- 1Department of Pediatrics, Tangdu Hospital, Air Force Medical University, Xi’an, China
- 2Department of Thoracic Surgery, Tangdu Hospital, Air Force Medical University, Xi’an, China
Pulmonary hemorrhage (PH) is a rare acute catastrophic event with high mortality among neonates, especially preterm infants. Primary treatments included pulmonary surfactant, high-frequency oscillatory ventilation, epinephrine, coagulopathy management, and intermittent positive pressure ventilation. However, there are still challenges in diagnosing and treating refractory or focal pulmonary hemorrhages. Ultra-slim bronchoscopy has been widely used in the field of critically ill children and is increasingly being done in neonates with critical respiratory disease in recent years. In this study, we report a case with refractory pulmonary hemorrhage in premature infants, which was finally diagnosed as localized hemorrhage in the upper left lobe and cured by ultra-slim bronchoscopy-guided topical hemostatic drug administration. Bronchoscopy is an optional, safe, and practicable technique for early diagnosis and direct injection therapy of neonatal PH in managing life-threatening PH.
Introduction
Pulmonary hemorrhage (PH) is a rare, life-threatening complication in premature infants with respiratory distress syndrome. The incidence of PH is 3 to 11 per 1,000 live births with PH occurring most commonly within the first few days of life (1–5). PH is typically seen in neonates weighing less than 1500 g, who often have a patent ductus arteriosus (PDA), infection complication, trauma, have been treated with surfactant, and are ventilated (4, 6, 7). Mortality rates of over 50% have been reported in premature infants (8), and as a result, PH does significantly increase the risk of later pulmonary or neurodevelopmental disabilities among those who survive (9, 10). There are several effective methods of managing PH in neonates, including surfactant, high-frequency oscillatory ventilation (HFOV), epinephrine, coagulopathy management, intermittent positive pressure ventilation, cocaine, and tolazoline (4). However, it remains challenging to diagnose and therapy precisely in refractory or focal PH cases. Here, we present a case of refractory PH in a preterm infant diagnosed by ultra-slim flexible bronchoscopy. Furthermore, video bronchoscopy provides accurate information about PH location and bronchoscopy-guided local hemostatic treatment. We aimed to provide a potentially effective treatment for refractory pulmonary hemorrhage in neonates.
Case report
A 1-h-old male neonate was born to an Asian mother through cesarean delivery at 33+3 weeks of gestation due to severe preeclampsia, weighed 1,870 g, and had Apgar scores of 9, 10, and 10 at 1, 5, and 10 min, respectively. The infant was artificial insemination-assisted reproduction due to the mother's tubal obstruction.
