- 1Department of Pediatrics, The Affiliated Hospital of Hangzhou Normal University, Hangzhou, China
- 2Department of Neonatology, Women's Hospital, Zhejiang University School of Medicine, Hangzhou, China
Objective: This paper studied the Epstein–Barr virus (EBV) infection status and influencing factors among children using a combined detection of specific antibodies and DNA.
Methods: We retrospectively analyzed children who visited the Affiliated Hospital of Hangzhou Normal University from January 2019 to December 2020, and correlations between the social environment and clinical data were analyzed.
Results: The cumulative positive rates of specific antibody, DNA, and combined detection of EBV were 52.4%, 39.5%, and 54.0% (P = 0.001), respectively. The current infection rate was 15.7%, and the peak of infection occurred in the preschool group (P = 0.021). After adjusting for confounding factors, the number of siblings (OR = 1.550) and family members who smoke (OR = 1.524) were independent risk factors for EBV infection, whereas parents with a higher education level (OR = 0.493, OR = 0.316), longer breastfeeding time (OR = 0.578) and dedicated tableware (OR = 0.573) were independent protective factors.
Conclusion: A combination of antibody and DNA tests may be beneficial for the diagnosis of EBV infection. The EBV infection rate in children at our hospital was lower than the national average. Furthermore, the infection rate is closely related to the number of siblings, regardless of whether family members smoke, the status of parents' education, breastfeeding duration, and meal patterns. Overall, prevention measures should focus on the preschoolers.
Introduction
The Epstein–Barr virus is a human lymphotropic herpes virus that causes a lifelong infection in approximately 95% of the global population (1). The primary EBV infection is more common in children and adolescents, and most cases are asymptomatic and atypical infections (2, 3), which are easily missed and misdiagnosed. Therefore, strengthening the monitoring of EBV in children can provide early clinical anti-infective treatment and prevention. Presently, an important method for judging EBV infection is to measure EBV-related antibodies. However, determining EBV infection by detecting EBV-DNA is gradually becoming more common in clinical practice. There is currently a lack of research on EBV infection in a large sample size of children with combined DNA and antibody detection in the Hangzhou area. This paper retrospectively analyzed the data of 1,744 children who were treated in the Affiliated Hospital of Hangzhou Normal University from January 2019 to December 2020 that were tested for EBV-specific antibodies and EBV-DNA.
Subjects and methods
Subjects
A total of 1,744 children aged 0–14 years who were tested for both EBV-specific antibodies and DNA participated in this study at the pediatrics department of the Affiliated Hospital of Hangzhou Normal University from January 2019 to December 2020. The subjects included 936 males and 808 females. The medical history and general information of the research subjects were recorded in detail including sex, age, siblings, family members' smoking status, breastfeeding duration, meal patterns, parents' educational levels, and housing conditions. Exclusion criteria included any antiviral drug treatment before the test, severe immunodeficiency or application of related immunosuppressants, a recent history of blood or blood product transfusion, basic diseases, such as malignant tumor, twins, premature birth; congenital malformation, and severe organ insufficiency. This study was approved by the medical ethics committee of the hospital, and all of the children's guardians signed the informed consent forms.
EBV-specific antibody test and EBV-DNA PCR (polymerase Chain reaction) test
Venous blood samples (4 mL each) were collected from the subjects and 2 mL was placed in a vacuum blood collection tube. We used an enzyme-linked immunosorbent assay (ELISA) (EUROIMMUN, Germany) to detect EBV capsid antigen IgM antibody (EBV-CA-IgM), EBV capsid antigen IgG antibody (EBV-CA- IgG), EBV early antigen IgM antibody (EBV-EA-IgM), and nuclear antigen IgG antibody (EBV-NA-IgG) to determine the infection stage as follows: current infection, previous infection and non-infection (4). The remaining 2 mL was placed in an EDTA tube (containing anticoagulant), and 1 mL of plasma was separated. The EBV-DNA in the plasma (1 mL) was measured by real-time fluorescence quantitative PCR technology, and 1 mL was used for detecting EBV-DNA in peripheral blood mononuclear cells (PBMCs). The reagents were provided by SANSURE BIOTECH INC. The analytical instrument was a SLAN-96S of Shanghai HONGSHI Medical Tech. EBV-DNA copies > 400 copies/mL were considered positive.
