
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 02 November 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.993167
This article is part of the Research Topic Respiratory Support Strategies in the Prevention and Treatment of Bronchopulmonary Dysplasia View all 14 articles
Introduction: Moderate and severe bronchopulmonary dysplasia (BPD) is a common pulmonary complication in premature infants, which seriously affects their survival rate and quality of life. This study aimed to describe the mechanical ventilation characteristics and evaluate their prediction performance for the risk of moderate and severe BPD in infants with gestational age <30 weeks and birth weight <1,500 g on postnatal Day 14.
Methods: In this retrospective cohort study, 412 infants with gestational age <30 weeks and birth weight <1,500 g were included in the analysis, including 104 infants with moderate and severe BPD and 308 infants without moderate and severe BPD (as controls). LASSO regression was used to optimize variable selection, and Logistic regression was applied to build a predictive model. Nomograms were developed visually using the selected variables. To validate the model, receiver operating characteristic (ROC) curve, calibration plot, and clinical impact curve were used.
Results: From the original 28 variables studied, six predictors, namely birth weight, 5 min apgar score, neonatal respiratory distress syndrome (≥Class II), neonatal pneumonia, duration of invasive mechanical ventilation (IMV) and maximum of FiO2 (fraction of inspiration O2) were identified by LASSO regression analysis. The model constructed using these six predictors and a proven risk factor (gestational age) displayed good prediction performance for moderate and severe BPD, with an area under the ROC of 0.917 (sensitivity = 0.897, specificity = 0.797) in the training set and 0.931 (sensitivity = 0.885, specificity = 0.844) in the validation set, and was well calibrated (PHosmer-Lemeshow test = 0.727 and 0.809 for the training and validation set, respectively).
Conclusion: The model included gestational age, birth weight, 5 min apgar score, neonatal respiratory distress syndrome (≥Class II), neonatal pneumonia, duration of IMV and maximum of FiO2 had good prediction performance for predicting moderate and severe BPD in infants with gestational age <30 weeks and birth weight <1,500 g on postnatal Day 14.
In preterm infants, bronchopulmonary dysplasia (BPD) is one of the most common and serious pulmonary complications, seriously affecting the quality of their lives. The definition of BPD is oxygen support as needed for at least 28 days (1). According to their required fraction of inspired oxygen (FiO2) at 36 weeks of corrected gestational age, infants with gestational age <32 weeks were categorized as mild (no oxygen requirement), moderate (21%–30%) or severe BPD (over 30% or positive pressure assistance) (1). With the improvement of the treatment success rate of very low birth weight infants (VLBWI, birth weight <1,500 g) and extremely low birth weight infants (ELBWI, birth weight <1,000 g), the incidence of BPD is also increasing. According to the studies from many countries, BPD’s incidence varies from 11 to 50%, due to the different diagnostic and treatment criteria (2). BPD incidence increases as the gestational age or birth weight decreases. Previous studies have reported that about 30% of VLBWI suffer from BPD (3), the incidence rate of BPD fluctuated between 12.9% and 41% in infants with gestational age <32 weeks, and the incidence rate can reach 80% in infants with gestational age <25 weeks (4). The quality of life for preterm infants with BPD is reduced due to a higher mortality rate and a higher incidence of pulmonary, cardiovascular, and neurodevelopmental disorders (5, 6). Moreover, preterm infants with moderate and severe BPD are more likely to suffer complications and comorbidities, including longer hospital stays, respiratory support after discharge and higher death risk (7, 8). Therefore, clarifying the predictors of moderate and severe BPD, early screening and prevention of moderate and severe BPD is of great significance not only for clinical control of moderate and severe BPD, but also for improving the prognosis of preterm infants.
Many clinical risk factors for BPD have been reported in infants with gestational age <32 weeks over the past few decades (9). To our knowledge, smaller gestational age and lower birth weight are proven risk factors for BPD (9). Other perinatal and postpartum factors can increase the risk of BPD, such as chorioamnionitis, patent ductus arteriosus (PDA), neonatal pneumonia, neonatal respiratory distress syndrome (10). Moreover, supportive care with mechanical ventilation is an essential strategy for managing severe neonatal respiratory failure. It is well known that the ventilator-induced lung injury is an important risk factor for BPD (11). In a study involving 17 centers, the authors developed and validated models for BPD risk at 6 postnatal ages using gestational age, birth weight, race and ethnicity, sex, respiratory support, and FiO2, and found that gestational age conveyed the most predictive information for BPD risk on Postnatal Days 1 and 3, and respiratory support on Days 7, 14, 21, and 28 (12). Although previous predictive models for BPD have been described (12–14), studies focused on the risk factors and prediction models for moderate and severe BPD are few, especially in infants with gestational age <30 weeks and birth weight <1,500 g. In the current study, based on the clinical characteristics and the different types of mechanical ventilation up to postnatal Day 14, we analysed and identified risk factors affecting moderate and severe BPD, and developed a meaningful risk prediction model for paediatricians and neonatologists to perform early screening of moderate and severe BPD in infants with gestational age <30 weeks and birth weight <1,500 g on postnatal Day 14. The prediction model could be used to target VLBWI at the highest risk of moderate and severe BPD, to tailor follow-up and preventive measures for VLBWI.
This was a retrospective study based on electronic medical records from NingBX neonatal perinatal network (http://www.ningbx.com). Infants with gestational age <30 weeks and birth weight <1,500 g were recruited at the Department of Pediatrics, Nanjing Maternity and Child Health Care Hospital from January 2018 to December 2021. This study was approved by the Ethics Committee of Nanjing Maternity and Child Health Care Hospital, and either a legal guardian or parent provided informed consent. Moderate and severe BPD is defined as oxygen requirement (FiO2 > 21%) for at least 28 days, and the need for any type of respiratory support at 36 weeks post-menstrual age (1, 15). The preterm infants with moderate and severe BPD were assigned to the case group, while preterm infants without moderate and severe BPD were assigned to the control group. Inclusion criteria were: (1) gestational age at birth <30 weeks and birth weight <1,500 g; (2) infants hospitalized within 1 day after birth. Whereas exclusion criteria were: (1) infants hospitalized for less than 28 days and lost to follow-up after discharge, who still required continued oxygen inhalation and hospitalization; (2) infants with severe congenital malformation; (3) infants with pneumothorax or pleural effusion; (4) infants undergoing surgery; (5) infants who died less than 28 days after birth; (6) incomplete data. At last, 412 infants with gestational age <30 weeks and birth weight <1,500 g were included in the analysis, including 104 infants with moderate and severe BPD and 308 controls.