The infant presented with symptoms of respiratory distress, moaning, and a few pink foamy secretions from the mouth after birth. Physical exam revealed low breath sounds and pulmonary moist rales. The infant was diagnosed with neonatal respiratory distress syndrome (NRDS) and continuos positive airway pressure (CPAP)-assisted ventilation was administered immediately (FiO2 25% PEEP 5 cmH2O), then the infant's condition gradually stabilized. However, 5 h later, plenty of blood gushed from the infant's mouth. Meanwhile, respiratory distress was notable for the respiratory rate of 60 per minute and SPO2 sat down to 82%. Therefore, we actively carried out endotracheal intubation and ventilator-assisted ventilation (synchronized intermittent mandatory ventilation (SIMV): FiO2 40%, positive end expiratory pressure (PEE) 6.0 cmH2O, peak inspiratory pressure (PIP) 16 cmH2O, I: E 1:1.5). Simultaneously, epinephrine hydrochloride 1:10,000 and hemocoagulase were administered intratracheally. His complete blood count was 11.24 × 109/L (with 80% neutrophils and 14.1% lymphocytes), Hb 185 g/L, and PLT 249 × 109/L. Arterial blood gas analysis was PH 7.33, PaCO2 40.1 mmHg, PaO2 34 mmHg, HCO3 19.6 mmol/L, and lactate 4.09 mmol/L, whereas his C-reactive protein levels were normal. FiO2 was increased from 40% to 60% after administration of vitamin K1, plasma, hemocoagulase, cefotaxime sodium, and penicillin. One hour later, the infant's breathing was gradually stabilized, and calf pulmonary surfactant was administered. However, 2 h later, large amounts of blood were again gushing out of the endotracheal tube. In addition to the above treatment, we switched to HFOV [Drager NV500: FiO2 100%, mean airway pressure (MAP) 12 cmH2O, frequency 11 Hz, amplitude 20 cmH2O]. An ultrasonic cardiogram showed patent ductus arteriosus (3.3 mm) and patent foramen ovale (2.2 mm), and a chest x-ray showed exudative changes in both lungs. On day 2, the infant had dyspnea and respiratory distress under high-frequency ventilator-assisted, mottled skin, purpura, lethargy, abdominal distention, and capillary refill time (CRT) > 3 s. Simultaneously, the white blood cell count decreased to 2.4 × 109/L (with 85% neutrophils and 13.3% lymphocytes), and a chest x-ray showed increased inflammation in the lower lobes of both lungs. In addition, arterial blood gas analysis was PH 7.32, PaCO2 29.3 mmHg, PaO2 54 mmHg, HCO3−14.5 mmol/L, and lactate 4.4 mmol/L, and his C-reactive protein levels increased to 42.47 mg/L. Therefore, meropenem was administered as further anti-infective treatment. On day 3, his white blood cell count increased to 12.74 × 109/L (with 69.9% neutrophils and 16.2% lymphocytes), and Hb and PLT decreased to 127 g/L and 70 × 109/L, respectively. Therefore, the antibiotic was gradually upgraded to vancomycin based on the infant's symptoms and the laboratory examination results. On day 5, the coagulation series of the infant seriously deteriorated, especially Fib, from 0.85 g/L (with prothrombin time (PT) 17.7 s, prothrombin activity (PTA) 42.9 s, activated partial thromboplastin time (APTT) 52 s, international normalized ratio (INR) 1.7, thrombin time (TT) 22.4 s, fibrinogen degradation products (FDP) 44.8 mg/L, and D-Dimer 13.43 mg/L) to 0.39 g/L (with PT 13.2 s, PTA 69.9 s, APTT 34.3 s, INR 1.2, TT 26.7 s, FDP 32.17 mg/L, and D-Dimer 3.78 mg/L). Therefore, human fibrinogen, cryoprecipitate (Cpp), plasma, and suspension red blood cells (RBCs) were administered. During the following 2 weeks, the infant continued to suffer from recurrent pulmonary bleeding to varying degrees with the assistance of a high-frequency ventilator (Figure 1).
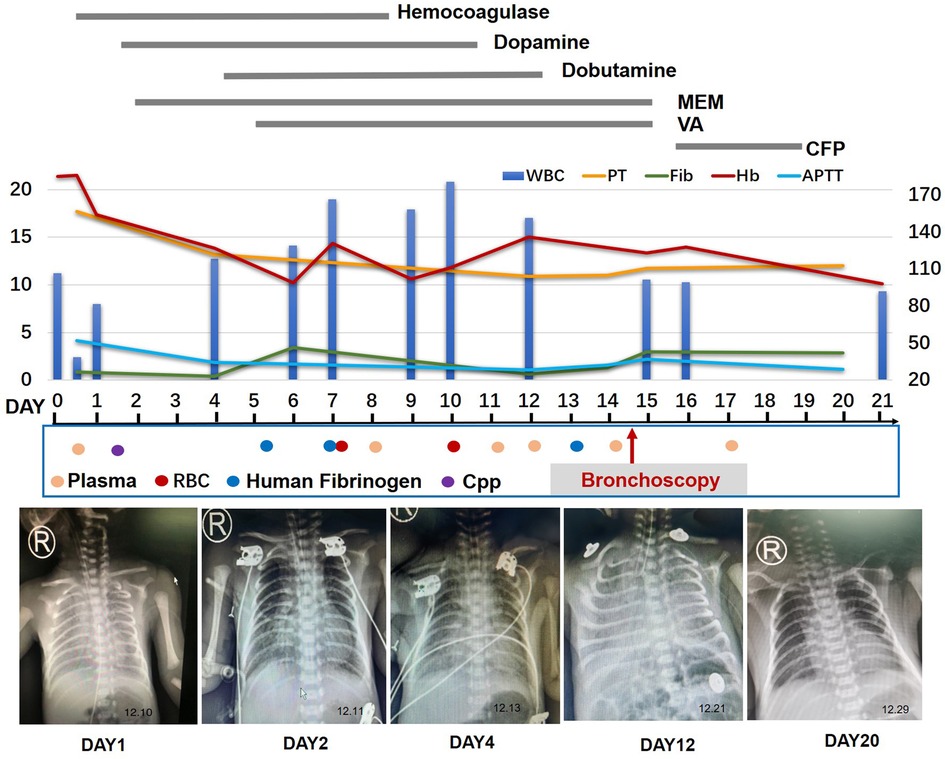
Figure 1. Timeline of the patient's clinical course and outcome. MEM, meropenem; VA, vancomycin; VOR, voriconazole; CFP, cefoperazone; RBC, red blood cells; Cpp, cryoprecipitate.
