
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 05 January 2023
Sec. Pediatric Infectious Diseases
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.988706
This article is part of the Research TopicImpact of SARS-CoV-2 and COVID-19 Pandemic in PediatricsView all 25 articles
 Sarah Khafaja1,2,†
Sarah Khafaja1,2,† Nour Youssef1,3,†
Nour Youssef1,3,† Zeinab El Zein1,2
Zeinab El Zein1,2 Celina F. Boutros1
Celina F. Boutros1 Samer Bou Karroum1
Samer Bou Karroum1 Nour Abdel-Halim3
Nour Abdel-Halim3 Rim Salameh3
Rim Salameh3 Daniella Hodroj3
Daniella Hodroj3 Nour El Meski4
Nour El Meski4 Oussama Nasrallah4
Oussama Nasrallah4 Aram Bidikian4
Aram Bidikian4 Ghassan Bou Saba4
Ghassan Bou Saba4 Mariam T. Arabi3,5
Mariam T. Arabi3,5 Rima Hanna-Wakim1,2,3
Rima Hanna-Wakim1,2,3 Ghassan S. Dbaibo1,2,3*
Ghassan S. Dbaibo1,2,3*
Introduction: Reports of multisystem inflammatory syndrome in children (MIS-C), following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, have been increasing worldwide, with an incidence varying significantly across studies based on the definition used for the diagnosis. At our tertiary medical center in Lebanon, we encountered several cases that presented a diagnostic challenge because they mimicked MIS-C but did not meet the US Centers for Disease Control and Prevention (CDC) definition. We decided to review these cases and describe their features in comparison with cases that met the CDC criteria of MIS-C and those that had an alternative diagnosis.
Methods: This is a retrospective chart review of subjects aged <19 years old admitted to the American University of Beirut Medical Center (AUBMC) between March 1, 2020, and May 31, 2021, with suspected or confirmed MIS-C, following documented COVID-19 infection, with sufficient or insufficient criteria for diagnosis. Subjects were classified into 3 groups: “MIS-C”, “Near MIS-C” and “Alternative Diagnosis”.
Results: A total number of 29 subjects were included in our cohort. Fever was present in all subjects. In the MIS-C group, evidence for cardiovascular system involvement was the most common feature followed by the mucocutaneous and gastrointestinal systems. In the “Near MIS-C” and “Alternative Diagnosis” group, gastrointestinal symptoms were the most common with only one patient with cardiac abnormalities and none with coagulopathy. Subjects with typical MIS-C presentation had higher inflammatory markers when compared to subjects in the other groups. Almost all the subjects had positive IgG for SARS-CoV-2. Of the 29 subjects, the Royal College of Paediatrics and Child Health (RCPCH) case definition would have identified all suspected cases without an alternative diagnosis as MIS-C, whereas the World Health Organization (WHO) and the CDC definitions would have excluded 6 and 10 subjects, respectively.
Conclusion: MIS-C presents a diagnostic challenge due to the nonspecific symptoms, lack of pathognomonic findings, and potentially fatal complications. More research is needed to fully understand its pathogenesis, clinical presentation spectrum, and diagnostic criteria. Based on our experience, we favor the hypothesis that MIS-C has a continuum of severity that necessitates revisiting and unifying the current definitions.
A novel coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was recognized in December 2019 in China following the emergence of unexplained severe lower respiratory infections in clusters of patients (1, 2). The World Health Organization (WHO) has declared the novel coronavirus (COVID-19) outbreak a global pandemic on March 11, 2020 (3). The COVID-19 pandemic continues to spread rapidly, presenting with a wide range of clinical manifestations from asymptomatic to severe acute respiratory distress syndrome, multiorgan failure and death (4). Initially, the pediatric patients were largely overlooked during the COVID-19 pandemic and were considered a low-risk population, as they accounted for less than 8% of the total cases, and the largest cohort with high morbidity and mortality was reported in the elderly (5–7). In late April 2020, reports from the United Kingdom emerged, describing children who required admission to intensive care units due to an unexplained multisystem inflammatory syndrome with features resembling Kawasaki disease and toxic shock syndrome (8). Subsequently, similarly affected children were reported across Europe and the United States, associated temporally and geographically with COVID-19 outbreaks (4). When an increase in the pediatric COVID-19 cases associated with hyperinflammation was described, the World Health Organization (WHO), the US Centers for Disease Control and Prevention (CDC), and the Royal College of Paediatrics and Child Health (RCPCH) developed overlapping definitions of the syndrome and named it multisystem inflammatory syndrome in children (MIS-C) or paediatric multisystem inflammatory syndrome—temporally associated with SARS-CoV-2 (PIMS-TS or PIMS) (8). These definitions have common elements, such as prolonged fever, multi-organ dysfunction, elevated inflammatory markers, and recent or current SARS-CoV-2 infection or exposure, however, they slightly differ in many other criteria (8–11).