Statistical analysis
We used Excel to organize the data and to establish a reliable database and SPSS 25.0 software for statistical analysis. The rate comparison was performed using the chi-squared test. We performed univariate analysis and multivariate logistic regression analysis on the related factors that could affect EBV infection. P < 0.05 was used to indicate that the difference was statistically significant.
Results
Epidemiological characteristics of EBV infection
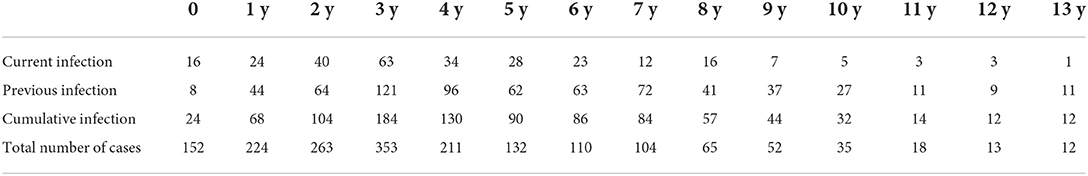
The cumulative infection rates of EBV-specific antibodies and DNA tests were 52.4% (914/1,744) and 39.5% (689/1,744), respectively, and the combined detection was 54.0% (941/1,744). The difference between the tests was statistically significant (P < 0.01). Additionally, the cumulative EBV infection rate increased with age; the EBV infection rate of 10-year-old children was 91.4%, and the cumulative infection rate of children under the age of 6 years was 47.5% (686/1,445). The current infection rate was 15.7% (275/1,744) (Table 1).
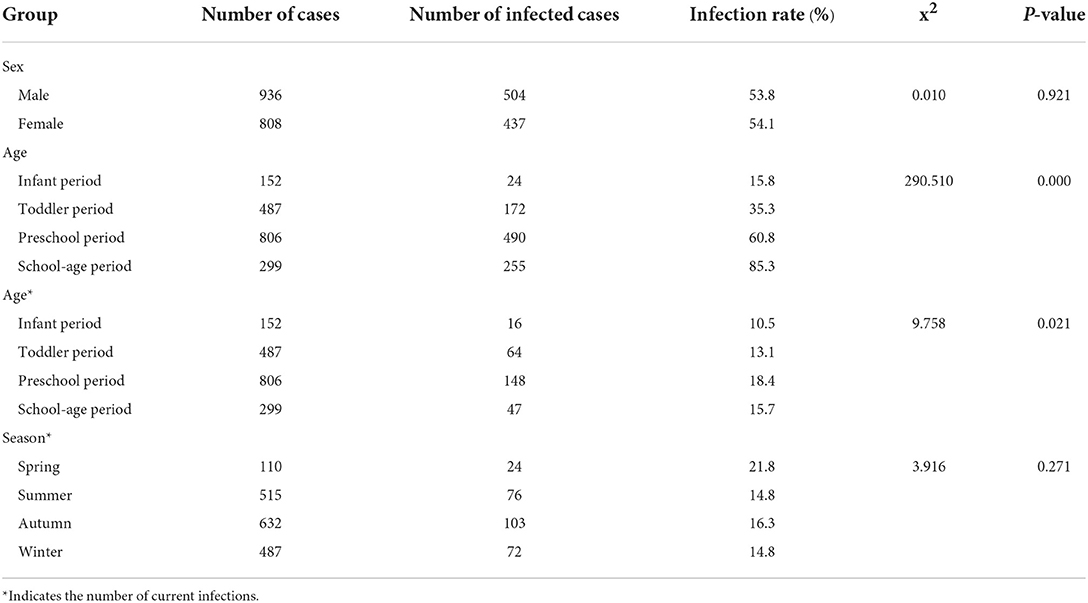
The subjects were divided by age into an infant group (0– <1-year-old), a toddler group (1–<3 years old), a preschool age group (3~ < 7 years old), and a school-age group (7–13 years old). The preschool group had the highest current infection rate (P < 0.05). Among the 1,744 children, the infection rate was 53.8% in boys and 54.1% in girls, and there was no significant difference between the male and female infection rates (P > 0.05). Furthermore, there was no significant difference in the current infection rate of EBV in different seasons (P > 0.05) (Table 2).