On day 14, the patient had recurrent symptoms of uncontrolled massive pulmonary hemorrhage, and vital signs were notable for the respiratory rate of 70 per minute and heart rate of 170 beats per minute. A physical exam revealed coarse breath sounds and pulmonary moist rales. The chest x-ray showed the range of high density of both lungs was larger than before, and the density increased. The dot and flake dense shadow, blurred boundary, and bronchial inflation sign were observed. With the informed consent of the parents, we performed an emergency ultra-slim flexible bronchoscopy (OLYMPUS BF-XP260F, outer diameter 2.8 mm, inner diameter 1.2 mm). Bronchoscopy entered the airway through the nose, pharynx, and larynx through the glottis. Furthermore, the trachea, right main bronchus, left main bronchus, right upper lobe, right middle lobe, right lower lobe, and left lower lobe were unobstructed with a small amount of blood (Figure 2). In addition, fresh blood was further observed in the upper lobe of the left lung. Therefore, hemostatic agents (adrenalin hydrochloride and hemagglutinin for injection) were injected after rapid suction and the removal of secretions from the upper lobe of the left lung. At the same time, a small number of deep secretions were collected under bronchoscopy and sent for etiological examination. The infant's respiration gradually stabilized after surgery, and we adjusted the ventilator mode to the non-invasive high-frequency ventilator (Drager NV500, FiO2 50%, MAP 12 cmH2O, frequency 11 Hz, amplitude 23 cmH2O). On the second day, nucleic acid tests of 14 respiratory pathogens were negative, so the anti-infection treatment was changed to cefoperazone. Four days later, the infant was taken off the ventilator and started to stammer milk. Informed consent was obtained from the infant's mother prior to the publication of this case report.
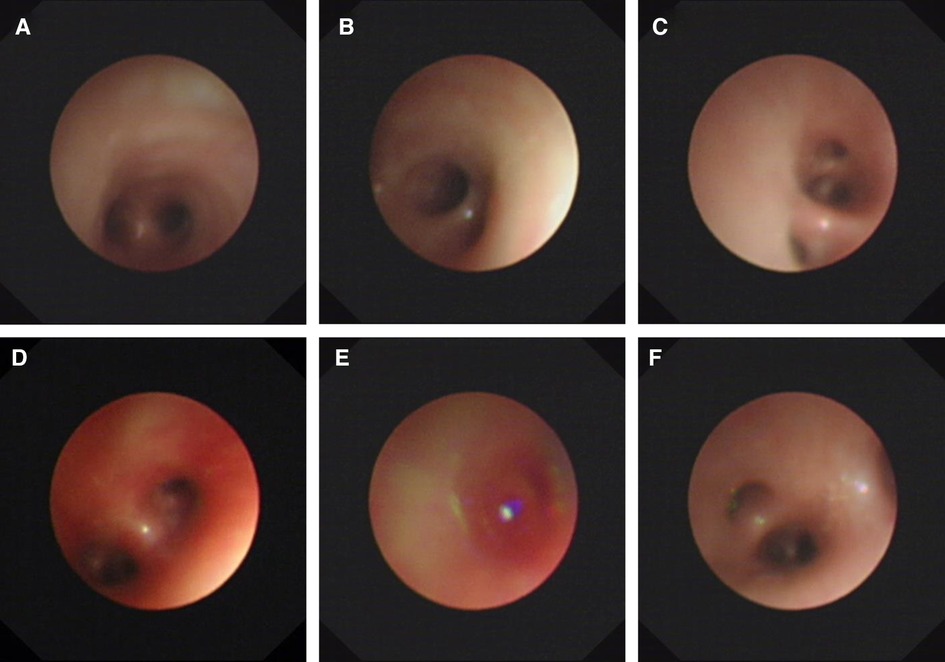
Figure 2. Ultra-slim flexible bronchoscopy showing pulmonary hemorrhage. (A) Carina. (B) Right main bronchus. (C) Right inferior lobar bronchus. (D) Left main bronchus. (E) Left superior lobar bronchus. (F) Left inferior lobar bronchus. Ultra-slim flexible video bronchoscopy showed a large outflow of fresh blood from the left upper lobe of the lung.
