
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Pediatr., 09 August 2022
Sec. Children and Health
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.981741
This article is part of the Research TopicPediatric Preventable DiseasesView all 10 articles
 Louis Bont1†
Louis Bont1† Catherine Weil Olivier2†
Catherine Weil Olivier2† Egbert Herting3†
Egbert Herting3† Susanna Esposito4†
Susanna Esposito4† Jose Antonio Navarro Alonso5†
Jose Antonio Navarro Alonso5† Federico Lega6†
Federico Lega6† Silke Mader7†
Silke Mader7† Ichiro Morioka8†
Ichiro Morioka8† Kunling Shen9†
Kunling Shen9† George A. Syrogiannopoulos10†
George A. Syrogiannopoulos10† Saul N. Faust11,12†
Saul N. Faust11,12† Elena Bozzola13*†
Elena Bozzola13*†Respiratory syncytial virus (RSV) infects nearly all infants at least once by their second birthday (1). It spreads through coughs, sneezes, or close physical contact (2). RSV infections are associated with morbidity and mortality, ranging from mild upper respiratory illness to life threatening lower respiratory tract infections (LRTIs). More than 97% of RSV-attributable deaths occur in low- and middle-income countries (LMICs) (3), reflecting that healthcare infrastructure and resources in these countries is limited and may present issues in dealing with RSV disease burden.
Severe RSV infection is more likely during the first months of life (4). RSV causes a substantial burden for infants below 12 months of age. It is estimated that 12.9 million RSV LRTI episodes, 2.2 million RSV-associated hospitalizations and 66,300 RSV-attributable deaths occurred globally in 2019 (3). RSV also causes a significant outpatient burden worldwide, with RSV being associated with 21% of infants aged <24 months brought to the emergency department and 18% in pediatric practices according to one study (5).
RSV is a leading cause of hospitalization among infants in their first year of life, whether they are born during or before the RSV season (6). Severe RSV cases are difficult to predict. Although preterm infants or infants with co-morbidity have a higher risk of having a severe infection, ~80% of infants hospitalized with RSV are otherwise healthy, i.e., with no underlying medical conditions, and born at term (7). The percentage can be even higher in some countries. For example, it was reported in Japan that 98% of infants hospitalized for RSV were otherwise healthy (8). In addition, roughly 50% of all children hospitalized with RSV are born outside of the RSV season (9). Quantifying individual RSV risk is far more complicated than assessing population level RSV risk due to a number of interrelated risk factors (10). Multiple factors appear to put some children at higher risk of a severe RSV infection, such as having older siblings; having a parent who smokes; exposure to pollution; poor living conditions; living in the suburbs or large communities; siblings attending school or daycare; socio-economic status and a low level of parental education; maternal age; and a familial history of atopy.
RSV represents a significant economic burden. RSV infection increases the length of hospital stay and admissions to the intensive care department compared to non-RSV-related infections (11). RSV in premature and at-risk infants (i.e., those with congenital heart disease, chronic lung disease, neuromuscular impairment, immunodeficiency and Down's syndrome) incurs an individual economic burden that is comparatively higher than a healthy, full-term infant (12, 13). However, in an annual cohort of infants, RSV disease burden and associated costs (including hospitalization and outpatient visit costs) are created mostly by RSV in infants who were healthy prior to the acute RSV illness, due to the far higher number of hospitalizations observed in this infant population (14). Infant RSV infection also resulted in significant 5-year long-term healthcare-resource utilization impact (15). The etiological link between RSV infection and the development of asthma has long been debated. Many studies assume that RSV infection is a trigger of a pre-existing predisposition to asthma and can trigger further economic burden (3, 14, 16, 17).
RSV infections also place a significant burden and emotional impact on affected families and caregivers (18–20). Parents may feel powerless due to a lack of knowledge on RSV and its related complications. Given the overwhelming service needs during RSV season, which may overlap with other infectious disease's seasonality, healthcare staff are also impacted, often manifesting as stress and burnout (21).
The COVID-19 pandemic has disrupted the epidemiology of many infectious diseases—including RSV. RSV usually peaks across the late autumn and winter in temperate countries (typically November to March) (22). While typically being more evenly distributed across the year in tropical regions, most countries in this region show a peak toward late summer (23). A minority of countries—almost all equatorial LMICs—report multiple peaks, or consistent RSV circulation year-round (23).