Influencing factors of EBV infection
Univariate analysis
There was no relationship between renting a house and EBV infection (P > 0.05). However, the number of siblings (P < 0.01), parents' educational level (all P < 0.01), the duration of breastfeeding (P < 0.05), smoking status of family members (P < 0.01), and meal patterns (P < 0.01) were all associated with EBV infection (Table 3).
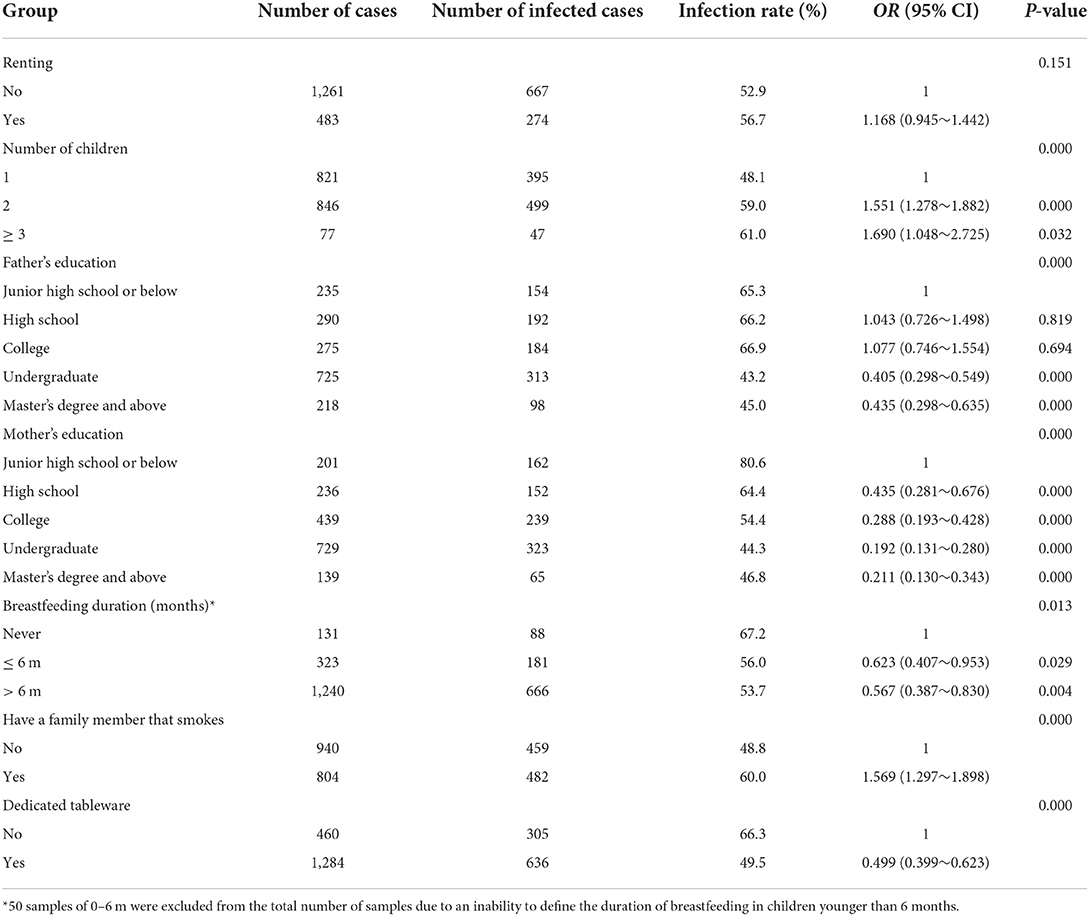
Table 3. Univariate logistic regression to determine the risk of EBV infection based on participant characteristics.