Discussion
The highest incidence of PH for preterm infants was 86.9 cases per 1,000 admissions at 24-week gestation, with a gradual decline at higher gestations. The incidence of PH for infants’ ≥32 weeks' gestation ranged from 0.6 to 1.9 cases per 1,000 admissions. Research has shown that the mortality is 40.6% within the first week after birth among infants <28 weeks' gestation (8), and the survivors have an increased risk of chronic lung disease and poor long-term outcomes (9, 10).
Risk factors for PH included severity of illness, intrauterine growth restriction, PDA, coagulopathy, the need for assisted ventilation, and a 10-min Apgar score (11). Accordingly, treatment of PH focuses on mechanical ventilation (especially HFOV), surfactant treatment, blood product (fresh frozen plasma and/or RBC), closure of PDA, nutritional support, analgesic, and sedative drugs (4, 12). Research has shown that HFOV is used to recruit compromised lungs, improve oxygenation through combined high MAPs and less tidal volumes, and eliminate carbon dioxide with few adverse cerebral side effects (13, 14). Therefore, HFOV was commonly used as the primary treatment method in PH. Ko et al. showed that 72% (13/18) newborn infants with PH responded to HFOV and survived (15), while 100% (6/6) survived without complications in the prospective observational study by Poddutoor et al. (16), although no cause for pulmonary hemorrhage was found in any of the infants (16). In this case, CPAP ventilation was administered after admission and adjusted to HFOV mode soon after an exacerbation. However, refractory pulmonary hemorrhage still occurred during HFOV application and when parameters were gradually decreased.
Pulmonary surfactant in the treatment of PH is controversial. On the one hand, PH appears to be a complication of surfactant therapy. In a meta-analysis, surfactant therapy was associated with an increased risk of PH (relative risk (RR) 1.47; 95% CI 1.05–2.07) indicating an increased risk for PH with surfactant therapy (17). In five multicenter, placebo-controlled trials of the synthetic surfactant in neonates, the incidence of clinical PH increased in those treated with the surfactant (18). These studies suggest that surfactant therapy may be a contributing factor, causing PH by inducing a rapid lowering of intrapulmonary pressure, which facilitates left-to-right shunting across a PDA and an increase in pulmonary blood flow (19). On the other hand, a prospective randomized controlled trial showed that natural surfactants improved oxygenation when administered for pulmonary hemorrhage in preterm infants (20). Amizuka et al. found that 21 of 26 neonates treated with single-dose surfactant 3.0 ± 1.3 h after the onset of hemorrhagic pulmonary edema exhibited a favorable response to exogenous surfactant, which was defined as a ventilatory index <0.047 at 1 h after surfactant administration (21). Similarly, a retrospective case series by Pandit et al. found that all 15 infants treated with surfactant had an improved ventilatory index and arterial/alveolar ratio (22). However, no recommendation for clinical practice based on randomized controlled trials can be presented. In our case, as a premature infant, the symptoms of respiratory distress appeared immediately after birth. Therefore, NRDS was diagnosed by combining relevant examinations, and CPAP-assisted ventilation was administered. However, 5 h after birth, the symptoms of respiratory distress worsened, and pulmonary hemorrhage occurred, so pulmonary surfactant (Ke-li-su, China) was administered according to the recommendation of the 2019 European NRDS Guidelines (23). Since the infant still had symptoms of respiratory distress and repeated pulmonary hemorrhage on the next day, porcine lung surfactant (Curosurf, Italy) was administrated 16 h after birth, considering its better solubility, while we found only about 3–4 h of symptom relief after every administration. Therefore, we believe that pulmonary surfactant has an adjunctive effect, while the efficacy for active bleeding is limited, and further research should be conducted.