We also collected multiple maternal and neonatal data according to electronic medical records up to postnatal Day 14, including maternal characteristics, medication use, neonatal characteristics, mechanical ventilation and oxygen requirement (Table 1). The maternal characteristics included gestational diabetes mellitus, maternal hypertension and chorioamnionitis. Medication use included prenatal use of glucocorticoids and magnesium sulfate. The neonatal characteristics included infant gender, gestational age at birth (weeks), birth weight (g), delivery mode, 1 min and 5 min apgar score, neonatal asphyxia, neonatal respiratory distress syndrome (≥Class II), neonatal pneumonia, patent ductus arteriosus (PDA), PDA size (mm), PDA treatment, PDA and ventricular septal defect, intraventricular hemorrhage (IVH III/IV), sepsis, and necrotizing enterocolitis (NEC ≥ Stage II). Neonatal asphyxia is defined as the inability of neonates to initiate and maintain breathing at birth, followed by impaired gas exchange, resulting in progressive hypoxemia, hypercapnia, and severe metabolic acidosis. Mild asphyxia is diagnosed by 1 min apgar score ≤7, or 5 min apgar score ≤7 and umbilical cord arterial pH <7.2; whereas severe asphyxia is diagnosed by 1 min apgar score ≤3, or 5 min apgar score ≤5 and umbilical cord arterial pH <7.0.
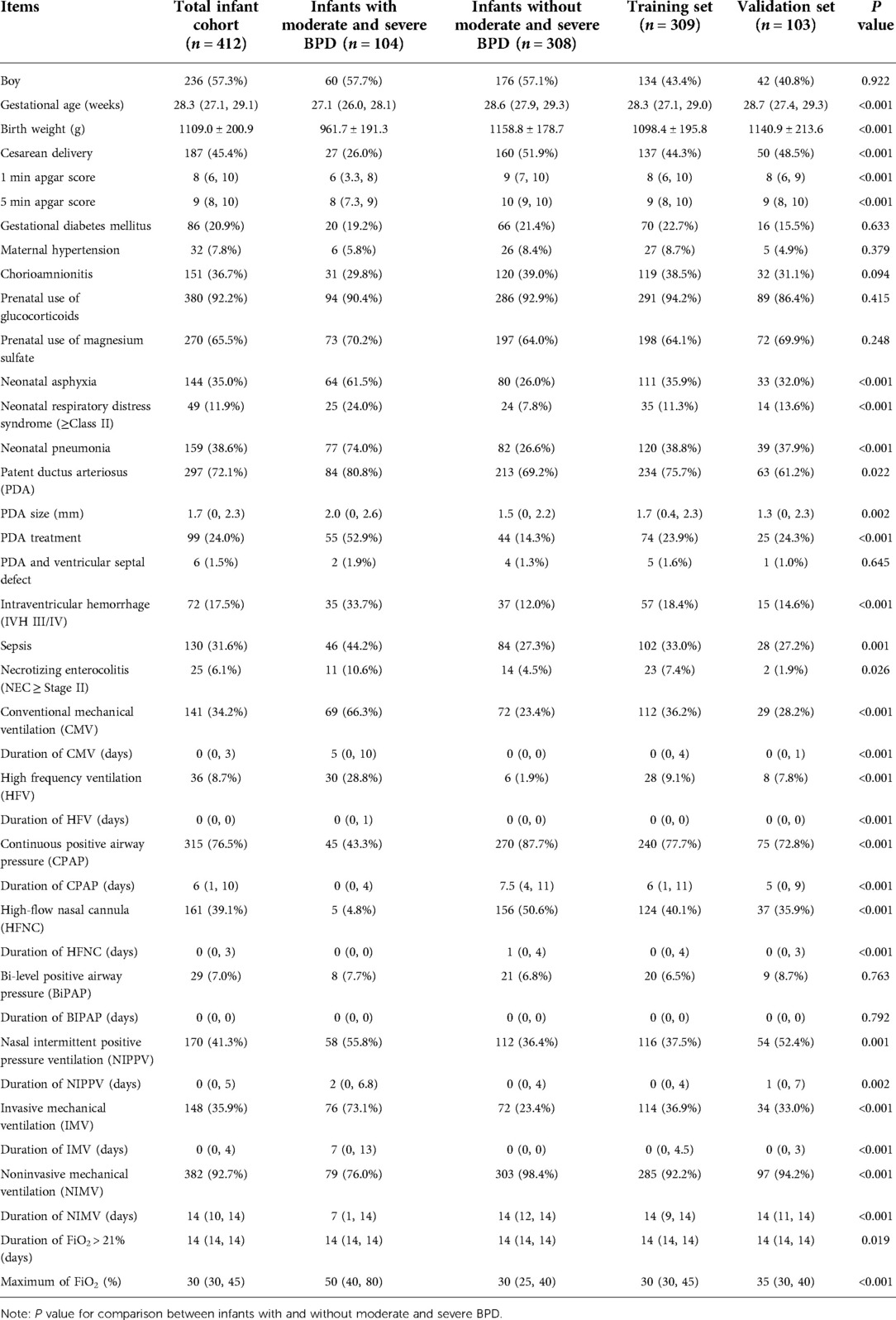
Table 1. Characteristics of the 412 preterm infants with gestational age <30 weeks and birth weight <1,500 g enrolled in the study according to presence/absence of moderate and severe bronchopulmonary dysplasia (BPD) and randomization to training set and validation set.
