencing cognitive outcome at 5 1/2 years of age in very-low-birthweight children. Dev Med Child Neurol. (2002) 44:508–16. doi: 10.1017/S001216220100247X
28. Altendahl M, Sim MS, Kokhanov A, Gundlach B, Tsui I, Chu A. Severe retinopathy of prematurity is not independently associated with worse neurodevelopmental outcomes in preterm neonates. Front Pediatr. (2021) 9:679546. doi: 10.3389/fped.2021.679546
29. Piris Borregas S, Torres Valdivieso MJ, Martín-Arriscado C, de la Cruz Bértolo J, Sierra García P, Pallás Alonso CR. Model that predicted death or disabilities in premature infants was valid at seven years of age. Acta Paediatr Int Acta Paediatrica. (2019) 108:1245–9. doi: 10.1111/apa.14679
30. Chou HD, Shih CP, Huang YS, Liu L, Lai CC, Chen KJ, et al. Cognitive outcomes following intravitreal bevacizumab for retinopathy of prematurity: 4- to 6-year outcomes in a prospective cohort. Am J Ophthalmol. (2022) 234:59–70. doi: 10.1016/j.ajo.2021.06.034
31. Ricci D, Lucibello S, Orazi L, Gallini F, Staccioli S, Serrao F, et al. Early visual and neuro-development in preterm infants with and without retinopathy. Early Hum Dev. (2020) 148:105134. doi: 10.1016/j.earlhumdev.2020.105134
32. Stephenson T, Wright S, O’Connor A, Fielder A, Johnson A, Ratib S, et al. Children born weighing less than 1701g: visual and cognitive outcomes at 11–14 years. Arch Dis Child Fetal Neonatal Ed. (2007) 92:F265–70. doi: 10.1136/adc.2006.104000
33. Quinn GE, Dobson V, Saigal S, Phelps DL, Hardy RJ, Tung B, et al. Health-related quality of life at age 10 years in very low-birth-weight children with and without threshold retinopathy of prematurity. Arch Ophthalmol. (2004) 122:1659. doi: 10.1001/archopht.122.11.1659
34. Drost FJ, Keunen K, Moeskops P, Claessens NHP, van Kalken F, Išgum I, et al. Severe retinopathy of prematurity is associated with reduced cerebellar and brainstem volumes at term and neurodevelopmental deficits at 2 years. Pediatr Res. (2018) 83:818–24. doi: 10.1038/pr.2018.2
35. Fan YY, Huang YS, Huang CY, Hsu JF, Shih CP, Hwang YS, et al. Neurodevelopmental outcomes after intravitreal bevacizumab therapy for retinopathy of prematurity: a prospective case-control study. Ophthalmology. (2019) 126:1567–77. doi: 10.1016/j.ophtha.2019.03.048
36. Hungerford J, Stewart A, Hope P. Ocular sequelae of preterm birth and their relation to ultrasound evidence of cerebral damage. Br J Ophthalmol. (1986) 70:463–8. doi: 10.1136/bjo.70.6.463
37. Al-Moujahed A, Azad A, Vail D, Ludwig CA, Callaway NF, Moshfeghi DM. Retinopathy of prematurity and neurodevelopmental outcomes in premature infants. Eye. (2021) 35:1014–6. doi: 10.1038/s41433-020-0941-x
38. Sugimoto S, Furukawa A, Hatsukawa Y, Yanagihara K, Saito Y. Weak association between retinopathy of prematurity and neurological disorders in childhood. Jpn J Ophthalmol (1998) 42:142–5. doi: 10.1016/s0021-5155(97)00114-7
39. Bae SP, Shin SH, Yoon YM, Kim EK, Kim HS. Association of severe retinopathy of prematurity and bronchopulmonary dysplasia with adverse neurodevelopmental outcomes in preterm infants without severe brain injury. Brain Sci. (2021) 11:699. doi: 10.3390/brainsci11060699
40. Todd DA, Goyen TA, Smith J, Rochefort M. Developmental outcome in preterm infants <29 weeks gestation with ≤stage 3 retinopathy of prematurity (ROP): relationship to severity of ROP. J Dev Orig Health Dis. (2012) 3:116–22. doi: 10.1017/S2040174411000766
41. Jacobson L, Vollmer B, Kistner A, Böhm B. Severity of retinopathy of prematurity was associated with a higher risk of cerebral dysfunction in young adults born extremely preterm. Acta Paediatr Int Acta Paediatrica. (2021) 110:528–36. doi: 10.1111/apa.15461