Research has shown that PDA is a risk factor for the development of pulmonary hemorrhage in preterm infants (8, 24–26). Currently, PH occurs in 3%–5% of preterm ventilated infants with severe RDS who often suffer PDA and have received surfactant. The cause of PH is thought to be due to the rapid lowering of intrapulmonary pressure, which facilitates left-to-right shunting across a PDA and an increase in pulmonary blood flow (27). Kluckow et al. showed that infants with large PDA and early indomethacin treatment had significantly fewer early pH compared with infants who were not treated with indomethacin (28). These studies confirmed the importance of closure of the PDA in preterm infants with a high risk of PH. In this case, hemodynamically significant PDA (3.3 mm, left atrium/aortic root diameter = 1.5) was diagnosed by ultrasonic cardiogram. Symptomatic treatment, including fluid management (60–70 ml/kg), respiratory support, diuretics, and avoidance of hyperoxia, was administered first (29). One week later, the second echocardiography indicated that the size of the PDA was 2.2 mm. Considering the temporary absence of active hemorrhage, ibuprofen was administered as nasogastric tube therapy on the 8th day after birth. Although the PDA was closed 2 days later, repeated pulmonary hemorrhage still occurred.
Refractory pulmonary hemorrhage prompted further consideration of neonatal bronchoscopy to determine the etiology. Ultra-slim flexible video bronchoscopy is small and soft and enters below the bronchial segment and subsegment of the lung, which is beneficial to observe whether there are malformations or abnormalities of the tracheal mucosa and luminal, as well as whether there are excreta, foreign bodies, bleeding points, fistulas, and secretions, etc., and is widely used in pediatric clinical practice (30, 31). Due to the poor tolerance of small-weight airway stenosis to hypoxia and high technical requirements, there are few related studies in neonates. However, a recent European survey concerning practices in pediatric bronchoscopy showed the average number of bronchoscopies performed annually per center was 96, and 16% of those were performed in pediatric and neonatal ICUs. These results suggest that pediatric bronchoscopy has become more widely available and established. Atag et al. found that bronchoscopy assessments revealed at least one abnormality in 90.8% of patients whose median age was 5 months (range 0.3–205 months), and there were no major complications (32). Although bronchoscopy is increasingly used in the neonatal ICU, the diagnosis and treatment of pulmonary hemorrhage in a preterm infant have not been reported. In this case, HFOV, closed artery catheter, blood products, adrenaline, and antibiotics were administered systematically, and the infant still has repeatedly unexplained recurrent pulmonary hemorrhage. Therefore, with the family's informed consent, ultra-slim flexible bronchoscopy was performed, and local pressure administration of epinephrine and hemagglutinin was administered intraoperatively. Surprisingly, the symptoms of dyspnea were relieved quickly after the surgery.
In conclusion, PH is a life-threatening catastrophic event associated with a high mortality rate in neonates. Although traditional, conservative treatment is effective for the disease, some refractory PH is still difficult to treat clinically. Bronchoscopy is an essential tool for the diagnosis and management of respiratory disorders in NICUs and is a safe procedure in this vulnerable population. Endobronchial administration of hemostatics during bronchoscopy may be a safe and accurate option in the treatment of refractory PH. Further research is required for the follow-up of patients after bronchoscopy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) and minor(s) legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent to participate in this study was provided by the participant's legal guardian/next of kin.