Multifactor analysis
Incorporating the above factors into a multivariate logistic regression analysis revealed that more siblings (OR = 1.550, 95% CI: 1.226–1.960) and family members smoking (OR = 1.524, 95% CI: 1.244–1.868) were EBV-infected independent risk factors. Higher parental education (OR = 0.493, 95% CI: 0.395–0.614; OR = 0.316, 95% CI: 0.216–0.462), longer breastfeeding duration (OR = 0.578, 95% CI: 0.396–0.844), and having dedicated tableware (OR = 0.573, 95% CI: 0.452–0.726) were independent protective factors for EBV infection (Table 4).

Table 4. Multivariate logistic regression of EBV infection risk based on participant characteristics.
Discussion
Specific antibodies and DNA detection of EBV infection have advantages and limitations, respectively. EBV infection produces a series of specific antibodies. When combined with the detection of multiple antibodies, a multidimensional data analysis can be performed to distinguish between current infection, previous infection, and non-infection status. However, 5–10% of infected people do not produce EBV-NA-IgG antibodies (5). Young children (especially those under the age of four), immunosuppressed, or immunodeficient children may not produce specific antibodies. Also, the virus sometimes cannot stimulate the body to produce corresponding viral antibodies that reach the lower limit of detection (6).
DNA testing is direct evidence of EBV infection and compensates for the shortage of EBV-specific antibody testing (7), but DNA testing is effective only when the virus is active. Most previous studies were only conducted through a single antibody or DNA detection method, which leads to misdiagnosis. Therefore, this study examined four specific antibodies and DNA tests, and demonstrated that this combined test can comprehensively detect and monitor EBV infection.
The cumulative infection rate of EBV in children in this study was 54.0%, which was lower than the EBV infection rate (84.93%) of 3,277 Jilin children as reported by Pan (8). This result may be related to the age distribution of the enrolled patients. Another reason for this might be the development of the economy and the improvement of living standards, which may causing the EBV infection rate to gradually decrease (9). In this study, the EBV infection rate of children under the age of six was 47.5%, which was lower than the EBV infection rate of 67.3% of children in a study in Beijing in 2013 and 83% in 2000 (10), although only a single antibody was selected in that study. This agrees with a decreasing yearly trend. In this study, the EBV infection rate of 10-year-old children exceeded 90% for the first time and reached the adult level, while Xiong suggested that the EBV infection rate of children in Guangzhou reached the adult level in children at the age of eight (11). We attribute this phenomenon to an increased attention to health. These differences may also be due to the methodologies used, and therefore, further research is needed to justify the validity of these findings with the same methodology. The current infection rate in this study was 15.7%. When comparing the current infection rates of each age group, we found that the preschool age group had the highest current infection rate, which was consistent with the results of most domestic studies (12, 13). This suggests that the preschool age group experienced a high incidence of EBV infection.
The fact that children who live in cities go to school at a younger age is related to an increased chance of exposure in kindergarten, which has important implications for the timing of vaccination. There was no significant difference in the EBV infection rate by sex, which was consistent with the literature (14). Li's investigation suggested that autumn and winter were the peak periods of EBV infection, which may be related to greater virus activity during these periods (15), but this study did not show this seasonal variation.
EBV infection presents a family clustering phenomenon, and 82% of siblings in families of EBV-infected children had consistent serum antibodies (16). This study shows that the EBV infection rate of children with siblings is significantly higher than that of only children. It is speculated that close contact between siblings may increase the risk of infection, but genetic factors cannot be ruled out (16). The smoking status of family members increases the rate of EBV infection in children, which is consistent with previous research (17). Additionally, providing a good living environment can reduce the risk of EBV infection. The EBV infection rate of children with dedicated tableware was significantly lower than that of children without dedicated tableware, which may be related to the main transmission route of EBV through saliva. Without their own dedicated tableware, children have a higher risk for EBV infection. This suggests the importance of strengthening the promotion of health and cultivating good hygiene.
Lifestyle is an important measure in controlling EBV infection, but parental education level is a more important independent protective factor for EBV infection in children, which is consistent with recent research (18). Due to the high prevalence of EBV infection in adults, breastfeeding increases the chance of infection in children (17). However, our study found that the EBV infection rate was lower in children breastfed for a longer time, which may indicate a correlation between EBV infection and nutritional status. This agrees with the work of Juan et al. (19), but more research is warranted.