The characteristics of mechanical ventilation and oxygen requirement included conventional mechanical ventilation (CMV), duration of CMV (days), high frequency ventilation (HFV), duration of HFV (days), continuous positive airway pressure (CPAP), duration of CPAP (days), high-flow nasal cannula (HFNC), duration of HFNC (days), Bi-level positive airway pressure (BiPAP), duration of BiPAP (days), nasal intermittent positive pressure ventilation (NIPPV), duration of NIPPV (days), invasive mechanical ventilation (IMV), duration of IMV (days), noninvasive mechanical ventilation (NIMV), duration of NIMV (days), duration of FiO2 > 21% (days), and maximum of FiO2 (%). IMV includes CMV and HFV, and NIMV includes CPAP, HFNC, BiPAP and NIPPV.
Statistical analyses were conducted by applying R (v4.1.3) software. Normally distributed continuous variables were displayed as mean (SD) and skewed distributed variables as median (25th, 75th), and Student's t-test or Mann-Whitney test were used to compare the two study groups. For categorical variables, frequency (percentage) was displayed and compared using χ2 test or Fisher exact test.
Based on the significantly different clinical characteristics (P < 0.05), we sought to build predictive model for moderate and severe BPD in infants with gestational age <30 weeks and birth weight <1,500 g on postnatal Day 14. First, conforming to a ratio of 3 : 1, we randomly divided the 412 preterm infants into a training set with 309 infants and a validation set with 103 infants. To identify the best predictors of current risk factors, LASSO (least absolute shrinkage and selection operator) regression was performed in the training set using R glmnet package (16). As the dependent variable is whether moderate and severe BPD is present or not, we set family = “binomial” and set type.measure = “deviance”. Based on the binomial family and the type measure of deviance, the LASSO regression analysis runs a ten-fold cross-validation for centralizing and normalizing the variables included, and then the best lambda value was picked. In terms of performance, Lambda.1se gives the best results, but with the fewest independent variables. Then the R rms package was used to run logistic regression to construct a prediction model for moderate and severe BPD by introducing the factors selected in the LASSO regression and the proven risk factors for moderate and severe BPD. All of the selected factors were applied to construct nomogram prediction model. In order to easily approximate the individual-specific risk of moderate and severe BPD in infants with gestational age <30 weeks and birth weight <1,500 g on postnatal Day 14, a graphical nomogram was also created (17).
Additionally, the accuracy of the model was estimated using several validation methods using the data of training set and validation set, respectively (16, 18). Receiver-operator characteristic (ROC) curve was constructed with the R pROC package, and the area under the ROC curve (AUC) provided good discrimination between true positives and false positives for the quality of the risk nomogram. Using the “best threshold” criteria of the ROC curve, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated to illustrate the model effects. To evaluate the calibration of the moderate and severe BPD risk nomogram, along with the Hosmer-Lemeshow test, the R rms package was used to draw the calibration curves. As well, clinical impact curves were drawn using the R rmda package to determine whether the nomogram is clinically practicable for predicting moderate and severe BPD. All statistical significance levels reported were two-sided, and P < 0.05 was considered to be significant.