Non-pharmaceutical COVID-19 interventions such as social distancing reduced transmission, leading to an unusual reduction of RSV cases throughout 2020 in many countries (22). In 2021, following the removal of lockdown measures, uncommon resurgences during spring and summer were first reported in southern hemisphere countries such as Australia (24). Similar trends were then observed in some northern hemisphere countries, such as France, Spain, Germany and the UK (25).
The out-of-season peaks resulted in a significant disruption of healthcare systems and overwhelmed pediatric services due to RSV-related complications and potential hospital-acquired infections. In Germany, children aged 6 months to 1 year were hospitalized due to RSV across the summer of 2021 at a higher rate than they had been in previous years (26). Due to reduced circulation of RSV during the winter months of 2020, older infants and toddlers showed an increased risk of severe RSV-associated illness in 2021 (up to 5 years of age) (27).
All infants should ideally be protected during their first RSV season as long as the prevention solution provided is proven safe and cost-effective. The only currently available preventative measure is a mAb, palivizumab (AstraZeneca) which is indicated and approved in many high-income countries for some infants born preterm, and/or who have existing heart or lung disease and must be injected monthly throughout the RSV season (28).
Several active and passive immunization options are in late-stage development. These include new monoclonal antibodies and both pediatric and maternal vaccines. Clinical trials are ongoing (29). It is of utmost importance to have data on the efficacy and duration of protection for these new immunization options in order for Immunization Technical Advisory Groups to assess them properly, with the objective of protecting all infants against RSV and reducing the overall RSV burden.
A long-acting mAb, which is likely to be the first licensed new preventative intervention, has shown promising results and there is a growing body of evidence suggesting it can protect all infants from RSV through their first RSV season with a single dose (30–34) (Figure 1). A baby born during or just before the RSV season should be immunized with a mAb as early as possible. The timing and location in which the immunization is administered is likely to differ depending on the country due to the differences in the postnatal practices and systems. Where possible, immunization should occur directly after birth, whether in the hospital, birth centers or in primary care settings (31, 35–37). Approaches that work within pre-existing immunization routine visits and structures are essential to ensuring uptake and to avoiding additional appointments for parents. The closest medical appointment to the start of the RSV season will be the optimal time for administration of the mAb to infants born before it (35). There is a degree of flexibility in the administration timing due to the rapid onset of protection with a mAb. Although acceptability of the RSV mAb palivizumab is currently high for infants at risk, it is unknown to what extent parents of healthy, full-term infants will accept long-acting mAbs against RSV (38–40).