The risk of EBV infection is higher for children in families living in rental properties (20). In this study, univariate and multivariate logistic regression models were used to analyze the data, and neither suggested this as an influencing factor of EBV infection. We speculate that renting was associated with EBV infection when more people live together in a smaller space. A smaaler space represents a higher density of people, which increases EBV infection rates. However, with the advancement of urban shantytown reform policies, rental properties may provide a better living environment and a lower density of people.
Our study had several limitations. One of the major results from the population under study is that the preschool age group had the highest acute infection rate. Indeed, we only included children who had undergone EBV-specific antibody and DNA tests; in other words, our population (hospital population) was not strictly representative of the population of children in Hangzhou. Different prevalence results might be observed if every child in Hangzhou had received EBV-related screening. Moreover, the preschool-aged children in our study were close to half of the population, and preschool-aged children are more susceptible to pathogen infection. All of the above may cause selection bias. Therefore, an unselected larger-sample clinical study is required to confirm this hypothesis. Finally, this study only contained 2 years of records (2019–2020), which showed a decreasing trend of the EBV infection rate but cannot be used to make a definitive conclusion.
Conclusions
In summary, we found that the EBV infection rates differ by mode of detection, and the two methods should be combined to improve the rate of infection detection. This preliminary study explored the low rate of EBV infection in children at the Affiliated Hospital of Hangzhou Normal University. The infection rate was closely related to the number of siblings, family members' smoking habits, parents' education level, breastfeeding duration, and meal patterns. Factors such as sex, season, and rental housing did not play a role in infection. Specific attention should be given to current infection in the preschool age group.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of Hangzhou Normal University Affiliated Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
YY: data collection, data analysis, manuscript writing, and manuscript revision. YZ manuscript revision and project supervision. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Hangzhou Science and Technology Project (20191203B107).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
EBV, Epstein-Barr virus; ELISA, Enzyme-linked immunosorbent assay; EBV-CA-IgM, EBV capsid antigen IgM antibody; EBV-CA-IgG, EBV capsid antigen IgG antibody; EBV-EA-IgM, EBV early antigen IgM antibody; EBV-NA-IgG, EBV nuclear antigen IgG antibody; PCR, Polymerase chain reaction; OR, Odds rations; PBMC, peripheral blood mononuclear cell.
References
1. Pukownik E, Kubicka M, Kurylo-Rafinska B, Debski R, Galazka P, Czyzewski K, et al. Impact of CMV and EBV on immune recovery after allogeneic hematopoietic cell transplantation in children. Anticancer Res. (2018) 38:6009–13. doi: 10.21873/anticanres.12950
2. Bolis V, Karadedos C, Chiotis I, Chaliasos N, Tsabouri S, et al. Atypical manifestations of Epstein–Barr virus in children: a diagnostic challenge. J Pediatria. (2016) 92:113–21. doi: 10.1016/j.jped.2015.06.007
3. Huang X, Nolte I, Gao Z, Vos H, Hepkema B, Poppema S, et al. Epidemiology of classical hodgkin lymphoma and its association with Epstein Barr Virus in Northern China. PLoS ONE. (2011) 6:e21152. doi: 10.1371/journal.pone.0021152
4. Chinese Medical Association, National Group of Epstein-Barr Virus Associated Diseases in Children; Editorial Board of Chinese Journal of Experimental and Clinical Virology. Expert consensus on laboratory diagnosis and clinical application of Epstein-Barr virus infection. Chin J Experi Clin Virol. (2018) 32:2–8. doi: 10.3760/cma.j.issn.1003-9279.2018.01.001
5. Smatti MK, Al-Sadeq DW, Hajali N, Pintus G, Abou-Saleh H, Nasrallah GK. EBV epidemiology, serology and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol. (2018) 8:211. doi: 10.3389/fonc.2018.00211
6. Topp SK, Rosenfeldt V, Vestergaard H, Christiansen CB, Von Linstow M-L. Clinical characteristics and laboratory findings in Danish children hospitalized with primary Epstein-Barr virus infection. Infect Dis. (2015) 47:908–14. doi: 10.3109/23744235.2015.1082036
7. Kasifoglu N, Oz S, Dinleyici EC, Us T, Bor O, Durmaz G, et al. Comparison of methods used for the diagnosis of epstein-barr virus infections in children. Polish J Microbiol. (2018) 67:81–8. doi: 10.5604/01.3001.0010.6287
8. Cui LY, Cui YS, Yan Y. Analysis on 3,277 pediatric patients with EB virus infection. China Continuing. Med Educ. (2019) 11:87–9.