In total, 519 infants with gestational age <30 weeks and birth weight <1,500 g were recruited in Nanjing Maternity and Child Health Care Hospital from 2018 to 2021. We excluded 79 uncured infants hospitalized for less than 28 days and lost to follow-up after discharge; 3 infants with severe congenital malformation; 7 infants with pneumothorax or pleural effusion; 13 infants undergoing surgery; and 5 infants who died less than 28 days after birth. At last, 412 infants were included in the analysis (Figure 1), including 104 infants with moderate and severe BPD and 308 infants without moderate and severe BPD (as controls). These infants were randomly divided into a training set with 309 infants and a validation set with 103 infants for external validation.
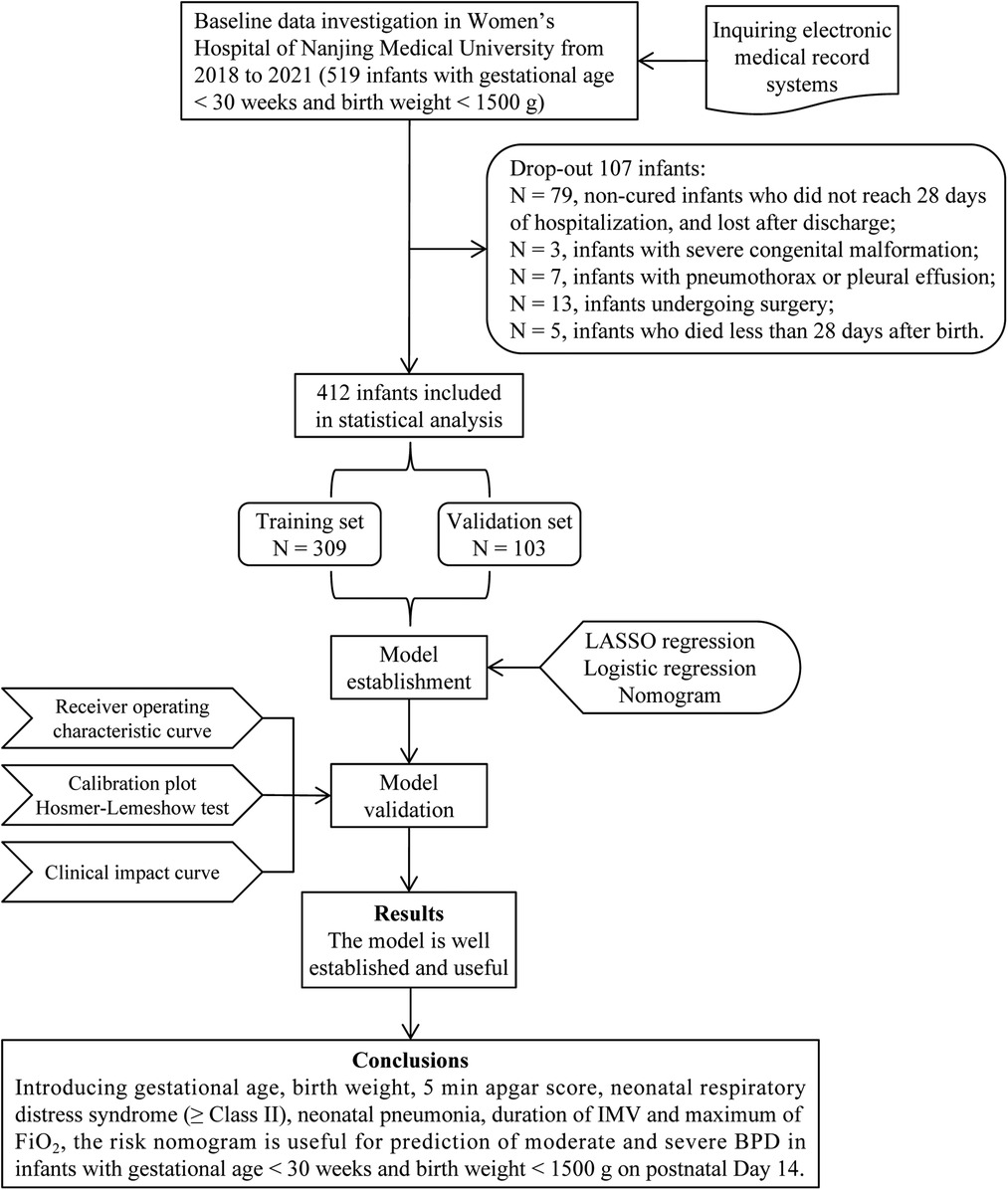
Figure 1. Flow diagram of study design. BPD, bronchopulmonary dysplasia; IMV, invasive mechanical ventilation; FiO2, fraction of inspiration O2.
The maternal characteristics, medication use, neonatal characteristics, mechanical ventilation and oxygen requirement up to postnatal Day 14 are shown in Table 1. In terms of maternal characteristics and medication use, the rates of gestational diabetes mellitus, hypertension, chorioamnionitis, prenatal use of glucocorticoids and magnesium sulfate were similar between the two study groups. In terms of neonatal characteristics, the gestational age, birth weight, cesarean delivery rate, 1 min and 5 min apgar score were significantly lower in infants with moderate and severe BPD than those in control group (all P < 0.001). Whereas the rates of neonatal asphyxia, neonatal respiratory distress syndrome (≥Class II), neonatal pneumonia, PDA, PDA treatment, IVH III/IV, sepsis, and NEC (≥Stage II) were significantly higher in infants with moderate and severe BPD (all P < 0.05), and the PDA size was also larger in infants with moderate and severe BPD (P = 0.002).
We then focused on the characteristics of mechanical ventilation and oxygen requirement up to postnatal Day 14 between the two study groups. In infants with moderate and severe BPD, the rates of CMV, HFV, NIPPV and IMV, and the duration of CMV, HFV, NIPPV and IMV were significantly higher than those in control group (all P < 0.01), whereas the rates of CPAP, HFNC and NIMV, and the duration of CPAP, HFNC and NIMV were significantly lower than that in control group (P < 0.001). For oxygen requirement, the maximum of FiO2 was significantly higher in infants with moderate and severe BPD (P < 0.001).
The significant items in Table 1 were selected for predictive variables using LASSO regression analysis. As a result, 6 of the original 28 variables were considered in the predictive model, including birth weight, 5 min apgar score, neonatal respiratory distress syndrome (≥Class II), neonatal pneumonia, duration of IMV and maximum of FiO2 (Figure 2). In the LASSO regression model, these 6 variables had non-zero coefficients. As smaller gestational age is proven risk factors for moderate and severe BPD, we also introduced this variable into the predictive model. In Table 2, we present the results of the logistic regression analysis for these 7 variables. Based on the predictive model, a nomogram was used to quantitatively predict the risk probability of moderate and severe BPD in infants with gestational age <30 weeks and birth weight <1,500 g on postnatal Day 14 (Figure 3A). As an example, an infant with gestational age of 25.7 weeks, birth weight of 780 g, 5 min apgar score of 9, without neonatal respiratory distress syndrome (≥Class II), with neonatal pneumonia, a duration of IMV for 13 days, and maximum of FiO2 of 55%, has an estimated probability of moderate and severe BPD of 0.879 (Figure 3B).
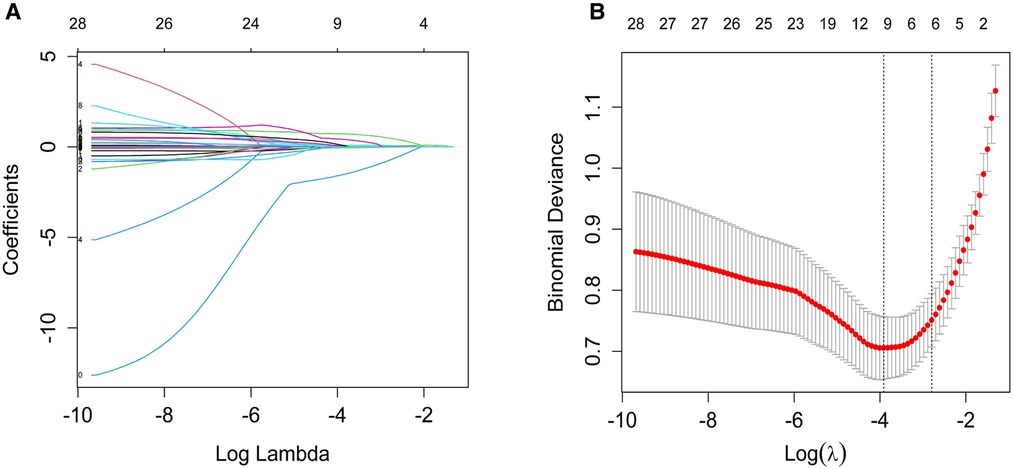
Figure 2. Variable selection by the LASSO binary logistic regression model. (A) A coefficient profile plot was constructed against the log(lambda) sequence. Twenty-eight variables with nonzero coefficients were selected by deriving the optimal lambda. (B) Following verification of the optimal lambda in the LASSO model, we plotted the binomial deviance curve versus log(lambda) and drew dotted vertical lines based on 1 standard error criteria. LASSO, Least absolute shrinkage and selection operator.