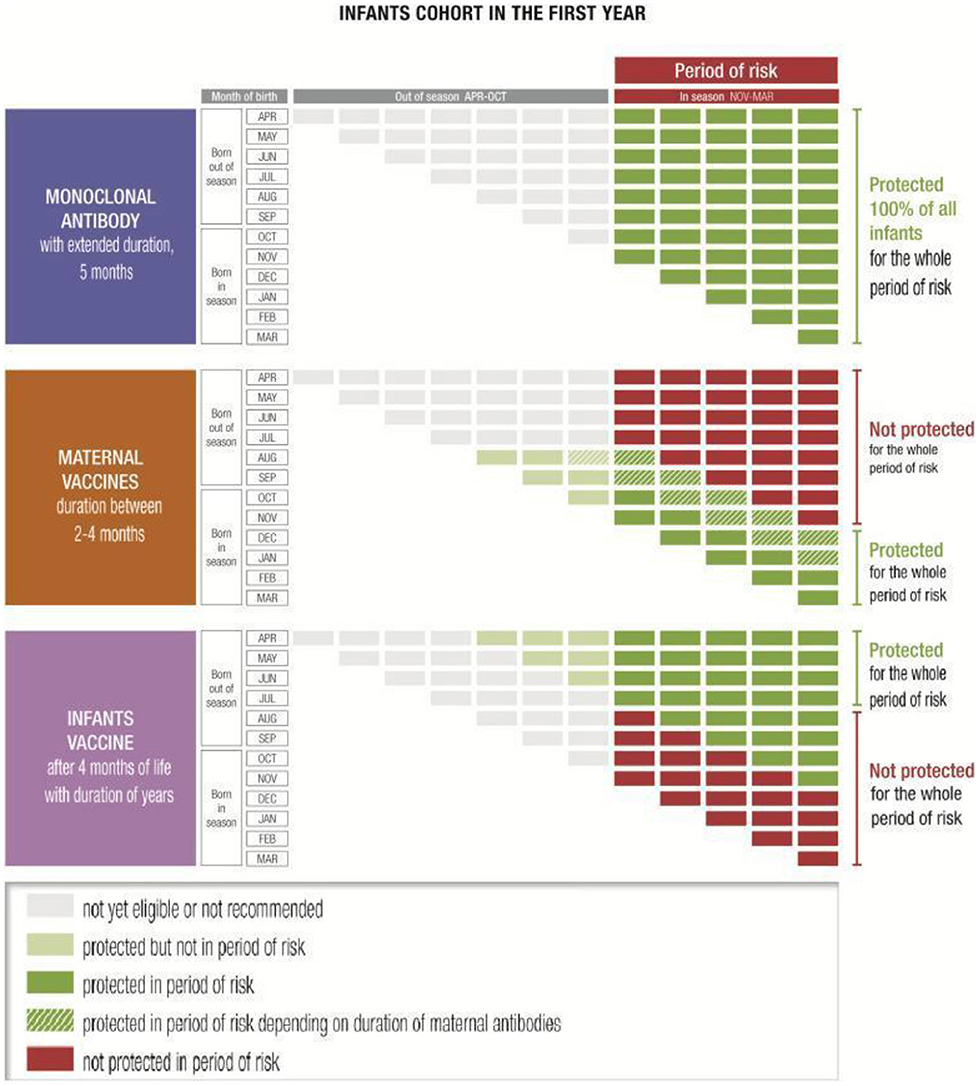
Figure 1. Examples of immunization coverage of infants through the first year of life against the November–March seasonality typical of temperate Northern hemisphere countries (35).
Maternal vaccination is another option which might have great potential (41–43), even though the protected population might be more limited in cases of extreme prematurity where the infant may not receive the full benefit of antibody transfer (44), and for infants born out of season (35) (Figure 1). It may be that some health systems will offer parents a choice of prevention technologies: if a maternal vaccine is licensed, for babies born close to or in season, the mother may be offered the choice of receiving a vaccine during the third trimester or of the baby receiving a mAb shortly after birth. In countries and communities with poor maternal vaccine coverage, implementation of a RSV maternal vaccine could be challenging.
Both options (mAbs and maternal immunization) could be combined with RSV pediatric vaccines—which will likely be available at a much later date—to extend the duration of RSV protection throughout childhood (35) (Figure 1). This combined approach would grant immune coverage throughout the first months of life before immune system maturity is developed.
Currently there is a general lack of education about the burden of RSV and especially about all infants' cohorts being at potential risk. Consequently, there is a lack of awareness about the differences and complementarities between future preventative solutions. Knowledge was even noted to be low among non-specialist medical practitioners (45).
Parents must be properly informed about the benefit and reassured about the safety profile of any preventative solution in appropriate language. Some of this education will be given by health practitioners including midwives, nurses, family doctors, pharmacists and pediatricians, depending on the country.
The current momentum of discussion surrounding infectious diseases must not be wasted. COVID-19 has sensitized policymakers to respiratory infectious diseases and their necessary prevention. In many countries, these are now high on the political agenda. Policymakers should be informed of the burden of RSV in all infants, the value of the different prevention solutions and how they can support public health objectives, and prioritize the assessment of new RSV immunization routine strategies. Overall, strategies to increase the uptake of immunization, as well as efforts to dispel harmful misinformation (46) must also be promoted.
Even though a lot of data is already available, there is still a need for improved national surveillance systems for respiratory viral infections. New epidemiological data will come from increased use of multiplex PCR and rapid tests in the wake of COVID-19. Policymakers and healthcare professionals should also consider collecting more data on rehospitalizations and outpatients visits due to the long-term sequelae of RSV infection in infancy, such as recurrent wheezing and asthma. These data may be important in aiding evidence-based decisions about prevention.