The epidemiology of MIS-C remains unclear, however it appears to be a rare condition with an incidence <1% in children infected with SARS-CoV-2 (12). The mortality and morbidity in those patients differ significantly from the benign course of COVID-19 in children. The majority had previous SARS-CoV-2 infection or known exposure or serologic evidence of SARS-CoV-2, supporting the hypothesis that MIS-C represents an immune-mediated and dysregulated post-infectious inflammatory response possibly triggered by SARS-CoV-2 (13, 14).
Obviously, epidemiological data including incidence of MIS-C and the clinical findings varied significantly across studies, based on the definition used for diagnosis, with broader criteria leading to reports of a higher incidence (8, 15). Despite the risk of overdiagnosis, the true incidence of MIS-C may be significantly higher due to the lack of clinical awareness and specific diagnosis. Furthermore, diagnostic criteria were based on clinical manifestations in children hospitalized with severe disease, which may neglect mild cases (8).
As more cases are described in the literature, the clinical heterogeneity of the disease and its wide spectrum are being further understood. At present, our knowledge of this inflammatory syndrome is incomplete and the available results from published studies provide insufficient insights into the full epidemiological, clinical, immunological, and prognostic spectrum of MIS-C. Therefore, it is pivotal to undergo further studies in order to obtain a better definition of MIS-C, to optimize the characterization and the diagnostic criteria, to assess its true impact and to generate the best clinical and therapeutic approach, in addition to clarifying short term and long-term outcomes.
Following a wave of COVID-19 infections in Lebanon, we encountered several patients suspected to have MIS-C at our tertiary medical center in Lebanon over a 15-month period. Some of these patients presented a diagnostic challenge because they mimicked MIS-C but did not meet the CDC definition (9). We decided to review these cases and describe their features in comparison with cases that met the CDC criteria of MIS-C or those that had an alternative diagnosis.
This is a retrospective chart review of patients with suspected or confirmed MIS-C, following current or recent SARS-CoV-2 infection or exposure to a person with suspected or confirmed COVID-19 infection, who had sufficient or insufficient criteria for diagnosis. The study was conducted at the American University of Beirut Medical Center (AUBMC), located in Beirut, Lebanon, and was approved by the institutional review board (IRB) at AUBMC (IRB ID: BIO- 2021- 0090). All patients were identified retrospectively, and their charts were reviewed through medical records by looking at the following ICD-10 codes for admission diagnosis: “Fever of other and unknown origin”, “fever” and “multisystem inflammatory disorder”. In addition, at least one of the authors on this paper was on the Pediatric Infectious Diseases (PID) service, which is consulted on cases suspected to have MIS-C, for the duration of the study period. Therefore, we used the PID service records to identify any patients that may have been missed by the hospital medical record review.
All children and adolescents <19 years of age, admitted to the hospital between March 1, 2020, to May 31, 2021 were included in the study if they had suspected MIS-C on admission. All included subjects had recent or past infection of SARS-CoV-2 confirmed by real-time reverse-transcriptase -polymerase -chain-reaction (RT-PCR) and/or serology. Notably, COVID-19 vaccines were not available for children and adolescents during the study period.
Included subjects were categorized into the “MIS-C group” when they fulfilled the criteria for MIS-C CDC case definition. Subjects who were thought to have MIS-C upon presentation but did not fulfill the CDC criteria and had no alternative diagnosis, were categorized into the “Near MIS-C” group. The third group “Alternative Diagnosis” included subjects who were initially worked up for MIS-C but ended up with an alternative diagnosis.
In this study, categorization of subjects was based on the CDC case definition for MIS-C throughout the whole analysis, except for the first table of the analysis where subjects were classified according to the three most recognized definitions, the RCPCH, the WHO and the CDC.