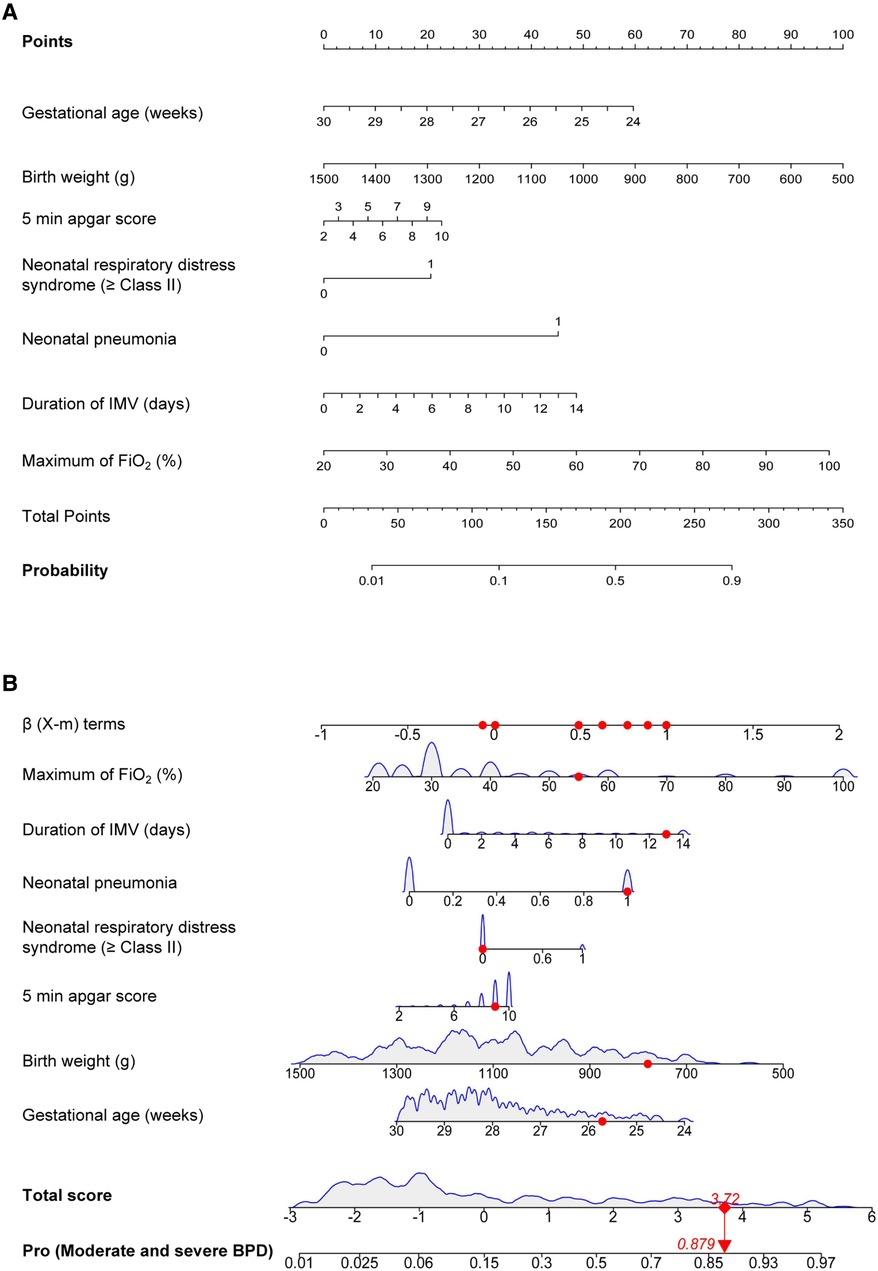
Figure 3. Moderate and severe BPD risk nomogram prediction model. (A) Risk factors of gestational age, birth weight, 5 min apgar score, neonatal respiratory distress syndrome (≥Class II), neonatal pneumonia, duration of IMV and maximum of FiO2 for nomogram prediction model. (B) Dynamic nomogram used as an example. BPD, bronchopulmonary dysplasia; IMV, invasive mechanical ventilation; FiO2, fraction of inspiration O2.
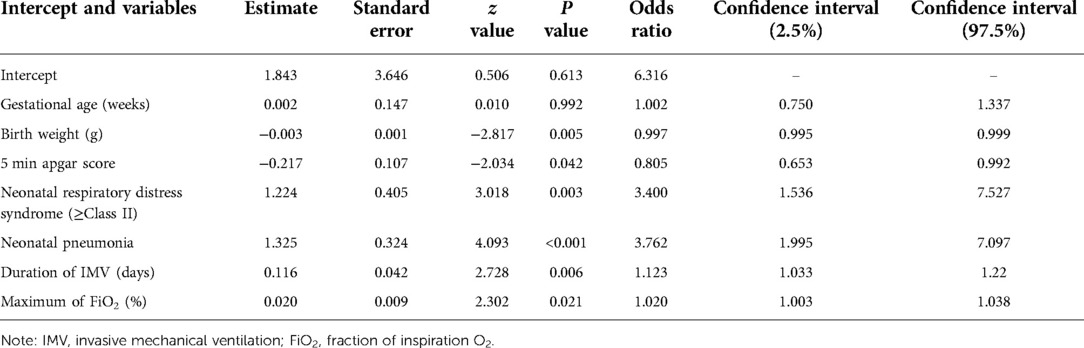
Table 2. Logistic regression analysis of the predictors for the risk of moderate and severe bronchopulmonary dysplasia (BPD) in infants with gestational age <30 weeks and birth weight <1,500 g on postnatal Day 14.
