
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 20 March 2023
Sec. Pediatric Nephrology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1038206
This article is part of the Research Topic Insights in Pediatric Nephrology: 2022 View all 6 articles
 Jaime M. Restrepo1,†
Jaime M. Restrepo1,† Laura Torres-Canchala2,3*
Laura Torres-Canchala2,3* Joseph V. Bonventre4,‡
Joseph V. Bonventre4,‡ Juan C. Arias5,‡
Juan C. Arias5,‡ Michael Ferguson6,‡
Michael Ferguson6,‡ Adriana Villegas7,‡
Adriana Villegas7,‡ Oscar Ramirez8,9,‡
Oscar Ramirez8,9,‡ Guido Filler10,11,†
Guido Filler10,11,†
Background: Preterm birth is associated with decreased nephron endowment. Currently, there is no reliable non-invasive biomarker to identify or monitor decreased nephron number in at-risk patients. Urinary Kidney Injury Molecule-1 (KIM-1) is a biomarker of acute and chronic renal injury. We measured urinary KIM-1 among a wide array of other potential biomarkers.
Methods: We conducted an ambispective cohort study of 5-years-old children born prematurely and healthy controls identified from city schools. Detailed anthropometrics, renal ultrasound dimensions, and biochemical parameters were measured. Urinary KIM-1 was measured using Luminex® technology. Age independent z-scores were calculated and compared. Spearman correlations were used for estimating the association between measures and KIM-1.
Results: We enrolled 129 children, 97 (75.2%) born pre-term and 32 (24.8%) healthy controls born at full-term. Pre-term patients had significantly lower weight and body surface area than controls. Pre-term patients and controls did not differ in current age, sex, race, height, blood pressure, urinary sodium, fractional sodium excretion, serum creatinine and estimated GFR. All spearman correlation between KIM-1 and gestational age, renal and serum measurements were weak without statistical significance
Conclusion: In 5-year-old children born prematurely, KIM-1 was not correlated with gestational age. Further prospective studies need to confirm this finding.
Prematurity leads to increased cardiovascular morbidity and mortality in adulthood (1), necessitating an approach for early diagnosis and intervention (2). It is generally believed that nephrogenesis, which normally continues until 36 weeks of gestation, is prematurely stopped, or at least altered with premature delivery (3, 4). Reduced nephron endowment is considered a risk factor for prenatally programmed adult morbidity, but we currently do not have a good tool for determining nephron endowment in vivo (5, 6). Traditionally, renal function is measured using serum creatinine, but creatinine is very insensitive to mildly decreased nephron endowment (3). Renal volumes have been proposed to assess nephron endowment in the neonatal period (7). However, after preterm birth, renal volume of the cortex increases rapidly (8) due to glomerular hyperfiltration (8). Renal volumes of prematurely born Swedish children (<28 weeks of gestation) are not different from healthy controls when corrected to body surface area (9) To identify children at risk, tubular function would have to be assessed, rather than estimate or measure glomerular filtration rate (GFR). In that context, it is postulated that the remaining tubules would have to excrete more urinary sodium and potassium to achieve salt and fluid homeostasis. In adults, the contralateral kidney significantly increased the fractional sodium excretion 90 days after nephrectomy (10). Moreover, urinary calcium excretion should be increased if urinary sodium excretion is increased since urinary calcium reabsorption in the distal tubule is impaired in the presence of a higher urinary sodium concentration (11, 12).
In a recent paper about reduced nephron endowment in a murine model of kidney fibrosis, urinary Kidney-Injury-Molecule-1 (KIM-1) was found to be elevated (13). Ideally, a biomarker of reduced nephron endowment should also be correlated with gestational age. Our study aim was to determine if KIM-1 (14, 15), a marker of tubular injury, would be different between 5-year old children born pre-term, compared to controls who were born at full-term. We hypothesized that urinary sodium excretion and urinary KIM-1 would be higher with a lower gestational age at birth as there are fewer tubules to handle the filtered load.
This study was performed in Cali, Colombia, using a sample of convenience served at two institutions: “Casa Madre Canguro Alfa” and Fundación Valle de Lili Hospital. “Casa Madre Canguro Alfa” is a nurse-led comprehensive program that provides longitudinal health care for preterm children. Fundación Valle del Lili is a tertiary care university with a pediatric nephology practice that serves 3,000 patients per year and a catchment area of 4.6 million people in the Southwest area of Colombia.