As the COVID-19 pandemic evolved in different countries, the RCPCH, the WHO and the CDC had developed, based on a limited number of cases, different definitions for the multisystem inflammatory syndrome in children (Table 1) (9–11, 16).
Different patterns of clinical presentation and organ system involvement were present in our subjects and ranged from mild to severe forms. Many subjects had mild upper or lower respiratory symptoms such as sore throat, nasal congestion, rhinorrhea or cough, or very mild mucocutaneous symptoms which were not clinically suggestive of significant organ involvement. We described organ system involvement based on the symptoms, clinical findings, and laboratory results (17).
The data collection was performed by the study team at the Center of Infectious Diseases Research Center (CIDR). Variables recorded from admission to discharge were extracted from the clinical records assuring anonymity and confidentiality. Variables included demographics, comorbidities, history of COVID-19 infection or exposure to a COVID-19 positive person, presenting signs and symptoms, associated comorbidities, physical examination findings, laboratory tests, imaging reports, viral testing with SARS-CoV-2 RT-PCR and serology tests, clinical course in the hospital, management, and outcomes.
Descriptive analyses were performed using numbers and percentages for qualitative variables and means with standard deviation (SD) or median with range for continuous variables. All statistical analyses were performed with the use of the Statistical Package for Social Sciences (SPSS) program, version 25.0 for Windows (Armonk, NY, IBM Corp).
In the 15-month study period, a total number of 29 subjects who presented with a clinical picture suspicious of MIS-C were included in our cohort, with a median age of 5 years.
Based on the RCPCH definition, 21 subjects fit the definition of PIMS (MIS-C), but when the WHO and the CDC case definitions were used, only 15 and 11 subjects, respectively, fit the MIS-C definition (Table 2). Most of our subjects were previously healthy; however, 24.1% of them had preexisting medical conditions and comorbidities including hematological malignancies, sickle cell disease, thalassemia, hemophagocytic lymphohistiocytosis (HLH) or obesity. The “Alternative Diagnosis” group included 8 subjects whose workup showed other etiologies including drug-induced pericarditis, salmonella or rotavirus gastroenteritis, adenovirus or rhinovirus/enterovirus infection documented by multiplex PCR respiratory panel, urinary tract infection (UTI), or brucellosis. Overall, there was a male predominance (69%), which was also noted in both MIS-C and “Near MIS-C” groups (81.8% and 80% respectively). Almost 90% of the subjects had history of recent SARS-CoV-2 infection by RT-PCR or exposure to a suspected or confirmed COVID-19-infected person within the 2 to 8 weeks prior to presentation (Table 3).
Table 4 summarizes the clinical presentation of the subjects. Fever was present in all subjects with a difference in the median duration between the MIS-C group (6 days), the near-MIS-C group (2 days) and the alternative diagnosis group (4 days). Gastrointestinal symptoms were prevalent in the 3 groups. Respiratory symptoms were most encountered in the MIS-C group. When comparing the 3 groups, mucocutaneous involvement was reported in 81.8% of subjects with MIS-C while only 20% and 37.5% of the subjects in the “Near MIS-C” group and “Alternative Diagnosis” group, respectively, had conjunctivitis, cracked lips, strawberry tongue or a rash reported during illness.
Out of 23 subjects who underwent imaging (chest x-ray or chest CT-scan), only 6 subjects had evidence of radiologically confirmed pneumonia, of which 4 subjects belonged to the MIS-C group. Echocardiography was performed in 25 subjects, where 9 out of 10 subjects in the MIS-C group had positive findings including 55.5% with reduced ejection fraction. One of these subjects had severe biventricular dysfunction (ejection fraction <20%) with increased pulmonary artery pressure requiring admission to the pediatric intensive care unit (ICU) and the use of vasopressors. Coronary artery dilation and pericardial effusion were noted, respectively, in one and 4 subjects in the MIS-C group. One subject from the “Alternative Diagnosis” group had moderate pericardial effusion. This subject, known to have hemophagocytic lymphohistiocytosis status four-months post hematopoietic stem cell transplant, showed typical findings of pericarditis for which he received steroids and non-steroidal anti-inflammatory agents. None of the subjects in the “Near MIS-C” group had cardiac involvement. Two subjects with MIS-C developed pleural effusion, whereas none of the subjects with “Near MIS-C” did. None of the subjects had neurological involvement.