Author contributions
YL and XJ conceptualized and designed the study, drafted the initial manuscript, and reviewed it. YL and BjX reviewed and revised the manuscript. YL, HfZ, MhX, and LcZ designed the data collection instruments, collected data, and carried out the initial analyses. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This work was sponsored by the National Natural Science Foundation of China (82000522) and the Shaanxi Provincial Science Foundation of China (2022SF-082).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen YY, Wang HP, Lin SM, Chang JT, Hsieh KS, Huang FK, et al. Pulmonary hemorrhage in very low-birthweight infants: risk factors and management. Pediatr Int. (2012) 54:743–7. doi: 10.1111/j.1442-200X.2012.03670.x
2. Ferreira CH, Carmona F, Martinez FE. Prevalence, risk factors and outcomes associated with pulmonary hemorrhage in newborns. J Pediatr (Rio J). (2014) 90:316–22. doi: 10.1016/j.jped.2013.12.008
3. Scholl JE, Yanowitz TD. Pulmonary hemorrhage in very low birth weight infants: a case-control analysis. J Pediatr. (2015) 166:1083–4. doi: 10.1016/j.jpeds.2014.12.032
4. Barnes ME, Feeney E, Duncan A, Jassim S, MacNamara H, O’Hara J, et al. Pulmonary haemorrhage in neonates: systematic review of management. Acta Paediatr. (2022) 111:236–44. doi: 10.1111/apa.16127
5. Yum SK, Moon CJ, Youn YA, Lee HS, Kim SY, Sung IK. Risk factor profile of massive pulmonary haemorrhage in neonates: the impact on survival studied in a tertiary care centre. J Matern Fetal Neonatal Med. (2016) 29:338–43. doi: 10.3109/14767058.2014.1000853
6. Lakshmi KS, Peterson RR, Saranadagouda PK, Kuruvilla A. A rare cause of pulmonary hemorrhage in an infant. Lung India. (2016) 33:242–3. doi: 10.4103/0970-2113.177450
7. Tomaszewska M, Stork E, Minich NM, Friedman H, Berlin S, Hack M. Pulmonary hemorrhage: clinical course and outcomes among very low-birth-weight infants. Arch Pediatr Adolesc Med. (1999) 153:715–21. doi: 10.1001/archpedi.153.7.715
8. Ahmad KA, Bennett MM, Ahmad SF, Clark RH, Tolia VN. Morbidity and mortality with early pulmonary haemorrhage in preterm neonates. Arch Dis Child Fetal Neonatal Ed. (2019) 104:F63–F8. doi: 10.1136/archdischild-2017-314172
9. Pandit PB, O’Brien K, Asztalos E, Colucci E, Dunn MS. Outcome following pulmonary haemorrhage in very low birthweight neonates treated with surfactant. Arch Dis Child Fetal Neonatal Ed. (1999) 81:F40–4. doi: 10.1136/fn.81.1.F40
10. Alfaleh K, Smyth JA, Roberts RS, Solimano A, Asztalos EV, Schmidt B, et al. Prevention and 18-month outcomes of serious pulmonary hemorrhage in extremely low birth weight infants: results from the trial of indomethacin prophylaxis in preterms. Pediatrics. (2008) 121:e233–8. doi: 10.1542/peds.2007-0028
11. Li J, Xia H, Ye L, Li X, Zhang Z. Exploring prediction model and survival strategies for pulmonary hemorrhage in premature infants: a single-center, retrospective study. Transl Pediatr. (2021) 10:1324–32. doi: 10.21037/tp-21-64
12. Yen TA, Wang CC, Hsieh WS, Chou HC, Chen CY, Tsao PN. Short-term outcome of pulmonary hemorrhage in very-low-birth-weight preterm infants. Pediatr Neonatol. (2013) 54:330–4. doi: 10.1016/j.pedneo.2013.04.005
13. Zannin E, Doni D, Ventura ML, Fedeli T, Rigotti C, Dellaca RL, et al. Relationship between mean airways pressure, lung mechanics, and right ventricular output during high-frequency oscillatory ventilation in infants. J Pediatr. (2017) 180:110–5. doi: 10.1016/j.jpeds.2016.09.015
14. Hsu JF, Yang MC, Chu SM, Yang LY, Chiang MC, Lai MY, et al. Therapeutic effects and outcomes of rescue high-frequency oscillatory ventilation for premature infants with severe refractory respiratory failure. Sci Rep. (2021) 11:8471. doi: 10.1038/s41598-021-88231-6
15. Ko SY, Chang YS, Park WS. Massive pulmonary hemorrhage in newborn infants successfully treated with high frequency oscillatory ventilation. J Korean Med Sci. (1998) 13:495–9. doi: 10.3346/jkms.1998.13.5.495