This was an ambispective study. The exposed cohort comprised of children who graduated from the “Casa Madre Canguro Alfa” program. Data was collected at 5 years of age (±3 months). The inclusion criteria included prematurity < 37 weeks of gestation and a birth weight < 2,500 grams. We excluded children with major congenital or renal abnormalities and/or those who were lost to follow-up. The enrolled children had their first visit to the Casa Madre Canguro Alfa program between 2006 and 2012. Healthy children with birth weight ≥ 2,500 grams and gestational age ≥ 37 weeks from different schools around the city were enrolled as controls. This study was in accordance with the Declaration of Helsinki and was approved by the Fundación Valle del Lili ethics board committee. Informed written consent was obtained from parents in each case.
The main outcome variables were urinary electrolyte concentrations and urinary KIM-1 levels and their respective urinary creatinine ratios at 5 years of age. Furthermore, each child had one renal ultrasound study interpreted by three independent radiologists for renal dimensions (with a General Electric, LOGIQ* E9) in triplicates. An age-appropriate 9–12 mHz curved array transducer was used with the participant lying in the supine position and scanned in the para-coronal view with the transducer positioned to obtain the longest kidney dimension. Kidney volume was calculated using the ellipsoid formula from the average of the 9 measurements in each dimension. Simultaneously, 3 repeated measurements for patient anthropometrics were obtained. One measure of standard serum and urine biochemistry tests were obtained.
Height was measured without shoes on a wall mounted stadiometer and weight was measured without heavy clothes using either a digital or balance-beam scale. Body mass index (BMI) was calculated as weight (kg)/height (m2). Age- and gender- independent height and weight z-scores were calculated as previously described, using the Ped(z) app with the WHO reference intervals (16).
Blood samples were collected in anticoagulant and lithium heparin tubes. Creatinine was measured using the calorimetric modified Jaffe method with alkaline picrate (17) and were isotope dilution-mass spectrometry traceable. Estimated GFR (eGFR) was calculated from the average of the three height measurements and one serum creatinine with the new modified Schwartz formula (18). Fractional excretion of sodium excretion was also calculated in percent using 100*(urinary sodium * serum creatinine)/(urinary creatinine * serum sodium) (19).
Urinary KIM-1 was measured using Luminex® technology immunoassay. Antibodies were obtained from R and D systems. The assay was run according to standardized protocol. For KIM-1 the lower and upper limits of quantitation on a seven-point calibration curve were 1.22 and 5000 pg/ml, respectively. Intra- and inter-assay precision were <6%, recovery of spiked samples was 104%–107%, and dilutional linearity was shown at 1 in 10, 1 in 100 and 1 in 1,000 dilutions in the assay diluent.
Anthropometric z-scores were calculated using the app Ped(z) based on WHO growth charts (16). Renal volume z-scores were calculated using the same app based on the body surface area nomograms based on Scholbach and Weitzel (20).
Calculations were always performed based on the mean of all repeated measurements. Continuous variables were analyzed for normal distribution using the D'Agostino-Pearson normality test (21). We used descriptive statistics wherever possible. For continuous variables we used linear regression throughout as most of the parameters were normally distributed. We also used multivariate regression with urinary calcium, serum sodium, renal length and gestational age as independent variables and urinary KIM-1 as dependent variable. No adjustments were made for missing data. All calculations were performed using GraphPad Prism version 5.0 for Mac, registered to GF, or STATA 14.0 for PC, registered to Fundación Valle de Lili.
Figure 1 demonstrates the flow of the patients included. Of the 154 eligible patients approached, 129 could be included into the study (32 full-term controls and 97 pre-term participants). Table 1 summarizes the patient characteristics.