It is difficult to identify which infants will experience the most severe RSV illness and the vast majority of infants hospitalized with severe RSV are otherwise healthy and born at term. Beyond hospitalizations, RSV causes a significant outpatient burden. Policy recommendations to protect all infants against RSV should be discussed by Immunization Technical Advisory Groups. In each country, the development of recommendations will demand a careful assessment of the new RSV immunization programs and an analysis on the ease of implementation of these measures.
New immunization perspectives can protect more infants compared with the current standard of care. New long-acting mAbs are likely to be licensed first and can protect all infants, through administration at or close to birth for those infants born during the RSV season or alongside pre-scheduled immunization visits for those born before the season. That point of view is supported by additional publications released since the RSV Experts Group Event held in October 2021 (47, 48). Reducing RSV could relieve recurrent pressure on healthcare systems, leading to more efficient use of resources and contributing to the sustainability of healthcare systems.
The uptake of new RSV immunization strategies depends on the level of awareness of RSV among healthcare professionals, policymakers, and parents.
Although more is needed, awareness of RSV is improving. COVID-19 has played a huge role in this, with multiplex testing also checking for RSV cases in some countries. The data generated have made healthcare workers more aware of the volume of cases. The pandemic has also highlighted how crucial adequate immunization coverage is to maintaining both the health of the targeted population and the functioning of healthcare systems. As a consequence, many governments are now prioritizing the prevention of respiratory infectious diseases. As the burden of RSV is much higher than that of other pediatric infectious diseases, it is crucial to make the assessment of new RSV preventative measures for all infants a priority.
All authors participated in the RSV Experts Group Event held in October 2021. The discussions during this meeting were used as the basis of the content of the article.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Sanofi and AstraZeneca facilitated and financially supported the RSV Experts Group Event, remuneration to some of the experts was agreed for the time spent for the meeting but the authors were not paid for writing the publication. Funding for the publication fees was provided by Sanofi and AstraZeneca. Hyderus CYF—was paid by Sanofi and AstraZeneca for the expert meeting organization, medical writing, and editorial support.
The authors thank Erin Sparrow, Technical Officer at the World Health Organization, for her participation as an observer. The authors also note Mark Chataway and Marion Zibelli for their role as moderators. The authors acknowledge Nicholas Parry and Mark Chataway, for providing editorial assistance with the preparation of this manuscript, funded by Sanofi and AstraZeneca; and acknowledge Dr. Sonali Kochhar for providing expert medical review.
LB has regular interaction with pharmaceutical and other industrial partners and has not received personal fees or other personal benefits and was the founding chairman of the ReSViNET Foundation. UMCU has received major funding (>€100,000 per industrial partner) for investigator initiated studies from AbbVie, MedImmune, AstraZeneca, Sanofi, Janssen, Pfizer, MSD and MeMed Diagnostics, has received major funding for the RSV GOLD study from the Bill and Melinda Gates Foundation, has received major funding as part of the public private partnership IMI-funded RESCEU and PROMISE projects with partners GSK, Novavax, Janssen, AstraZeneca, Pfizer and Sanofi, has received major funding by Julius Clinical for participating in clinical studies sponsored by MedImmune and Pfizer, and received minor funding (€1,000–25,000 per industrial partner) for consultation and invited lectures by AbbVie, MedImmune, Ablynx, Bavaria Nordic, MabXience, GSK, Novavax, Pfizer, Moderna, Astrazeneca, MSD, Sanofi, Genzyme, Janssen. EH has received speaking/advisory fees/travel support from Abbott (Astra Zeneca, Medimmune), Sanofi and Merck and has supported the German and the European (EFCNI) parents organization in the preparation of information material concerning RSV. UKSH has received a grant (no personal money for EH) to conduct a study (BRICE study) on the prevalence of RSV in Europe. SE reports Advisory Board Participation and Honoraria for Lectures: GSK, Janssen, Pfizer, Moderna, MSD, Qiagen, Sanofi, Genzyme, Janssen. SM reports a sponsorship agreement between SP and EFCNI. IM has received lecture fees from AstraZeneca K.K., MSD Co., Ltd., and Sanofi K.K. An honorarium was paid to SFs institution for his participation in the expert group by Sanofi but SF received no personal payments of any kind. SF has acted as clinical trial investigator on behalf of his hospital for GSK, Janssen (J&J), Regeneron and Medimmune (AstraZeneca) in the field of RSV vaccines and monoclonal antibodies but SF received no personal payment of any kind.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ALRI, Acute lower respiratory illness; mAbs, monoclonal antibodies; RSV, Respiratory syncytial virus; RSV-ALRI, RSV-associated acute lower respiratory illness; WHO, World Health Organization; ICU, Intensive Care Units; LMICs, Low- and middle-income countries.