42. Msall ME, Phelps DL, DiGaudio KM, Dobson V, Tung B, McClead RE, et al. Severity of neonatal retinopathy of prematurity is predictive of neurodevelopmental functional outcome at age 5.5 years. Pediatrics. (2000) 106:998–1005. doi: 10.1542/peds.106.5.998
43. Ahn J-H, Lee KM, Kim MJ, Park H-K, Kim YJ, Ahn SJ, et al. Neurodevelopmental outcomes in very low birthweight infants with retinopathy of prematurity in a nationwide cohort study. Sci Rep. (2022) 12:5053. doi: 10.1038/s41598-022-09053-8
44. Brumbaugh JE, Bell EF, Hirsch SC, Crenshaw EG, DeMauro SB, Adams-Chapman IS, et al. Relationships between retinopathy of prematurity without ophthalmologic intervention and neurodevelopment and vision at 2 years. Pediatr Res. (2021):10.1038/s41390-021-01778. doi: 10.1038/s41390-021-01778-y
45. Beligere N, Perumalswamy V, Tandon M, Mittal A, Floora J, Vijayakumar B, et al. Retinopathy of prematurity and neurodevelopmental disabilities in premature infants. Semin Fetal Neonatal Med. (2015) 20:346–53. doi: 10.1016/j.siny.2015.06.004
46. Allred EN, Capone A, Fraioli A, Dammann O, Droste P, Duker J, et al. Retinopathy of prematurity and brain damage in the very preterm newborn. J AAPOS. (2014) 18:241–7. doi: 10.1016/j.jaapos.2014.01.014
47. Glass TJA, Chau V, Gardiner J, Foong J, Vinall J, Zwicker JG, et al. Severe retinopathy of prematurity predicts delayed white matter maturation and poorer neurodevelopment. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F532–7. doi: 10.1136/archdischild-2016-312533
48. Chen YH, Lien RI, Lai CC, Chao AN, Chen KJ, Hwang YS, et al. Retinopathy of prematurity in neonatal patients with birth weight greater than 1500 g in Taiwan. Biomed J. (2013) 36:84–9. doi: 10.4103/2319-4170.110399
49. Chang Y-S, Chen Y-T, Lai T-T, Chou H-C, Chen C-Y, Hsieh W-S, et al. Involution of retinopathy of prematurity and neurodevelopmental outcomes after intravitreal bevacizumab treatment. PLoS One. (2019) 14:e0223972. doi: 10.1371/journal.pone.0223972
50. Choi Y-J, Hong EH, Shin YU, Bae GH, Kim I, Cho H. Severe retinopathy of prematurity associated with neurodevelopmental disorder in children. Front Pediatr. (2022) 10:816409. doi: 10.3389/fped.2022.816409
51. Arima M, Akiyama M, Fujiwara K, Mori Y, Inoue H, Seki E, et al. Neurodevelopmental outcomes following intravitreal bevacizumab injection in Japanese preterm infants with type 1 retinopathy of prematurity. PLoS One. (2020) 15:e0230678. doi: 10.1371/journal.pone.0230678. [Epub ahead of print]32196539
52. Celik P, Ayranci Sucakli I, Kara C, Petricli IS, Kavurt S, Celik IH, et al. Bevacizumab and neurodevelopmental outcomes of preterm infants with retinopathy of prematurity: should we still worry? J Matern-Fetal Neonatal Med. (2022) 35:415–22. doi: 10.1080/14767058.2021.1888913
53. Zayek M, Parker K, Rydzewska M, Rifai A, Bhat R, Eyal F. Bevacizumab for retinopathy of prematurity: 2-year neurodevelopmental follow-up. Am J Perinatol. (2021) 38:1158–66. doi: 10.1055/s-0040-1710556
54. Kennedy KA, Mintz-Hittner HA. Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J AAPOS. (2018) 22:61–65.e1. doi: 10.1016/j.jaapos.2017.10.006
55. Zhang MH, Blair MP, Ham SA, Rodriguez SH. Two-year outcomes comparing anti-vegf injections to laser for ROP using a commercial claims database. Ophthalmic Surg Lasers Imaging Retina. (2020) 51:486–93. doi: 10.3928/23258160-20200831-02