Laboratory parameters were reviewed as shown in Table 5. Compared with the subjects with “Near MIS-C” and those with alternative diagnoses, subjects with typical MIS-C presentation had higher leukocytosis, platelet counts and acute inflammatory markers, specifically C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), ferritin and D-dimer (Table 5). Elevated troponin and pro-B-type natriuretic peptide (Pro-BNP) levels were mainly detected in the MIS-C group with mean values that were significantly higher than the values reported for the remaining subjects. Among the subjects in the “Near MIS-C” and the alternative diagnoses groups who were serologically tested for SARS-CoV-2, 100% had positive serology test results, mainly IgG, with or without positive RT-PCR test results at one point during their illness whether prior to their presentation or concomitantly. As for the MIS-C group, out of the 11 tested subjects, SARS-CoV-2 IgG was detected in 10 subjects. The remaining subject had positive SARS-CoV-2 IgM and negative IgG as he developed MIS-C around 2 weeks after infection and therefore SARS-CoV-2 IgG did not rise yet.
The outcome and management of patients are shown in Table 6.
None of the 29 subjects required mechanical ventilation or extracorporeal membrane oxygenation. On admission, empiric intravenous broad-spectrum antibiotics were initiated in 91% of the subjects with MIS-C vs. 30% of the subjects with “Near MIS-C”. Blood and urine cultures were negative for all subjects. Upon presentation, 3 subjects in the MIS-C group presented with hypotension and/or cardiogenic shock requiring ICU admission and inotropic support. In the MIS-C group, all subjects (100%) were treated with intravenous immunoglobulins (IVIG), 81.8% received methylprednisolone, which was later shifted to oral prednisolone and tapered over 3 to 4 weeks. None of the subjects in the two other groups received IVIG nor vasopressors. Enoxaparin was given to 63.6% of subjects with MIS-C who had severe ventricular dysfunction, dilated coronary arteries, or markedly elevated D-dimer level. The only subject in the “Near MIS-C” group who received enoxaparin had a hematological malignancy and markedly elevated D-dimer level. No mortality was documented, all subjects recovered.
The clinical course of two representative subjects is shown in Figures 1, 2, respectively. The first subject is a 4-year-old patient with thalassemia trait who returned from a trip to Turkey 3 weeks prior to presentation. He presented to our center with high-grade fever of 6 days duration, nasal congestion, and rash. Two nasopharyngeal RT-PCR tests for SARS-CoV-2 in the preceding few days were negative. On physical examination, he had bilateral nonexudative conjunctivitis with limbic sparing and progressive erythematous maculopapular blanching rash over his upper and lower extremities. Laboratory work up was significant for leukocytosis (26,000/mm3) with neutrophil predominance, microcytic anemia, low albumin, prolonged prothrombin time and International Normalized Ratio (INR), and elevated inflammatory markers including CRP, procalcitonin, ferritin and D-dimer. His SARS-CoV-2 serology yielded a positive IgG indicating a previous infection. Echocardiography showed mildly reduced ejection fraction, prominent coronary arteries, as well as mild tricuspid and mitral regurgitation. He was initially hemodynamically unstable requiring admission to the ICU and inotropic support. He was started empirically on broad spectrum antibiotics (vancomycin and ceftriaxone). The patient was diagnosed with MIS-C based on the CDC case definition and received IVIG, steroids and low-dose aspirin.
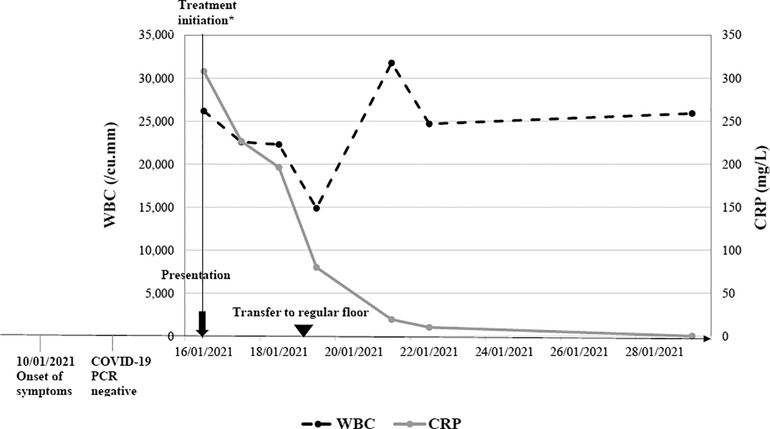
Figure 1. Clinical course of a 4-year-old with confirmed MIS-C. *Admission to Pediatric Intensive care unit and treatment administration including IVIG, steroids, aspirin and antibiotics.