The results of all measurements are summarized in Table 1 for the entire cohort. We compared parameters between five-years-old pre-term patients and healthy age-matched controls. While gestational age was significantly different between the pre-term babies (median 32 weeks, range 25–36) and the healthy full-term controls (median 38 weeks, range 37–42), p < 0.0001, Mann-Whitney test), we found significantly lower weight and body surface area between the pre-term and full-term children at age 5-years. Blood pressure measurements, blood pressure z-scores, serum creatinine, eGFR, renal volumes and renal volume z-scores did not differ significantly between pre-term and full-term participants at 5 years of age. The only exception was the right renal length, but not the right renal length z-score.
Median urinary KIM-1 was 17.8 pg/ml (interquartile range 4.6, 59.0 pg/ml) in the pre-term group and was not significantly different from the full-term group at 5-years (median 17.3 (interquartile range 12.3, 50.7 pg/ml).
There was a trend towards higher urinary protein in the pre-term group. Median urinary protein concentration was 7.35 mg/dl (IQR 4.57, 11.39) in the pre-term group and 5.54 mg/dl (4.01, 7.81) in the control group, p = 0.0745. There was a trend towards higher urinary creatinine in the pre-term group, median creatinine concentration was 49.9 mg/dl (IQR 24.4, 81.6) in the premature group and 34.2 (19.9, 57.85), p = 0.0879. However, urinary sodium did not differ. The urinary sodium/potassium ratio and the fractional sodium excretion did not differ between the groups. There was a trend towards higher urinary calcium in the pre-term group. In the pre-term group, median calcium concentration was 5.98 mg/dl (IQR 1.70, 10.4) and 3.16 (1.39, 6.65) in the control group (p = 0.0523). The urinary calcium/creatinine ratio, and urinary pH however, did not differ between the groups.
Table 2 describes the spearman correlation between urinary KIM-1 and the other measurements taken from the patients. In patients full-term five-years old patients, average left renal length and serum chloride had a strong correlation with serum KIM-1 concentration (n = 7 Spearman 0.786, p = 0.036). In moderate preterm patients, there was a moderate correlation with z-score for height and KIM-1 (n = 33, Spearman 0.408, p = 0.020). In the 5-year extreme preterm patients, diastolic blood pressure z-score was strongly correlated with serum KIM-1 concentration (n = 11, Spearman 0.770. p = 0.004). There was no correlation between gestational age at born and KIM-1 serum concentration (n = 73, Spearman −0.081, p = 0.423).
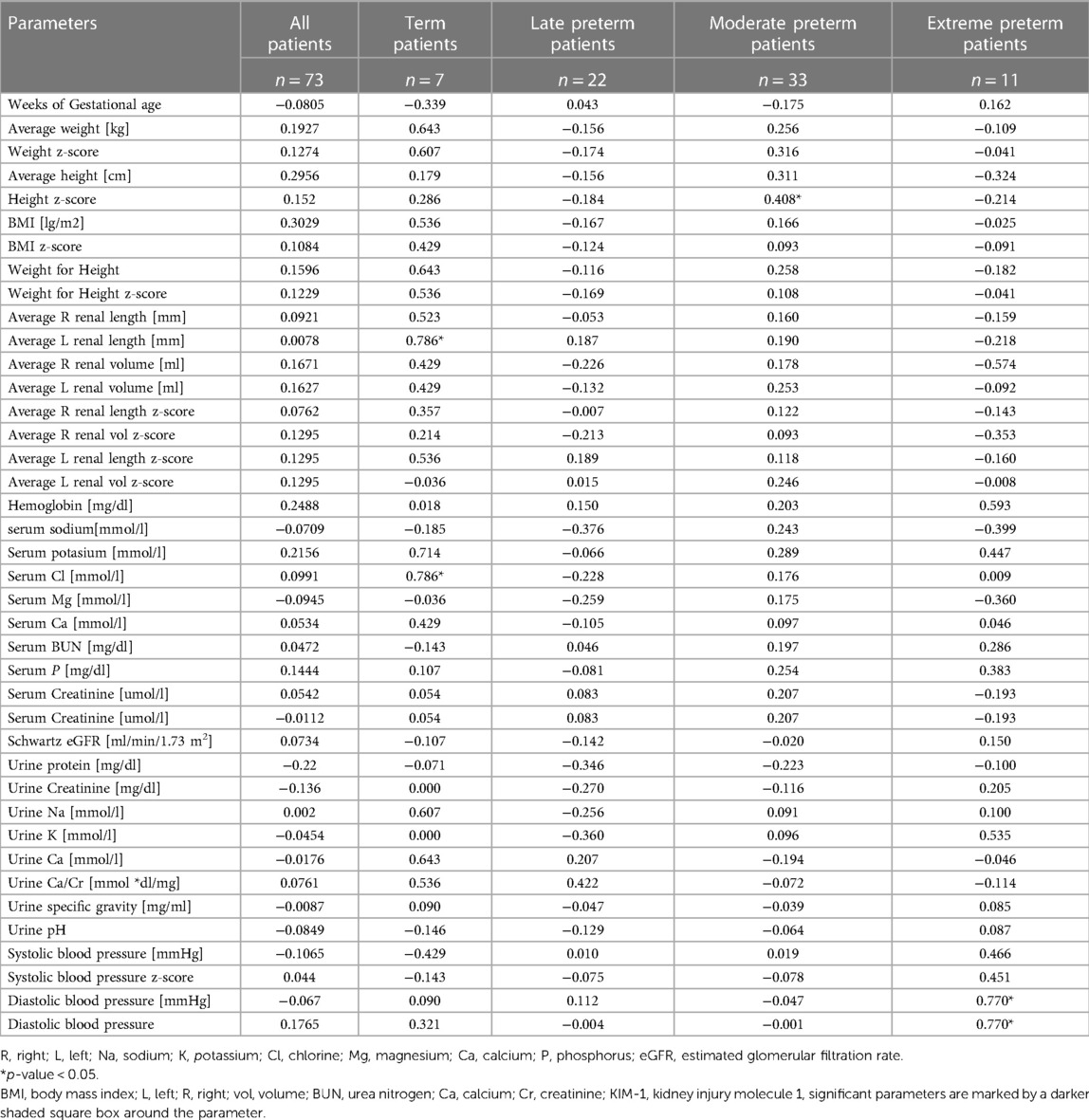