In order to evaluate the predictive model's discriminatory capacity, the ROC curve was used. The pooled AUC of the predictive model is 0.917 (sensitivity = 0.897, specificity = 0.797) in the training set and 0.931 (sensitivity = 0.885, specificity = 0.844) in the validation set, which indicates good performance (Figure 4). We also conducted a sensitivity analysis for severe BPD alone, with the pooled AUC of 0.933 (sensitivity = 0.900, specificity = 0.847) in the total data set. Afterwards, the predictive model was calibrated using a calibration plot and Hosmer–Lemeshow test. According to the calibration curves, the predictive model fit the data very well (Figure 5). Moreover, the Hosmer–Lemeshow test showed high consistency between actual and predicted probabilities (P = 0.727 for training set, P = 0.809 for validation set). Figure 6 shows the predictive model's clinical impact curves. The red solid line shows the total number of patients deemed high risk at each risk threshold out of 1,000. The blue dashed line indicates how many of those were true positives.
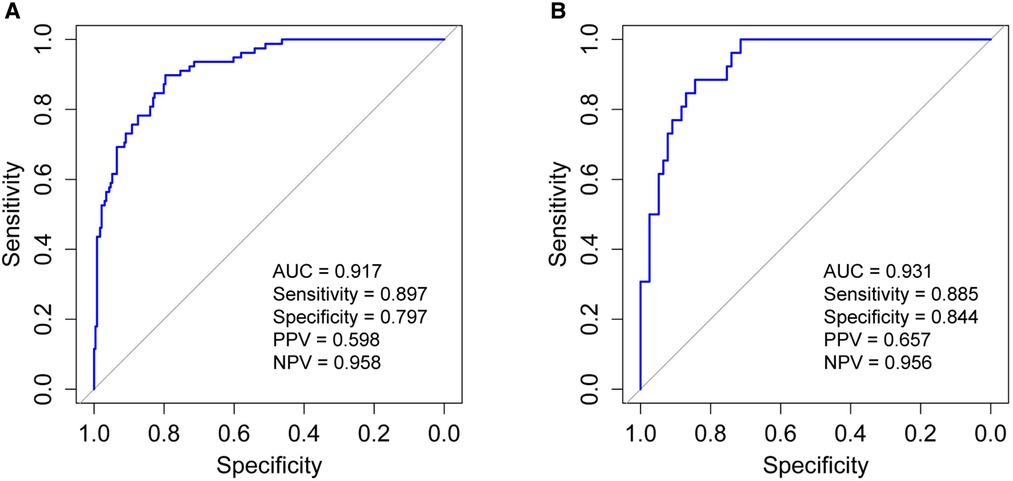
Figure 4. Receiver operating characteristic curve (ROC) validation of the moderate and severe BPD risk nomogram prediction. The y-axis represents the true positive rate of the risk prediction, the x-axis represents the false positive rate of the risk prediction. (A) The performance in the training set; (B) the performance in the validation set. BPD, bronchopulmonary dysplasia; AUC, area under the ROC curve; PPV, positive predictive value; NPV, negative predictive value.

Figure 5. Calibration curves of the moderate and severe BPD risk nomogram. The y-axis represents actual diagnosed cases of moderate and severe BPD, the x-axis represents the predicted risk of moderate and severe BPD. The diagonal dotted line represents a perfect prediction by an ideal model, the solid line represents the performance of the moderate and severe BPD risk nomogram in the training set (A) and validation set (B), with the results indicating that a closer fit to the diagonal dotted line represents a better prediction. BPD, bronchopulmonary dysplasia.
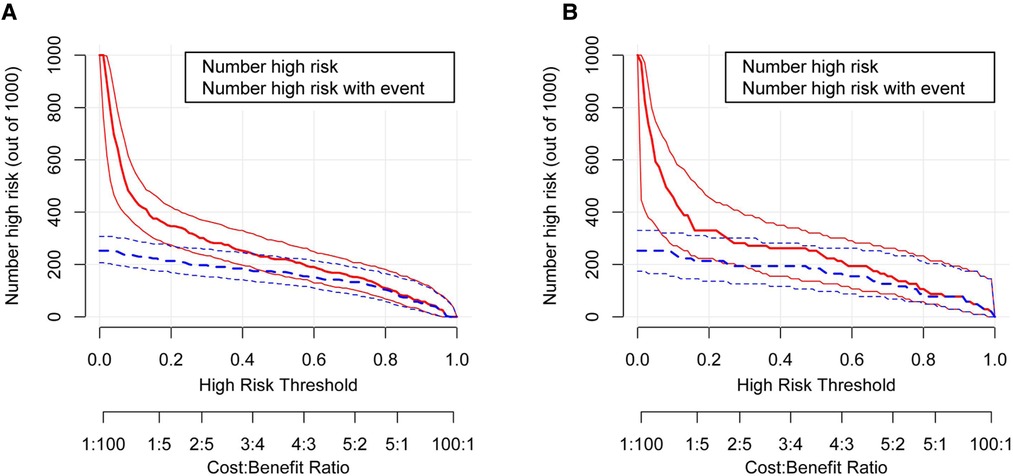
Figure 6. The clinical impact curve for the moderate and severe BPD predictive model. Of 1,000 patients, the red solid line shows the total number who would be deemed high risk for each risk threshold. The blue dashed line shows how many of those would be true positives (cases). (A) From the training set; (B) from the validation set. BPD, bronchopulmonary dysplasia.