The second subject is a 12-month-old boy who presented to the emergency department with a 2-day history of high-grade fever, associated with vomiting, decreased oral intake and urine output. His physical examination was remarkable for pharyngeal exudates, but no cervical adenopathy. The patient's parents had COVID-19 infection around 3 weeks prior to the onset of his symptoms, during which the patient had one episode of high-grade fever and diarrhea but was not tested. COVID-19 RT-PCR done on admission was negative while SARS-CoV-2 serology yielded a positive IgG and negative IgM. He had a WBC count of 13,900 /mm3 and CRP level of 112.7 mg/L. He was admitted to the pediatric unit for intravenous hydration and was started on aspirin as his echocardiography revealed prominent distal left anterior descending (LAD) coronary artery, with good biventricular function, and no pericardial effusion. This patient did not meet the full criteria for MIS-C and therefore was considered as a case of “Near MIS-C”. It is remarkable to mention that white count, platelet count, and inflammatory markers improved significantly at two, five, and twelve days later. In addition, the prominent distal LAD improved gradually on follow up echocardiography evaluations performed at one, three, and five months. However, it is worth mentioning that no further investigations to rule out a possible viral etiology were done since the patient clinically improved within two days without substantial intervention and that the initial presentation did not qualify for the diagnosis of Kawasaki disease (only 2 days of fever, no lymphadenopathy, mucositis, rash and never developed desquamation, or laboratory findings of thrombocytosis or hypoalbuminemia).
In the current series of patients with, or suspected to have, MIS-C, fever was present in all subjects including those from the “Near MIS-C” and “Alternative Diagnosis” groups. Cardiovascular system involvement was the most reported in our MIS-C group followed by mucocutaneous and gastrointestinal systems involvement. A recent systematic review also showed that cardiovascular and gastrointestinal symptoms were most commonly seen in MIS-C patients (15), in addition an Italian single-center study reported that 81% of their MIS-C patients had cardiac involvement, confirming that the heart along with the gastrointestinal system are most involved in MIS-C patients (18). Gastrointestinal symptoms were similarly encountered in the “Near MIS-C” and “Alternative Diagnosis” group but none had clinical evidence of coagulopathy or cardiac abnormalities except for one patient who was suspected to have cyclosporin-induced pericarditis. Hence, subjects with “Near MIS-C” did not meet the CDC case definition criteria but were hospitalized for close monitoring lest they develop additional organ involvement after their initial presentation.
MIS-C presents a diagnostic challenge for the physician due to the nonspecific early symptoms, lack of pathognomonic findings, and potentially fatal complications. Our understanding of the pathogenesis of this syndrome is evolving but remains limited. Whereas genetic predisposition to developing severe MIS-C has been demonstrated (19), the true triggers for the severe inflammatory response seen in otherwise healthy individuals with MIS-C and characterized by increased plasma cytokine levels including IL-6, IL-8, IFNγ, IL-17, TNFα, and IL-10 and the multilineage immune cell activation remain unknown (20–24). A specific immunopathologic signature and association with HLA alleles were identified recently that may pave the way for the development of more specific biomarkers (25). Importantly, the magnitude of the inflammatory response in MIS-C correlates with disease severity (23) and this raises the possibility that this syndrome is actually a continuum of clinical severity rather than an all-or-none response as the current definitions seem to imply.