9. Balfour HH, Sifakis F, Sliman JA, Knight JA, Schmeling DO, Thomas W. Age-specific prevalence of Epstein-Barr virus infection among individuals aged 6-19 years in the United States and factors affecting its acquisition. J Infect Dis. (2013) 208:1286–93. doi: 10.1093/infdis/jit321
10. Zhang B. Study on Epstein-Barr Virus Seroepidemiology of Children Visiting Peking Union Medical College Hospital. Peking Union Medical College (2014).
11. Xiong G, Zhang B, Huang M-Y, Zhou H, Fenf Q-S, Luo X, et al. Epstein-Barr Virus (EBV) infection in Chinese children: A retrospective study of age-specific prevalence. PLoS ONE. (2014) 9:e99857. doi: 10.1371/journal.pone.0099857
12. Cui J, Yan W, Xu S, Wang Q, Zhang W, Liu W, et al. Anti-Epstein–Barr virus antibodies in Beijing during 2013–2017: What we have found in the different patients. PLoS ONE. (2018) 13:e0193171. doi: 10.1371/journal.pone.0193171
13. Shi T, Huang L, Chen Z, Tian J. Characteristics of primary Epstein-Barr virus infection disease spectrum and its reactivation in children, in Suzhou, China. J Med Virol. (2021) 93:5048–57. doi: 10.1002/jmv.26941
14. Beader N, Kolarić B, Slačanac D, Tabain I, Vilibić-Cavlek T. Seroepidemiological study of Epstein-Barr virus in different population groups in croatia. Israel Med Assoc J. (2018) 20:86–90.
15. Chuan-Di L. Analysis of clinical features of EB viral infection onset of children of different age groups. China Foreign Med Treatment. (2018) 37:7–9. doi: 10.16662/j.cnki.1674-0742.2018.32.001
16. Viljoen J, Tuaillon E, Nagot N, Danaviah S, Peries M, Padayachee P, et al. Cytomegalovirus, and possibly Epstein–Barr virus, shedding in breast milk is associated with HIV-1 transmission by breastfeeding. Aids. (2015) 29:145. doi: 10.1097/QAD.0000000000000527
17. Levine H, Balicer RD, Rozhavski V, Halperin T, Shreberk M, Davidovitch N, et al. Seroepidemiology of Epstein-Barr virus and cytomegalovirus among Israeli male young adults. Ann Epidemiol. (2012) 22:783–8. doi: 10.1016/j.annepidem.2012.06.099
18. Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein-Barr virus infection in U.S. Children ages 6-19, 2003-2010. PLoS ONE. (2013) 8:e64921. doi: 10.1371/journal.pone.0064921
19. Li J, Xu PR. Analysis on the status of Epstein-Barr virus infection in children in Urumqi. J Mu Dan Jiang Med University. (2016) 37:99–101. doi: 10.13799/j.cnki.mdjyxyxb.20160302.001
Keywords: EBV, antibody, EBV-DNA, children, influencing factors
Citation: Yang Y and Zhu Y (2022) A combined antibody and DNA assay for EBV infection in children. Front. Pediatr. 10:989193. doi: 10.3389/fped.2022.989193
Received: 20 July 2022; Accepted: 11 August 2022;
Published: 25 August 2022.
Edited by:
Zhongjie Shi, Wayne State University, United StatesReviewed by:
Shuai Mao, Xi'an Jiaotong University, ChinaLu Wang, University of Michigan, United States
Copyright © 2022 Yang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yafei Zhu, bHN6aHV5YWZlaSYjeDAwMDQwO3NpbmEuY29t
†ORCID: Yulu Yang orcid.org/0000-0003-0230-8407
Yafei Zhu orcid.org/0000-0003-0718-9961
 Yulu Yang
Yulu Yang Yafei Zhu
Yafei Zhu