Table 2. Spearman correlation of urinary KIM-1 with individual measurements according to gestational age.
In this ambispective study of pre-term born 5-year-old children and healthy full-term controls, we found that KIM-1 was not related with gestational age and birth weight. Also, urinary KIM-1 concentrations did not differ between pre-term and full-term born at age 5-year-old. We found no differences between both groups for the large array of other clinical, ultrasound and biochemical tests assessing renal function, including fractional sodium excretion. No other biochemical or clinical or radiological parameter gave a signal that could be related to lower nephron endowment. We must reject the hypothesis that urinary sodium excretion was related to gestational age in 5-year-old children born prematurity.
Developmental programming of a reduced nephron endowment is more than just a infant's birth weight (22). About 30 years ago, David Barker postulated that low birth weight is associated with increased cardiovascular morbidity and mortality in adults, which was later linked to renal disease (23). The hypothesis of developmental origin and fetal programming of adult disease is considered proven and low nephron endowment may play a major role in this association (24). As outlined in the introduction, nephrogenesis ceases at 36 weeks of gestation and is likely reduced with premature delivery (2). To date, we don't have a precise tool for estimating nephron endowment in vivo. Experimental methods using magnetic resonance imaging in mice (25) or in rabbits (26) have not yet been established in humans. Effective renal plasma flow with concurrent measurement of glomerular filtration rate may reveal hyperfiltration and thus reduced nephron endowment (27); however, nuclear medicine measurements of effective renal plasma flow remain available only in the research setting. A biomarker has been lacking. KIM-1 seemed to be positioned as a promising biomarker to detect chronic kidney failure promptly (28). However, in this paper, there was no correlation between urinary KIM-1 concentrations and gestational age in five-year-old children; therefore KIM-1 could not be a surrogate for renal tubular dysfunction at five years of age. Our findings agree with a similar sized study from India where there was also no difference between 100 low birth weight and 66 normal birth weight children at three different time points during the dynamic phase of renal maturation in infancy (29). Similarly Askenazi et al., in a prospective cohort study on 113 VLBW infants (weight < 1200 g or <31 weeks' gestation) using urinary biomarkers, didn't find KIM-1 as an early tubular marker in patients with AKI neither without AKI (30).