In the present study, we compared the RCPCH, the WHO and the CDC case definitions (9–11). We found that the RCPCH definition was more inclusive and identified all subjects with MIS-C and “Near MIS-C” and excluded those who had features of MIS-C but were found to have an alternative diagnosis. The WHO and the CDC definitions are more stringent concerning clinical manifestations and the relationship with SARS-CoV-2 infection. In our case series, the WHO definition identified more subjects in the MIS-C group compared to the CDC definition, with few subjects whom we classified as “Near MIS-C” as they did not present multisystem dysfunction or did not have persistent fever for ≥3 days as per the WHO definition. In contrast to that of the WHO, the CDC definition excluded few subjects of those included in the MIS-C group based on the WHO definition, all failing to achieve the CDC criterion concerning the multi-organ (≥2 organ systems) dysfunction. Similarly, in a systematic review, Hoste et al. observed that although the RCPCH definition included all cases, nevertheless both the CDC and WHO case definitions were more selective, requiring multisystem involvement and a proven association with SARS-CoV-2, with the WHO definition including 97% of cases and the CDC definition 62% (15). Clearly a more precise definition for MIS-C based on a better understanding of the pathogenesis and the identification of more specific clinical and laboratory markers is needed.
The dynamic nature of this syndrome presents a further challenge to the physician. In the literature, several cases were reported to present with incomplete criteria initially but to later develop typical findings including cardiac involvement (16, 19). This has led us to hospitalize patients when the diagnosis of MIS-C was highly suspected, to provide close monitoring and rapid intervention when needed. This is further confounded by the frequent presence of another infection at the time of presentation. All the current definitions include the lack of “alternative plausible diagnoses (CDC)” (9), “other obvious microbial cause of inflammation (WHO)” (11), or “any other microbial cause (RCPCH)” (10). In our series, eight subjects ended up with an alternative diagnosis: drug-induced pericarditis, UTI, respiratory tract infections caused by adenovirus or rhinovirus/enterovirus, or infection with brucella, salmonella, or rotavirus. In these cases, the suspicion of MIS-C arose due to the temporal association with COVID-19 infection or exposure and the presence of commensurate clinical and laboratory findings including elevated inflammatory markers and D-dimers. In this regard, several questions present themselves for treating physicians: (1) how extensively should they work up the patient looking for an alternative diagnosis? (2) once identified, is the alternative diagnosis sufficient to explain the clinical presentation? (3) are the laboratory findings in keeping with the alternative diagnosis, e.g., does a rhinoviral infection explain the significantly elevated D-dimer level? Finally, (4) does the identification of an alternative diagnosis rule out the possibility of concomitant MIS-C? These questions present dilemmas for the treating physician who must decide on further treatment but has no discernible answers. An extensive work up to exclude a microbial infection may be necessary to rule out the majority of the alternative diagnoses, e.g., multiplex PCR respiratory and gastrointestinal panels that may not be readily available at most hospitals especially in underprivileged locations. Moreover, these additional tests present a significant economic burden on the healthcare system. On the other hand, not performing the extensive work up may lead to overdiagnosis of MIS-C as patients may satisfy all the criteria in the absence of an alternative diagnosis as reported previously (26).
Whether the identification of an alternative diagnosis is sufficient to explain the clinical and laboratory findings is not always straightforward. For example, all our subjects demonstrated elevation in inflammatory markers specifically CRP, ESR and D-dimer, which are nonspecific and overlap with many other infectious and inflammatory diseases (27). In the pre-COVID-19 era, and before the emergence of MIS-C, obtaining laboratory values such as ferritin and D-dimer for evaluation of febrile children was uncommon. In pediatric patients, D-dimer is requested for a select group of diagnoses including pulmonary embolism, disseminated intravascular coagulation, or hyperthrombotic states (28), however this is not the practice for other causes such as rhinovirus, rotavirus, or salmonella infections. For that reason, expected values for such tests in children with common febrile illnesses remain unknown (27). This is illustrated in the current series where we could not be certain whether the alternative diagnoses found in some of our subjects were associated intrinsically with elevated D-dimer or this finding was due to concomitant MIS-C or a recent COVID-19 infection. Not performing the D-dimer level doesn't seem to be a viable option as an increased level is sometimes the only clue for MIS-C and coronary artery dilatation (29). Thus, trending these inflammatory markers in subjects with suspected MIS-C, even in the presence of an alternative diagnosis, may help identify concomitant diagnoses of MIS-C, prevent disease progression, and multi-organ involvement (30).