56. Rodriguez SH, Peyton C, Lewis K, Andrews B, Greenwald MJ, Schreiber MD, et al. Neurodevelopmental outcomes comparing bevacizumab to laser for type 1 ROP. Ophthalmic Surg Lasers Imaging Retina. (2019) 50:337–43. doi: 10.3928/23258160-20190605-01
57. Marlow N, Stahl A, Lepore D, Fielder A, Reynolds JD, Zhu Q, et al. 2-Year outcomes of ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW extension study): prospective follow-up of an open label, randomised controlled trial. Lancet Child Adolesc Health. (2021) 5:698–707. doi: 10.1016/S2352-4642(21)00195-4
58. Morin J, Luu TM, Superstein R, Ospina LH, Lefebvre F, Simard MN, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. (2016) 137:e20153218. doi: 10.1542/peds.2015-3218. [Epub ahead of print]27244705
59. Murakami T, Sugiura Y, Okamoto F, Okamoto Y, Kato A, Hoshi S, et al. Comparison of 5-year safety and efficacy of laser photocoagulation and intravitreal bevacizumab injection in retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. (2021) 259:2849–55. doi: 10.1007/s00417-021-05137-9
60. Raghuram K, Isaac M, Yang J, AlAli A, Mireskandari K, Ly LG, et al. Neurodevelopmental outcomes in infants treated with intravitreal bevacizumab versus laser. J Perinatol. (2019) 39:1300–8. doi: 10.1038/s41372-019-0420-z
61. Chen TA, Schachar IH, Moshfeghi DM. Outcomes of intravitreal bevacizumab and diode laser photocoagulation for treatment-warranted retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina. (2018) 49:126–31. doi: 10.3928/23258160-20180129-07
62. Lien R, Yu M-H, Hsu K-H, Liao P-J, Chen Y-P, Lai C-C, et al. Neurodevelopmental outcomes in infants with retinopathy of prematurity and bevacizumab treatment. PLoS One. (2016) 11:e0148019. doi: 10.1371/journal.pone.0148019
63. Natarajan G, Shankaran S, Nolen TL, Sridhar A, Kennedy KA, Hintz SR, et al. Neurodevelopmental outcomes of preterm infants with retinopathy of prematurity by treatment. Pediatrics. (2019) 144:e20183537. doi: 10.1542/peds.2018-3537. [Epub ahead of print]31337693
64. Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C, et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. (2012) 153:327–333.e1. doi: 10.1016/j.ajo.2011.07.005
65. Kong L, Bhatt AR, Demny AB, Coats DK, Li A, Rahman EZ, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci. (2015) 56:956–61. doi: 10.1167/iovs.14-15842
66. Wu W-C, Shih C-P, Lien R, Wang N-K, Chen Y-P, Chao A-N, et al. Serum vascular endothelial growth factor after bevacizumab treatment for retinopathy of prematurity. Retina. (2017) 37:694–701. doi: 10.1097/IAE.0000000000001209
67. Kaushal M, Razak A, Patel W, Pullattayil AK, Kaushal A. Neurodevelopmental outcomes following bevacizumab treatment for retinopathy of prematurity: a systematic review and meta-analysis. J Perinatol. (2021) 41:1225–35. doi: 10.1038/s41372-020-00884-9
68. Tsai CY, Yeh PT, Tsao PN, Chung YCE, Chang YS, Lai TT. Neurodevelopmental outcomes after bevacizumab treatment for retinopathy of prematurity: a meta-analysis. Ophthalmology. (2021) 128:877–88. doi: 10.1016/J.OPHTHA.2020.11.012
69. Bowen JR, Starte DR, Arnold JD, Simmons JL, Ma PJ, Leslie GI. Extremely low birthweight infants at 3 years: a developmental profile. J Paediatr Child Health. (1993) 29:276–81. doi: 10.1111/j.1440-1754.1993.tb00511.x
70. Sveinsdóttir K, Ley D, Hövel H, Fellman V, Hüppi PS, Smith LEH, et al. Relation of retinopathy of prematurity to brain volumes at term equivalent age and developmental outcome at 2 years of corrected age in very preterm infants. Neonatology. (2018) 114:46–52. doi: 10.1159/000487847