Our study limitations include the sample size. Only 73 patients had urinary KIM-1 measured, of which only 7 patients were born at term. Future, larger studies must confirm these empirical findings. There were challenges with the recruitment of the control group and the healthy children do not have prospective data in this ambispective study. The data are also limited to predominantly Latino children and while a small proportion of patients were African descendants (11.5%, data not shown), the number was quite small to perform a subgroup analysis. Moreover, there was significant attrition in the prospectively recruited premature cohort, which introduces an unclear bias. The data may therefore not be generalizable and certainly must be confirmed in other ethnic backgrounds. Also, other urinary tubular markers such as Neutrophil gelatinase-associated lipocalin (31) and biomarkers of glomerular filtration rate such as cystatin C (32) were not employed.
Iyengar's study also did not demonstrate any differences when measuring estimated GFR with cystatin C (29). Strengths of the study include the wide array of clinical, anthropological, biochemical, and imaging modalities to assess these patients. The prospective nature of the prematurely born cohort is another strength.
Taken together, we found that urinary KIM-1 was not significantly correlated with gestational age and birth weight in a cohort of 5-year-old children born at term and prematurely, our findings need to be confirmed in a larger prospective study.
As is known, renal growth in both cortical and medullary parts increase significantly during pre and adolescence. Our study aimed to evaluate the impact of prematurity on renal function measured with urinary KIM-1 at the middle age of 5 years. Although this study did not show significant differences, it is a starting point for adolescent prospective evaluation, age of high metabolic demand. The metabolic challenge generated by body growth and the requirement for kidney growth added to the history of prematurity can be reflected in the tubular injury expressed in alterations in markers such as KIM-1, NGAL and cystatin C.
The data that support the findings of this study are available on request from the corresponding author, LTC. The data are not publicly available due to the international ethical regulations that protect the data security of the subjects.
The Institutional Review Board (IRB) of Fundación Valle del Lili approved this study with the approval letter No. 043-2010, and the study number 444 was assigned. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
JMR: conceived the study, obtained ethics approval, was involved in all aspects of the paper generation, and revised each draft, contributed to the statistical analysis, and coordinated all coauthors’ activities. JMR and OR: designed the study. JCA LT and AV: collected all the data. JVB: processed al the samples. MR and other two radiologist performed the renal ultrasounds. LTC and GF: performed the statistical analysis and wrote the different versions. LTC and GF: also prepared the tables and figures. All authors interpreted the data. All authors provided critical input into all aspects of the design and execution of the study and participated in all phases of the paper writing. All authors participated in revising the manuscript critically for important intellectual content and approved the final version to be submitted to the journal. All authors contributed to the article and approved the submitted version.
This study was generously supported by the International Society of Nephrology (ISN) with $10,000 US and Wendy Hoy with $10,000 US through the ISN Sister Renal Center Program: Boston Children’s Hospital USA—Fundación Valle del Lili, Cali, Colombia. JVB is supported by NIH grants DK39773 and DK72381