1. Scheltema N, Gentile A, Lucion F, Nokes D, Munywoki P, Madhi S et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Global Health. (2017) 5:e984–91. doi: 10.26226/morressier.5ad774e1d462b80296ca6e1d
3. Li Y, Wang X, Blau D, Caballero M, Feikin D, Gill C et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. (2022) 399:2047–64. doi: 10.1016/S0140-6736(22)00478-0
4. Openshaw P, Chiu C, Culley F, Johansson C. Protective and harmful immunity to RSV infection. Annu Rev Immunol. (2017) 35:501–32. doi: 10.1146/annurev-immunol-051116-052206
5. Lively J, Curns A, Weinberg G, Edwards K, Staat M, Prill M et al. Respiratory syncytial virus-associated outpatient visits among children younger than 24 months. J Pediatric Infect Dis Soc. (2019) 8:284–6. doi: 10.1093/jpids/piz011
6. McLaurin K, Farr A, Wade S, Diakun D, Stewart D. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol. (2016) 36:990–6. doi: 10.1038/jp.2016.113
7. Hall C, Weinberg G, Blumkin A, Edwards K, Staat M, Schultz A et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. (2013) 132:e341–8. doi: 10.1542/peds.2013-0303
8. Seimiya A, Morioka I, Okahashi A, Nagano N, Yoda H. Survey of infants hospitalized for respiratory syncytial virus disease in Tokyo, 2018. Pediatr Int. (2021) 63:219–21. doi: 10.1111/ped.14381
9. Demont C, Petrica N, Bardoulat I, Duret S, Watier L, Chosidow A et al. Economic and disease burden of RSV-associated hospitalizations in young children in France, from 2010 through 2018. BMC Infect Dis. (2021) 21:730. doi: 10.1186/s12879-021-06399-8
10. Bont L, Checchia P, Fauroux B, Figueras-Aloy J, Manzoni P, Paes B et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in Western Countries. Infectious Diseases and Therapy. (2016) 5:271–98. doi: 10.1007/s40121-016-0123-0
11. Hervás D, Reina J, Yañez A, Valle J, Figuerola J, Hervás J. Epidemiology of hospitalization for acute bronchiolitis in children: differences between RSV and non-RSV bronchiolitis. Eur J Clin Microbiol Infect Dis. (2012) 31:1975–81. doi: 10.1007/s10096-011-1529-y
12. Bowser D, Gervasio R, Glaser E, Harihan D, Rowlands K, Buckley L et al. The economic impact of respiratory syncytial virus (RSV) in infants in the United States: systematic literature review. Open Forum Infect Dis. (2020) 7(Supplement_1):S764. doi: 10.1093/ofid/ofaa439.1706
13. Bowser D, Shepard D, Rowlands K, Gervasio R, Glaser E, Buckley L et al. PRS11 Cost per hospitalization of respiratory syncytial virus in US infants: literature review and synthesis. Value Health. (2021) 24:S214–5. doi: 10.1016/j.jval.2021.04.1075
14. Simões E, Chirikov V, Botteman M, Kwon Y, Kuznik A. Long-term assessment of healthcare utilization 5 years after respiratory syncytial virus infection in US infants. J Infect Dis. (2020) 221:1256–70. doi: 10.1093/infdis/jiz278
15. Zhang S, Akmar L, Bailey F, Rath B, Alchikh M, Schweiger B et al. Cost of respiratory syncytial virus-associated acute lower respiratory infection management in young children at the regional and global level: a systematic review and meta-analysis. J Infect Dis. (2020) 222(Supplement_7):S680–S687. doi: 10.1093/infdis/jiz683
16. Driscoll A, Arshad S, Bont L, Brunwasser S, Cherian T, Englund J et al. Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? Critical review of the evidence and guidance for future studies from a World Health Organization-sponsored meeting. Vaccine. (2020) 38:2435–48. doi: 10.1016/j.vaccine.2020.01.020