In the prediction model we constructed for moderate and severe BPD, seven variables, namely gestational age, birth weight, 5 min apgar score, neonatal respiratory distress syndrome (≥Class II), neonatal pneumonia, duration of IMV and maximum of FiO2, are important predictors for moderate and severe BPD in infants with gestational age <30 weeks and birth weight <1,500 g on postnatal Day 14. Using these 7 predictors, the constructed model displayed good prediction performance for moderate and severe BPD, with an AUC of 0.917 in the training set and 0.931 in the validation set, and was well calibrated. Further sensitivity analysis for severe BPD alone showed the pooled AUC of the model is 0.933 in the total data set. Previously identified risk factors for BPD include gestational age, birth weight, oxygen therapy, mechanical ventilation, and duration of assisted ventilation (12). These factors measured up to postnatal Day 14 were included in our model. Besides, we added 5 min apgar score, neonatal respiratory distress syndrome (≥Class II) and neonatal pneumonia into our model, which have improved the prediction performance for the risk of moderate and severe BPD. Risk factors that were reported to be significant in previous studies but that were not included in our final model include male sex, PDA, NEC, and sepsis (12). These factors were considered in this study and were significantly different between the two study groups, but were not selected by further LASSO regression. Medical resource and management practices for preterm infants, as well as risk factors for BPD, vary from region to region, which may partly explain the differences between models.
In this study, a novel statistical method (LASSO regression) was used to identify the predictors for moderate and severe BPD. LASSO regression analysis minimizes the prediction error of quantitative response variables by imposing constraints on model parameters and reducing the regression coefficients of some variables to zero (16), thus providing more accurate results. A graphical nomogram was produced for obstetricians to easily use the constructed model to quantitatively predict the risk probability of moderate and severe BPD in preterm infants. Moreover, ROC, calibration and clinical impact curves were constructed to verify the model’s stability and accuracy. However, the limitations of this study need to be acknowledged. Firstly, this is a retrospective study. Secondly, due to the limited infants with gestational age <30 weeks and birth weight <1,500 g, the sample size of the training set and validation set was relatively small. Researchers will investigate more thoroughly in the future by involving a larger sample of preterm infants and assessing a broader range of risk factors.
In the comparison of mechanical ventilation characteristics and oxygen requirement between the two study groups, we found that duration of IMV, duration of FiO2 > 21%, and maximum of FiO2 in the infants with moderate and severe BPD were significantly higher than those in the control group, which was consistent with most studies (19, 20). Mechanical ventilation and high concentration of oxygen can cause increased reactive oxygen species and alveolar hyper-expansion, resulting in local oxidative stress injury, pressure and volume injury (20, 21). Interestingly, only duration of IMV and maximum of FiO2 were identified in further LASSO regression analysis. It is estimated that 65% of the preterm infants with birth weight <1,500 g received IMV support in the delivery room and/or during their admission (22). Although IMV can be lifesaving and improve the preterm infants’ respiratory status, it is related with increased risks of BPD, air leak syndrome and neurodevelopmental impairment, and may result in long-lasting consequences (20, 23). To reduce these adverse effects of IMV, preterm infants are increasingly received non-invasive respiratory support, often beginning in the delivery room (22). In general, NIMV refers to any ventilator support technique that does not require tracheal intubation but uses constant or variable pressure. In modern neonatal ventilators, improvements in the measurement of flow and volume have led to a variety of alternative NIMV procedures, such as CPAP, HFNC, BiPAP and NIPPV. In clinical practice, restricting IMV usage has been shown to be feasible and reduce the incidence of BPD and neurodevelopmental impairments (19, 24, 25). In addition, risk for respiratory disease has often been quantified by duration and concentration of supplemental oxygen, both of which could contribute to oxygen toxicity and serve as a marker for severity of disease (21, 26). Cumulative supplemental oxygen has been shown to be independently associated with BPD or death (21). In this study, based on the clinical characteristics and the different types of mechanical ventilation, we developed a meaningful risk prediction model for paediatricians and neonatologists to perform early screening of moderate and severe BPD in infants with gestational age <30 weeks and birth weight <1,500 g on postnatal Day 14. The prediction model could be used to target VLBWI at the highest risk of moderate and severe BPD, to tailor follow-up and preventive measures for VLBWI.
The 7 predictors verified by nomogram, including gestational age, birth weight, 5 min apgar score, neonatal respiratory distress syndrome (≥Class II), neonatal pneumonia, duration of IMV and maximum of FiO2, are very meaningful in identifying risk of moderate and severe BPD in infants with gestational age <30 weeks and birth weight <1,500 g on postnatal Day 14. Also, these indicators are helpful for early screening of moderate and severe BPD and provide prognostic information for families and clinicians.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics Committee of Nanjing Maternity and Child Health Care Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
XC and SH initiated, conceived and supervised the study. JY, LL and HL did data collection and performed the data analysis. All authors contributed to the article and approved the submitted version.