We thank the Lilibeth Caberto Kidney Clinical Research Unit, University of Western Ontario, in London Ontario, for the generous assistance with research space and infrastructure. We thank the Centro de Investigaciones Clinicas of Fundación Valle del Lili for the strong support given during the entire research process. Dra. Laura Torres-Canchala was generously supported by the International Society of Nephrology for a two-months research sabbatical at the University of Western Ontario. We thank Maria Diaz-González de Ferris who kindly edited the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chehade H, Simeoni U, Guignard J-P, Boubred F. Preterm birth: long term cardiovascular and renal consequences. Curr Pediatr Rev. (2018) 14(4):219–26. doi: 10.2174/1573396314666180813121652
2. Luyckx V, Bertram J, Brenner B, Fall C, Hoy W, Ozanne S, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet. (2013) 382:273–83. doi: 10.1016/S0140-6736(13)60311-6
3. Filler G, Lopes L, Awuku M. The importance of accurately assessing renal function in the neonate and infant. Adv Clin Chem. (2015) 71:141–56. doi: 10.1016/BS.ACC.2015.06.004
4. Filler G, Guerrero-Kanan R, Alvarez-Elías AC. Assessment of glomerular filtration rate in the neonate: is creatinine the best tool? Curr Opin Pediatr. (2016) 28:173–9. doi: 10.1097/MOP.0000000000000318
5. Puelles VG, Kanzaki G, Bertram JF. Indirect estimation of nephron number: a new tool to predict outcomes in renal transplantation? Nephrol Dial Transplant. (2016) 31:1378–80. doi: 10.1093/NDT/GFW034
6. Filler G, Yasin A, Medeiros M. Methods of assessing renal function. Pediatr Nephrol. (2014) 29:183–92. doi: 10.1007/S00467-013-2426-7
7. Abitbol CL, Seeherunvong W, Galarza MG, Katsoufis C, Francoeur D, Defreitas M, et al. Neonatal kidney size and function in preterm infants: what is a true estimate of glomerular filtration rate? J Pediatr. (2014) 164(5):1026–1031.e2. doi: 10.1016/J.JPEDS.2014.01.044
8. Li J, Guandalini M, Mcinnes H, Kandasamy Y, Trnka P, Moritz K. The impact of prematurity on postnatal growth of different renal compartments. Nephrology. (2020) 25:116–24. doi: 10.1111/NEP.13623
9. Rakow A, Laestadius Å, Liliemark U, Backheden M, Legnevall L, Kaiser S, et al. Kidney volume, kidney function, and ambulatory blood pressure in children born extremely preterm with and without nephrocalcinosis. Pediatr Nephrol. (2019) 34:1765–76. doi: 10.1007/S00467-019-04293-9
10. Tantisattamo E, Dafoe DC, Reddy UG, Ichii H, Rhee CM, Streja E, et al. Current management of patients with acquired solitary kidney. Kidney Int Rep. (2019) 4:1205. doi: 10.1016/J.EKIR.2019.07.001
11. Osorio Av, Alon US. The relationship between urinary calcium, sodium, and potassium excretion and the role of potassium in treating idiopathic hypercalciuria. Pediatrics. (1997) 100:675–81. doi: 10.1542/PEDS.100.4.675
12. Rodriguez Cuellar CI, Wang PZT, Freundlich M, Filler G. Educational review: role of the pediatric nephrologists in the work-up and management of kidney stones. Pediatr Nephrol. (2020) 35:383–97. doi: 10.1007/S00467-018-4179-9
13. Humphreys BD, Xu F, Sabbisetti V, Grgic I, Naini SM, Wang N, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. (2013) 123:4023–35. doi: 10.1172/JCI45361
14. Kaddourah A, Goldstein SL, Basu R, Nehus EJ, Terrell TC, Brunner L, et al. Novel urinary tubular injury markers reveal an evidence of underlying kidney injury in children with reduced left ventricular systolic function: a pilot study. Pediatr Nephrol. (2016) 31:1637–45. doi: 10.1007/S00467-016-3360-2
15. de Silva PMCS, Mohammed Abdul KS, Eakanayake EMDV, Jayasinghe SS, Jayasumana C, Asanthi HB, et al. Urinary biomarkers KIM-1 and NGAL for detection of chronic kidney disease of uncertain etiology (CKDu) among agricultural communities in Sri Lanka. PLoS Negl Trop Dis. (2016) 10(9):e0004979. doi: 10.1371/JOURNAL.PNTD.0004979
16. de Onis M, Onyango A, Borghi E, Siyam A, Blössner M, Lutter C, et al. Worldwide implementation of the WHO child growth standards. Public Health Nutr. (2012) 15:1603–10. doi: 10.1017/S136898001200105X
17. Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, et al. OPTN/SRTR 2012 annual data report: kidney. Am J Transplant. (2014) 14:11–44. doi: 10.1111/ajt.12579
18. Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. (2012) 82:445–53. doi: 10.1038/ki.2012.169
19. Torrubia Romero FJ, García Matilla JMM, Roa Romero LM, Cantero Rodríguez A, Fernandez Santiago E. Changes in creatinine clearance and fractional sodium excretion by the remaining kidney after nephrectomy of the contralateral diseased kidney in the adult. Arch Esp Urol. (1990) 43(1):5–11. 2331165. https://pubmed.ncbi.nlm.nih.gov/2331165/ [Accessed September 4, 2022]2331165
20. Scholbach T, Weitzel D. Body-surface-area related renal volume: a common normal range from birth to adulthood. Scientifica. (2012) 2012:1–4. doi: 10.6064/2012/949164
21. D’Agostino RB, Belanger A, D’Agostino RB. A suggestion for using powerful and informative tests of normality. Am Stat. (1990) 44:316. doi: 10.2307/2684359
22. Moritz KM, Singh RR, Probyn ME, Denton KM. Developmental programming of a reduced nephron endowment: more than just a baby's Birth weight. Am J Physiol Renal Physiol. (2009) 296(1): F1–F9. doi: 10.1152/AJPRENAL.00049.2008
23. Hoy WE, Rees M, Kile E, Mathews JD, Wang Z. A new dimension to the barker hypothesis: low birthweight and susceptibility to renal disease. Kidney Int. (1999) 56:1072–7. doi: 10.1046/j.1523-1755.1999.00633.x
24. Filler G, Yasin A, Kesarwani P, Garg AX, Lindsay R, Sharma AP. Big mother or small baby: which predicts hypertension? J Clin Hypertens. (2011) 13:35–41. doi: 10.1111/J.1751-7176.2010.00366.X
25. Baldelomar EJ, Charlton JR, DeRonde KA, Bennett KM. In vivo measurements of kidney glomerular number and size in healthy and Os /+ mice using MRI. Am J Physiol Renal Physiol. (2019) 317:F865–73. doi: 10.1152/AJPRENAL.00078.2019
26. Charlton JR, Baldelomar EJ, deRonde KA, Cathro HP, Charlton NP, Criswell SJ, et al. Nephron loss detected by MRI following neonatal acute kidney injury in rabbits. Pediatr Res. (2020) 87:1185–92. doi: 10.1038/S41390-019-0684-1
27. Huang SHS, Sharma AP, Yasin A, Lindsay RM, Clark WF, Filler G. Hyperfiltration affects accuracy of creatinine eGFR measurement. Clin J Am Soc Nephrol. (2011) 6:274. doi: 10.2215/CJN.02760310
28. Moresco RN, Bochi GV, Stein CS, de Carvalho JAM, Cembranel BM, Bollick YS. Urinary kidney injury molecule-1 in renal disease. Clin Chim Acta. (2018) 487:15–21. doi: 10.1016/J.CCA.2018.09.011
29. Iyengar A, Nesargi S, George A, Sinha N, Selvam S, Luyckx VA. Are low birth weight neonates at risk for suboptimal renal growth and function during infancy? BMC Nephrol. (2016) 17(1):100. doi: 10.1186/s12882-016-0314-7
30. Askenazi DJ, Koralkar R, Patil N, Halloran B, Ambalavanan N, Griffin R. Acute kidney injury urine biomarkers in very low-birth-weight infants. Clin J Am Soc Nephrol. (2016) 11:1527–35. doi: 10.2215/CJN.13381215
31. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, Bagshaw SM, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. (2009) 54:1012–24. doi: 10.1053/J.AJKD.2009.07.020
Keywords: KIM-1 (kidney injury molecule 1), gestational age (GA), renal volume z-scores, specific gravity (density), prematurity and low birth weight
Citation: Restrepo JM, Torres-Canchala L, Bonventre JV, Arias JC, Ferguson M, Villegas A, Ramirez O and Filler G (2023) Urinary KIM-1 is not correlated with gestational age among 5-year-old children born prematurely. Front. Pediatr. 11:1038206. doi: 10.3389/fped.2023.1038206
Received: 6 September 2022; Accepted: 25 January 2023;
Published: 20 March 2023.
Edited by:
Jacqueline Ho, University of Pittsburgh, United StatesReviewed by:
Vasiliki Karava, Aristotle University of Thessaloniki, Greece© 2023 Restrepo, Torres-Canchala, Bonventre, Arias, Ferguson, Villegas, Ramirez and Filler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Torres-Canchala bGF1cmEudG9ycmVzQGZ2bC5vcmcuY28=; bGF1cmFsdG9ycmVzY2FAZ21haWwuY29t
†These authors share senior authorship
‡These authors have contributed equally to this work and share last authorship
Specialty Section: This article was submitted to Pediatric Nephrology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.