17. Chawes B, Poorisrisak P, Johnston S, Bisgaard H. Neonatal bronchial hyperresponsiveness precedes acute severe viral bronchiolitis in infants. J Allergy Clin Immunol. (2012) 130:354–361.e3. doi: 10.1016/j.jaci.2012.04.045
18. Díez-Gandía E, Gómez-Álvarez C, López-Lacort M, Muñoz-Quiles C, Úbeda-Sansano I, Díez-Domingo J et al. The impact of childhood RSV infection on children's and parents' quality of life: a prospective multicenter study in Spain. BMC Infect Dis. (2021) 21:924. doi: 10.1186/s12879-021-06629-z
19. Glaser E, Bowser D, Hariharan D, Gervasio R, Rowlands K, Buckley L et al. POSC343 impact of respiratory syncytial virus on child, caregiver, and family quality of life (QOL) in the United States (US): systematic literature review and synthesis. Value Health. (2022) 25:S239. doi: 10.1016/j.jval.2021.11.1168
20. Mitchell I, Defoy I, Grubb E. Burden of respiratory syncytial virus hospitalizations in Canada. Can Respir J. (2017) 2017:1–9. doi: 10.1155/2017/4521302
21. EHMA. The Health System Burden of RSV in Europe: EHMA's White Paper. EHMA (2022). Available online at: https://www.shorturl.at/ (accessed June, 2022).
22. Baker R, Park S, Yang W, Vecchi G, Metcalf C, Grenfell B. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc Nat Acad Sci USA. (2020) 117:30547–53. doi: 10.1073/pnas.2013182117
23. Li Y, Hodgson D, Wang X, Atkins K, Feikin D, Nair H. Respiratory syncytial virus seasonality and prevention strategy planning for passive immunisation of infants in low-income and middle-income countries: a modelling study. Lancet Infect Dis. (2021) 21:1303–12. doi: 10.1016/S1473-3099(20)30703-9
24. Eden J, Sikazwe C, Xie R, Deng Y, Sullivan S, Michie A et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. medRxiv 2021.07.21.21260810. doi: 10.1101/2021.07.21.21260810
25. Bozzola E. 2021 respiratory syncytial virus resurgence in italy: the need to protect all neonates and young infants. Int J Environ Res Public Health. (2022) 19:380. doi: 10.3390/ijerph19010380
26. DGPI. RSV Coverage Update: 2022, Calendar Week 09. Available online at: https://dgpi.de/rsv-survey-update/ (accessed March 2022).
27. Lavoie P, Reicherz F, Solimano A, Langley J. Potential resurgence of respiratory syncytial virus in Canada. Can Med Assoc J. (2021) 193:E1140–1. doi: 10.1503/cmaj.210919
28. Mac S, Sumner A, Duchesne-Belanger S, Stirling R, Tunis M, Sander B. Cost-effectiveness of palivizumab for respiratory syncytial virus: a systematic review. Pediatrics. (2019) 143:e20184064. doi: 10.1542/peds.2018-4064
29. Eichinger K, Kosanovich J, Lipp M, Empey K, Petrovsky N. Strategies for active and passive pediatric RSV immunization. Therap Adv Vaccines Immunotherapy. (2021) 9:251513552098151. doi: 10.1177/2515135520981516
30. Hammitt L, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi S et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. New Engl J Med. (2022) 386:837–46. doi: 10.1056/NEJMoa2110275
31. Griffin M, Yuan Y, Takas T, Domachowske J, Madhi S, Manzoni P et al. Single-dose nirsevimab for prevention of RSV in preterm infants. New Engl J Medicine. (2020) 383:415–25. doi: 10.1056/NEJMoa1913556
32. Clinicaltrials.gov. A Study to Evaluate the Safety and Efficacy of MEDI8897 for the Prevention of Medically Attended RSV LRTI in Healthy Late Preterm and Term Infants (MELODY). Available online at: https://clinicaltrials.gov/ct2/show/NCT03979313 (accessed May, 2022)
33. Clinicaltrials.gov. A Study to Evaluate the Safety and Efficacy of MEDI8897 for the Prevention of Medically Attended RSV LRTI in Healthy Preterm Infants (MEDI8897 Ph2b). https://clinicaltrials.gov/ct2/show/results/NCT02878330 (accessed May 2022).