Clearly, more research is needed to fully understand the pathogenesis of MIS-C and to fully appreciate its clinical presentation spectrum. Based on our experience reported in the current series, we favor the hypothesis that MIS-C has a continuum of severity where the most severe cases present as Kawasaki disease-like picture or shock/myocarditis and the less severe cases present with various degrees of fever and hyperinflammation. The current study is limited by its retrospective nature from a single center. As a result, not all cases suspected to have MIS-C were worked up to the same extent. Thus, larger prospective studies are required to verify this hypothesis to provide unambiguous answers. For example, current databases recruiting MIS-C patients (31–33) should expand to include subjects with “Near MIS-C” and even those with an alternative diagnosis that may not fully explain all the clinical and laboratory findings. This is going to be more important as the pandemic wanes and the disease becomes more sporadic presenting an even more difficult diagnostic challenge for treating physicians.
The datasets presented in this article are not readily available because of privacy and ethical restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethical clearance was obtained from the Institutional Review Board (IRB) at the American University of Beirut Medical Center (BIO-2021-0090) in line with the World Medical Association, Declaration of Helsinki in 2013. A waiver from obtaining consent from subjects was granted by IRB for this study since we were reviewing medical records retrospectively, all data collected were stripped of patient identifiers, and there is no risk to subjects.
SK, NY, RHW and GD contributed to conception and design of the study. SK, NY, SBK, NAH, RS, DH, NEM, ON, AB and GBS collected data. SK and CB performed the statistical analysis and participated in the results writing. SK, NY wrote the initial draft of manuscript. ZEZ wrote sections of the manuscript. RHW and GD critically revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382(8):727–33. doi: 10.1056/NEJMoa2001017
3. Cucinotta D, Vanelli M. WHO Declares COVID-19 a pandemic. Acta Biomed. (2020) 91(1):157–60. doi: 10.23750/abm.v91i1.9397
4. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. (2020) 34:101623. doi: 10.1016/j.tmaid.2020.101623
5. Toraih EA, Hussein MH, Elshazli RM, Kline A, Munshi R, Sultana N, et al. Multisystem inflammatory syndrome in pediatric COVID-19 patients: a meta-analysis. World J Pediatr. (2021) 17(2):141–51. doi: 10.1007/s12519-021-00419-y
6. Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69(15):458–64. doi: 10.15585/mmwr.mm6915e3
7. Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. (2020) 20(11):e276–88. doi: 10.1016/S1473-3099(20)30651-4
8. Esposito S, Principi N. Multisystem inflammatory syndrome in children related to SARS-CoV-2. Paediatr Drugs. (2021) 23(2):119–29. doi: 10.1007/s40272-020-00435-x
9. CDC. Multisystem Inflammatory Syndrome (MIS) (2021). Available from: https://www.cdc.gov/mis/mis-c/hcp/index.html (Accessed May 11, 2022).
10. Health RCoPaC. Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS) - guidance for clinicians (2020). Available from: https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance (Accessed May11, 2022).
11. Organization WH. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19 (2020). Available from: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (Accessed May 11, 2022).
12. Panaro S, Cattalini M. The Spectrum of manifestations of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV2) infection in children: what we can learn from multisystem inflammatory syndrome in children (MIS-C). Front Med (Lausanne). (2021) 8:747190. doi: 10.3389/fmed.2021.747190
13. Alkan G, Sert A, Oz SKT, Emiroglu M, Yılmaz R. Clinical features and outcome of MIS-C patients: an experience from Central Anatolia. Clin Rheumatol. (2021) 40(10):4179–89. doi: 10.1007/s10067-021-05754-z
14. Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. (2021) 180(2):307–22. doi: 10.1007/s00431-020-03766-6
15. Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. (2021) 180(7):2019–34. doi: 10.1007/s00431-021-03993-5
16. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324(3):259–69. doi: 10.1001/jama.2020.10369
17. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N Engl J Med. (2020) 383(4):334–46. doi: 10.1056/NEJMoa2021680
18. Mannarino S, Raso I, Garbin M, Ghidoni E, Corti C, Goletto S, et al. Cardiac dysfunction in multisystem inflammatory syndrome in children: an Italian single-center study. Ital J Pediatr. (2022) 48(1):25. doi: 10.1186/s13052-021-01189-z
19. Roarty C, Waterfield T. Review and future directions for PIMS-TS (MIS-C). Arch Dis Child. (2022):archdischild-2021-323143. doi: 10.1136/archdischild-2021-323143
20. Glover HA, Davis AB. A case of multisystem inflammatory syndrome in children following SARS-CoV-2 infection in a rural emergency department. Adv Emerg Nurs J. (2021) 43(2):114–22. doi: 10.1097/TME.0000000000000346
21. Reiff DD, Cron RQ. Who Would Have Predicted Multisystem Inflammatory Syndrome in Children? Current Rheumatology Reports. (2022).