This work was supported by National Natural Science Foundation of China (82001597).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163:1723–9. doi: 10.1164/ajrccm.163.7.2011060
2. Ferre C, Callaghan W, Olson C, Sharma A, Barfield W. Effects of maternal age and age-specific preterm birth rates on overall preterm birth rates - United States, 2007 and 2014. MMWR Morb Mortal Wkly Rep. (2016) 65:1181–4. doi: 10.15585/mmwr.mm6543a1
3. Garcia-Munoz Rodrigo F, Losada Martinez A, Elorza Fernandez MD, Moreno Hernando J, Figueras Aloy J, Vento Torres M. The burden of respiratory disease in very-low-birth-weight infants: changes in perinatal care and outcomes in a decade in Spain. Neonatology. (2017) 112:30–9. doi: 10.1159/000455966
4. Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. (2017) 376:617–28. doi: 10.1056/NEJMoa1605566
5. Cheong JLY, Doyle LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol. (2018) 42:478–84. doi: 10.1053/j.semperi.2018.09.013
6. De Jesus LC, Pappas A, Shankaran S, Kendrick D, Das A, Higgins RD, et al. Risk factors for post-neonatal intensive care unit discharge mortality among extremely low birth weight infants. J Pediatr. (2012) 161:70–74.e71–72. doi: 10.1016/j.jpeds.2011.12.038
7. Jackson W, Hornik CP, Messina JA, Guglielmo K, Watwe A, Delancy G, et al. In-hospital outcomes of premature infants with severe bronchopulmonary dysplasia. J Perinatol. (2017) 37:853–6. doi: 10.1038/jp.2017.49
8. Kim DH, Kim HS, Choi CW, Kim EK, Kim BI, Choi JH. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. (2012) 101:40–6. doi: 10.1159/000327891
9. Yang T, Shen Q, Wang S, Dong T, Liang L, Xu F, et al. Risk factors that affect the degree of bronchopulmonary dysplasia in very preterm infants: a 5-year retrospective study. BMC Pediatr. (2022) 22:200. doi: 10.1186/s12887-022-03273-7
10. Kalikkot Thekkeveedu R, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir Med. (2017) 132:170–7. doi: 10.1016/j.rmed.2017.10.014
11. Kalikkot Thekkeveedu R, El-Saie A, Prakash V, Katakam L, Shivanna B. Ventilation-Induced lung injury (VILI) in neonates: evidence-based concepts and lung-protective strategies. J Clin Med. (2022) 11:557. doi: 10.3390/jcm11030557
12. Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. (2011) 183:1715–22. doi: 10.1164/rccm.201101-0055OC
13. Alonso-Ojembarrena A, Mendez-Abad P, Alonso-Quintela P, Zafra-Rodriguez P, Oulego-Erroz I, Lubian-Lopez SP. Lung ultrasound score has better diagnostic ability than NT-proBNP to predict moderate-severe bronchopulmonary dysplasia. Eur J Pediatr. (2022) 181:3013–21. doi: 10.1007/s00431-022-04491-y
14. Gentle SJ, Carper B, Laughon MM, Jensen EA, Williams A, Travers CP, et al. Duration of noninvasive respiratory support and risk for bronchopulmonary dysplasia or death. J Perinatol. (2022) 42:454–60. doi: 10.1038/s41372-021-01269-2
15. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the national institutes of health consensus definition of bronchopulmonary dysplasia. Pediatrics. (2005) 116:1353–60. doi: 10.1542/peds.2005-0249
16. Zhang Y, Shi R, Yu L, Ji L, Li M, Hu F. Establishment of a risk prediction model for non-alcoholic fatty liver disease in type 2 diabetes. Diabetes Ther. (2020) 11:2057–73. doi: 10.1007/s13300-020-00893-z
17. Wen J, Song X, Ding H, Shen X, Shen R, Hu LQ, et al. Prediction of vaginal birth after cesarean delivery in Chinese parturients. Sci Rep. (2018) 8:3084. doi: 10.1038/s41598-018-21488-6
18. Bi S, Zhang L, Chen J, Huang L, Zeng S, Jia J, et al. Development and validation of predictive models for vaginal birth after cesarean delivery in China. Med Sci Monit. (2020) 26:e927681. doi: 10.12659/MSM.927681
19. Mulder EE, Lopriore E, Rijken M, Walther FJ, Te Pas AB. Changes in respiratory support of preterm infants in the last decade: are we improving? Neonatology. (2012) 101:247–53. doi: 10.1159/000334591
20. Miller JD, Carlo WA. Pulmonary complications of mechanical ventilation in neonates. Clin Perinatol. (2008) 35:273–81 x–xi. doi: 10.1016/j.clp.2007.11.004
21. Wai KC, Kohn MA, Ballard RA, Truog WE, Black DM, Asselin JM, et al. Early cumulative supplemental oxygen predicts bronchopulmonary dysplasia in high risk extremely low gestational age newborns. J Pediatr. (2016) 177:97–102.e102. doi: 10.1016/j.jpeds.2016.06.079
22. Soll RF, Edwards EM, Badger GJ, Kenny MJ, Morrow KA, Buzas JS, et al. Obstetric and neonatal care practices for infants 501 to 1,500 g from 2000 to 2009. Pediatrics. (2013) 132:222–8. doi: 10.1542/peds.2013-0501
23. Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. (2005) 146:798–804. doi: 10.1016/j.jpeds.2005.01.047
24. Vliegenthart RJS, Onland W, van Wassenaer-Leemhuis AG, De Jaegere APM, Aarnoudse-Moens CSH, van Kaam AH. Restricted ventilation associated with reduced neurodevelopmental impairment in preterm infants. Neonatology. (2017) 112:172–9. doi: 10.1159/000471841
25. Vendettuoli V, Bellu R, Zanini R, Mosca F, Gagliardi L, Italian Neonatal N. Changes in ventilator strategies and outcomes in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2014) 99:F321–324. doi: 10.1136/archdischild-2013-305165
Keywords: mechanical ventilation, bronchopulmonary dysplasia, preterm infants, predictive model, newborn
Citation: Yin J, Liu L, Li H, Hou X, Chen J, Han S and Chen X (2022) Mechanical ventilation characteristics and their prediction performance for the risk of moderate and severe bronchopulmonary dysplasia in infants with gestational age <30 weeks and birth weight <1,500 g. Front. Pediatr. 10:993167. doi: 10.3389/fped.2022.993167
Received: 13 July 2022; Accepted: 10 October 2022;
Published: 2 November 2022.
Edited by:
Robin McKinney, Brown University, United StatesReviewed by:
Audrey Miller, The Ohio State University, United States© 2022 Yin, Liu, Li, Hou, Chen, Han and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Chen Y2hlbnhpYW9odWlAbmptdS5lZHUuY24= Shuping Han c2h1cGluZ2hhbkBuam11LmVkdS5jbg==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.