71. Löfqvist C, Engström E, Sigurdsson J, Hård A-L, Niklasson A, Ewald U, et al. Postnatal head growth deficit among premature infants parallels retinopathy of prematurity and insulin-like growth factor-1 deficit. Pediatrics. (2006) 117:1930–8. doi: 10.1542/peds.2005-1926
72. Logan JW, Allred EN, Fichorova RN, Engelke S, Dammann O, Leviton A. Endogenous erythropoietin varies significantly with inflammation-related proteins in extremely premature newborns. Cytokine. (2014) 69:22–8. doi: 10.1016/j.cyto.2014.04.009
73. Leviton A, Allred EN, Fichorova RN, Kuban KCK, Michael O’Shea T, Dammann O. Systemic inflammation on postnatal days 21 and 28 and indicators of brain dysfunction 2 years later among children born before the 28th week of gestation. Early Hum Dev. (2016) 93:25–32. doi: 10.1016/j.earlhumdev.2015.11.004
74. Holm M, Morken TS, Fichorova RN, VanderVeen DK, Allred EN, Dammann O, et al. Systemic inflammation-associated proteins and retinopathy of prematurity in infants born before the 28th week of gestation. Invest Ophthalmol Vis Sci. (2017) 58:6419. doi: 10.1167/iovs.17-21931
75. Korzeniewski SJ, Allred E, Logan JW, Fichorova RN, Engelke S, Kuban KCK, et al. Elevated endogenous erythropoietin concentrations are associated with increased risk of brain damage in extremely preterm neonates. PLoS One. (2015) 10:e0115083. doi: 10.1371/journal.pone.0115083
76. Hellström A, Engström E, Hård A-L, Albertsson-Wikland K, Carlsson B, Niklasson A, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. (2003) 112:1016–20. doi: 10.1542/peds.112.5.1016
77. Jensen AK, Ying G, Huang J, Quinn GE, Binenbaum G. Postnatal serum insulin – like growth factor I and retinopathy of prematurity. Retina. (2017) 37:867–72. doi: 10.1097/IAE.0000000000001247
78. Hellgren G, Löfqvist C, Hansen-Pupp I, Gram M, Smith LE, Ley D, et al. Increased postnatal concentrations of pro-inflammatory cytokines are associated with reduced IGF-I levels and retinopathy of prematurity. Growth Horm IGF Res. (2018) 39:19–24. doi: 10.1016/j.ghir.2017.11.006
79. Das A, Mhanna M, Sears J, Houdek JW, Kumar N, Gunzler D, et al. Effect of fluctuation of oxygenation and time spent in the target range on retinopathy of prematurity in extremely low birth weight infants. J Neonatal Perinatal Med. (2018) 11:257–63. doi: 10.3233/NPM-1757
Keywords: retinopathy of prematurity, preterm, cerebral palsy, bevacizumab, ranibizumab, anti-VEGF, behavioural issues
Citation: Diggikar S, Gurumoorthy P, Trif P, Mudura D, Nagesh NK, Galis R, Vinekar A and Kramer BW (2023) Retinopathy of prematurity and neurodevelopmental outcomes in preterm infants: A systematic review and meta-analysis. Front. Pediatr. 11:1055813. doi: 10.3389/fped.2023.1055813
Received: 28 September 2022; Accepted: 16 January 2023;
Published: 15 March 2023.
Edited by:
Karel Allegaert, University Hospitals Leuven, BelgiumReviewed by:
Hercília Guimarães, University of Porto, PortugalPia Lundgren, University of Gothenburg, Sweden
© 2023 Diggikar, Gurumoorthy, Trif, Mudura, Nagesh, Galis, Vinekar and Kramer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shivashankar Diggikar c2hpdi5kaWdnaWthckBnbWFpbC5jb20=
†ORCID Shivashankar Diggikar orcid.org/0000-0002-6259-3118 Paula Trif orcid.org/0000-0003-0967-9892 Diana Mudura orcid.org/0000-0001-5225-1758 Radu Galis orcid.org/0000-0002-4165-8816 Boris W. Kramer orcid.org/000-0002-8153-7103
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Shivashankar Diggikar
Shivashankar Diggikar Puvaneswari Gurumoorthy2
Puvaneswari Gurumoorthy2 Paula Trif
Paula Trif Radu Galis
Radu Galis