22. Waseem M, Shariff MA, Tay ET, Mortel D, Savadkar S, Lee H, et al. Multisystem inflammatory syndrome in children. J Emerg Med. (2022) 62(1):28–37. doi: 10.1016/j.jemermed.2021.07.070
23. Vella LA, Rowley AH. Current insights into the pathophysiology of multisystem inflammatory syndrome in children. Curr Pediatr Rep. (2021) 9:1–10. doi: 10.1007/s40124-021-00257-6
24. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. (2022) 23(2):177–85. doi: 10.1038/s41590-021-01123-9
25. Sacco K, Castagnoli R, Vakkilainen S, Liu C, Delmonte OM, Oguz C, et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat Med. (2022) 28:1050–62. doi: 10.1038/s41591-022-01724-3
26. KC S, Awasthi P, Kumar S, Angurana SK, Nallasamy K, Angrup A, et al. MIS-C mimickers: a case series of bacterial enteritis and sepsis mistaken as MIS-C. Indian J Pediatr. (2022) 89(2):206. doi: 10.1007/s12098-021-04019-6
27. Dworsky ZD, Roberts JE, Son MBF, Tremoulet AH, Newburger JW, Burns JC. Mistaken MIS-C: a case series of bacterial enteritis mimicking MIS-C. Pediatr Infect Dis J. (2021) 40(4):e159–61. doi: 10.1097/INF.0000000000003050
28. Kanis J, Hall CL, Pike J, Kline JA. Diagnostic accuracy of the D-dimer in children. Arch Dis Child. (2018) 103(9):832–4. doi: 10.1136/archdischild-2017-313315
29. Acharya S, Saxena A, John BM, Tewari VV. Atypical multisystem inflammatory syndrome in children (MIS-C). Indian J Pediatr. (2021) 88(7):715. doi: 10.1007/s12098-021-03774-w
30. Han P, Douillard J, Cheng J, Ramanathan A, Tieu D, Degner T. Multisystem inflammatory syndrome in children in a 15-year-old male with a retropharyngeal phlegmon. Case Rep Pediatr. (2020) 2020:6668371. doi: 10.1155/2020/6668371
31. Nygaard U. Incidence of MIS-C Following SARS-CoV-2 Infection. Identifier: NCT05186597. Available from: https://clinicaltrials.gov/ct2/show/study/NCT05186597
32. (NIAID) NIoAaID. COVID-19: Pediatric Research Immune Network on SARS-CoV-2 and MIS-C. Identifier: NCT04588363. Available from: https://clinicaltrials.gov/ct2/show/record/NCT04588363
33. HealthCore-NERI. Long-Term Outcomes After the Multisystem Inflammatory Syndrome in Children (MUSIC). Available from: https://clinicaltrials.gov/ct2/show/record/NCT05287412
Keywords: fever, COVID-19, SARS-CoV-2, multisystem inflammatory syndrome in children (MIS-C), Lebanon, children, adolescents
Citation: Khafaja S, Youssef N, El Zein Z, Boutros CF, Bou Karroum S, Abdel-Halim N, Salameh R, Hodroj D, El Meski N, Nasrallah O, Bidikian A, Bou Saba G, Arabi MT, Hanna-Wakim R and Dbaibo GS (2023) Multisystem inflammatory syndrome in children (MIS-C) and “Near MIS-C”: A continuum?. Front. Pediatr. 10:988706. doi: 10.3389/fped.2022.988706
Received: 7 July 2022; Accepted: 8 December 2022;
Published: 5 January 2023.
Edited by:
Hulya Bukulmez, Case Western Reserve University, United StatesReviewed by:
Wail Hayajneh, Cardinal Glennon Children's Medical Center, United States© 2023 Khafaja, Youssef, El Zein, Boutros, Bou Karroum, Abdel-Halim, Salameh, Hodroj, El Meski, Nasrallah, Bidikian, Bou Saba, Arabi, Hanna-Wakim and Dbaibo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghassan Dbaibo Z2RiYWlib0BhdWIuZWR1Lmxi
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Pediatric Infectious Diseases, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.