16. Pappas MD, Sarnaik AP, Meert KL, Hasan RA, Lieh-Lai MW. Idiopathic pulmonary hemorrhage in infancy: Clinical features and management with high frequency ventilation. Chest. (1996) 110(2):553–5. doi: 10.1378/chest.110.2.553
17. Raju TN, Langenberg P. Pulmonary hemorrhage and exogenous surfactant therapy: a metaanalysis. J Pediatr. (1993) 123:603–10. doi: 10.1016/s0022-3476(05)80963-1
18. van Houten J, Long W, Mullett M, Finer N, Derleth D, McMurray B, et al. Pulmonary hemorrhage in premature infants after treatment with synthetic surfactant: an autopsy evaluation. The American Exosurf Neonatal Study Group I, and the Canadian Exosurf Neonatal Study Group. J Pediatr. (1992) 120:S40–4. doi: 10.1016/s0022-3476(05)81232-6
19. Wiswell TE. Expanded uses of surfactant therapy. Clin Perinatol. (2001) 28:695–711. doi: 10.1016/s0095-5108(05)70113-5
20. Bozdağ Ş, Dilli D, Gökmen T, Dilmen U. Comparison of two natural surfactants for pulmonary hemorrhage in very low-birth-weight infants: a randomized controlled trial. Am J Perinatol. (2015) 32:211–8. doi: 10.1055/s-0034-1389090
21. Amizuka T, Shimizu H, Niida Y, Ogawa Y. Surfactant therapy in neonates with respiratory failure due to haemorrhagic pulmonary oedema. Eur J Pediatr. (2003) 162:697–702. doi: 10.1007/s00431-003-1276-x
22. Pandit PB, Dunn MS, Colucci EA. Surfactant therapy in neonates with respiratory deterioration due to pulmonary hemorrhage. Pediatrics. (1995) 95:32–6. doi: 10.1542/peds.95.1.32
23. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Te Pas A, et al. European Consensus guidelines on the management of respiratory distress syndrome—2019 update. Neonatology. (2019) 115:432–50. doi: 10.1159/000499361
24. Lin TW, Su BH, Lin HC, Hu PS, Peng CT, Tsai CH, et al. Risk factors of pulmonary hemorrhage in very-low-birth-weight infants: a two-year retrospective study. Acta Paediatr Taiwan. (2000) 41:255–8. PMID: 11100523
25. Garland J, Buck R, Weinberg M. Pulmonary hemorrhage risk in infants with a clinically diagnosed patent ductus arteriosus: a retrospective cohort study. Pediatrics. (1994) 94:719–23. doi: 10.1542/peds.94.5.719
26. Kluckow M, Evans N. Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J Pediatr. (2000) 137:68–72. doi: 10.1067/mpd.2000.106569
27. Aziz A, Ohlsson A. Surfactant for pulmonary haemorrhage in neonates. Cochrane Database Syst Rev. (2020) 2:CD005254. doi: 10.1002/14651858.CD005254.pub4
28. Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal and Neonatal Ed. (2014) 99:F99–104. doi: 10.1136/archdischild-2013-304695
29. Ohlsson A, Shah PS. Paracetamol (Acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst Rev. (2020) 1:CD010061. doi: 10.1002/14651858.CD010061.pub4
30. Simma L, Cincotta D, Sabato S, Long E. Airway emergencies presenting to the paediatric emergency department requiring advanced management techniques. Arch Dis Child. (2017) 102:809–12. doi: 10.1136/archdischild-2016-311945
31. Schramm D, Yu Y, Wiemers A, Vossen C, Snijders D, Krivec U, et al. Pediatric flexible and rigid bronchoscopy in European centers—availability and current practice. Pediatr Pulmonol. (2017) 52:1502–8. doi: 10.1002/ppul.23823
Keywords: pulmonary hemorrhage, preterm infant, refractory, HFOV, flexible bronchoscopy
Citation: Lin Y, Zhao H, Xue M, Xie B, Zeng L and Jiang X (2022) Ultra-slim flexible bronchoscopy-guided topical hemostatic drugs administration for the management of life-threatening refractory pulmonary hemorrhage in a preterm infant: Case report. Front. Pediatr. 10:981006. doi: 10.3389/fped.2022.981006
Received: 29 June 2022; Accepted: 26 September 2022;
Published: 18 October 2022.
Edited by:
Anne B. Chang, Charles Darwin University, AustraliaReviewed by:
Warwick Wolf Butt, Royal Children’s Hospital, AustraliaHaifeng Zong, Southern Medical University, China
© 2022 Lin, Zhao, Xue, Xie, Zeng and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Lin yiyilin19800823@126.com Xun Jiang jiangx@fmmu.edu.cn
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Pediatric Pulmonology, a section of the journal Frontiers in Pediatrics
 Yan Lin
Yan Lin