34. Clinicaltrials.gov. A Study to Evaluate the Safety of MEDI8897 for the Prevention of Medically Attended Respiratory Syncytial Virus (RSV) Lower Respiratory Track Infection (LRTI) in High-risk Children. Available online at: https://clinicaltrials.gov/ct2/show/NCT03959488 (accessed May 2022).
35. Azzari C, Baraldi E, Bonanni P, Bozzola E, Coscia A, Lanari M et al. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital J Pediatr. (2021) 47:198. doi: 10.1186/s13052-021-01148-8
36. Alonso S, Vidal M, Ruiz-Olalla G, González R, Jairoce C, Manaca M et al. HIV infection and placental malaria reduce maternal transfer of multiple antimalarial antibodies in Mozambican women. J Infect. (2021) 82:45–57. doi: 10.1016/j.jinf.2021.02.024
37. Zhu Q, McLellan J, Kallewaard N, Ulbrandt N, Palaszynski S, Zhang J et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med. (2017) 9:388. doi: 10.1126/scitranslmed.aaj1928
38. Skjefte M, Ngirbabul M, Akeju O, Escudero D, Hernandez-Diaz S, Wyszynski D et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. (2021) 36:197–211. doi: 10.1007/s10654-021-00728-6
39. Path. RSV Vaccine and mAb Snapshot. (2021). Available online at: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ (accessed December 2021).
40. Stark L, Power M, Turrentine M, Samelson R, Siddiqui M, Paglia M et al. Influenza vaccination among pregnant women: patient beliefs and medical provider practices. Infect Dis Obstet Gynecol. (2016) 2016:1–8. doi: 10.1155/2016/3281975
41. Glezen W, Paredes A, Allison J, Taber L, Frank A. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. (1981) 98:708–15. doi: 10.1016/S0022-3476(81)80829-3
42. Ogilvie M, Santhire Vathenen A, Radford M, Codd J, Key S. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol. (1981) 7:263–71. doi: 10.1002/jmv.1890070403
43. Stensballe L, Ravn H, Kristensen K, Agerskov K, Meakins T, Aaby P et al. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J Allergy Clin Immunol. (2009) 123:398–403. doi: 10.1016/j.jaci.2008.10.043
44. Twisselmann N, Bartsch Y, Pagel J, Wieg C, Hartz A, Ehlers M et al. IgG Fc glycosylation patterns of preterm infants differ with gestational age. Front Immunol. (2019) 9:e3166. doi: 10.3389/fimmu.2018.03166
45. Walsh E. Respiratory syncytial virus infection: an illness for all ages. Clin Chest Med. (2017) 38:29–36. doi: 10.1016/j.ccm.2016.11.010
46. Davidson M. Vaccination as a cause of autism-myths and controversies. Dialogues Clin Neurosci. (2017) 19:403–7. doi: 10.31887/DCNS.2017.19.4/mdavidson
47. Esposito S, Abu Raya B, Baraldi E, Flanagan K, Martinon Torres F, Tsolia M et al. RSV prevention in all infants: which is the most preferable strategy? Front Immunol. (2022) 13:880368. doi: 10.3389/fimmu.2022.880368
Keywords: infant and child health, infant mortality, respiratory syncytial virus (RSV), RSV-acute lower respiratory illness, monoclonal antibody, infant hospitalization, infant immunization, respiratory disease
Citation: Bont L, Weil Olivier C, Herting E, Esposito S, Navarro Alonso JA, Lega F, Mader S, Morioka I, Shen K, Syrogiannopoulos GA, Faust SN and Bozzola E (2022) The assessment of future RSV immunizations: How to protect all infants? Front. Pediatr. 10:981741. doi: 10.3389/fped.2022.981741
Received: 29 June 2022; Accepted: 19 July 2022;
Published: 09 August 2022.
Edited and reviewed by: Ting Fan Leung, The Chinese University of Hong Kong, Hong Kong SAR, China
Reviewed by:
Katherine Atkins, University of Edinburgh, United KingdomCopyright © 2022 Bont, Weil Olivier, Herting, Esposito, Navarro Alonso, Lega, Mader, Morioka, Shen, Syrogiannopoulos, Faust and Bozzola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Bozzola, ZWxlbmEuYm96em9sYUBvcGJnLm5ldA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.