
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 03 November 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.980725
 Matheus F. P. T. van Rens1*†,‡
Matheus F. P. T. van Rens1*†,‡ Mohammad A. A. Bayoumi1,†,‡
Mohammad A. A. Bayoumi1,†,‡ Agnes van de Hoogen2,‡
Agnes van de Hoogen2,‡ Airene L. V. Francia1,‡
Airene L. V. Francia1,‡ Irian J. Cabanillas1,‡
Irian J. Cabanillas1,‡ Fredericus H. J. van Loon3,‡
Fredericus H. J. van Loon3,‡ Timothy R. Spencer4,‡
Timothy R. Spencer4,‡
Background: Venous access devices (VADs) play a vital role within the neonatal intensive care unit. However, there are significant risks associated with the use of VADs, with complications such as infection, thrombosis, device occlusion, and infiltration/extravasation frequently contributing to device-related failures and increasing the risk of significant patient harm or injury. This study aimed to explore the relationships between risk factors and different venous access device complications in the neonatal setting, and then use that evidence to develop an algorithm based on observational data.
Methods: This is a retrospective, single-center cohort study that was conducted in a large 112-bed neonatal intensive care unit in Qatar. We examined venous access device data from January 2016 to December 2018 for all term and preterm neonates. Descriptive statistics were used to summarize the outcomes, which included a mean and its standard deviation or median and an interquartile range for continuous variables regarding normal distribution, and absolute numbers with percentages for discrete variables.
Results: The authors recorded a total of 23,858 VADs inserted during the study period. Of these, 21,313 (89%) were peripheral intravenous catheters, 689 (3%) were extended dwell-peripheral intravenous catheters, 1,335 (6%) were epicutaneo-caval catheters, and 521 (2%) were umbilical venous catheters. In total, 51,179 catheter days were registered, with 2.17 catheter days reported per patient. Peripheral device dwell times were significantly shorter when compared with central venous catheter devices (P < 0.001), with mean dwell times of 22 days ± 23 h and 236 days ± 183 h, respectively. After insertion, a complication occurred in 11,177 (51%) of peripheral VADs and 221 (12%) of central VADs. The type of device inserted [P < 0.001, hazard ratio (HR) = 0.52, 95% confidence interval (CI): 0.50–0.54], reason/indication for intravenous therapy (P < 0.001, HR = 0.85, 95% CI: 0.82–0.87), and the side of insertion of the device (P < 0.001, HR = 1.25, 95% CI: 1.24–1.27) had a significant relationship with outcomes.
Conclusions: Four subgroups of VADs were identified (peripheral intravenous catheters, extended dwell-peripheral intravenous devices, epicutaneo-caval catheters, and umbilical venous catheters) with outcome-related differences. Central venous access devices (epicutaneo-caval catheters and umbilical venous catheters) had lower complications compared with peripheral VADs. Proper venous access device selection, early insertion, and early removal approaches remain crucial to preventing venous access device complications. Peripheral intravenous devices should be used carefully and closely watched for early detection of complications.
According to reports, intravenous (IV) access is the most commonly performed invasive procedure in clinical settings worldwide. The support and management of neonatal patient conditions rely tremendously upon the provision of stable venous access for the administration of required and essential fluids, medications, nutrition, and blood products (1). Venous access devices (VADs), either central or peripheral, play a vital role within the neonatal intensive care unit (NICU). However, there are significant risks associated with VAD use, with complications such as infections, phlebitis, thrombosis, device occlusion, and peripheral intravenous infiltration/extravasation (PIVIE) frequently resulting in device failures and increasing the significant risk of patient harm or injury (2). Observational studies have found a total of peripheral IV-related complications including phlebitis, infections, and occlusions as high as 75% (3), and 0%–3% of central catheters in neonates resulted in therapy-related complications and device failure.
Preterm and critically ill neonates are at a higher risk of complications, owing to an immature immune system, an underdeveloped skin barrier, smaller, more fragile blood vessels, and exposure to invasive diagnostic and therapeutic procedures (4–6). Choices in VADs, various catheter insertion techniques, along with care and management strategies, are all complex and multifaceted aspects of care that frequently involve a multidisciplinary team. Evidence-based VAD insertion and maintenance strategies including appropriate skin/insertion site antisepsis and dressings have been developed to reduce the preventable causes of VAD failure and complications; however, evidence in the neonatal population is limited (7, 8). Approaches to VAD selection, insertion, and maintenance in neonates frequently vary among caregivers, with practice often based on traditional practices, anecdotal evidence, or practices recommended for adults and adolescents (9, 10). Undertaking clinical research can help in the identification of evidence-based practices that demonstrate effective methods for reducing preventable causes of patient harm or injury, as well as provide recommendations and guidelines for healthcare practitioners to focus on providing better and safer solutions and outcomes.
Comparing Pettit's benchmark studies (11, 12) with recent literature demonstrates the incidence of neonatal venous access device-related complications has remained persistent over the recent decades, regardless of newer clinical innovations and changes in practices. Infection rates are highly variable for both peripheral and central VADs but have been documented to range between 0.43 and 49 incidents/1,000 catheter days (13–15).
Currently, peripheral intravenous catheters (PIVCs) are most commonly used to provide infusion therapy (16–20). It seems that the presumption of high rates of central line-associated bloodstream infections (CLABSI) plays a significant role in making, often inappropriate, choices of VADs. Comparison studies between CLABSI and peripheral line-associated bloodstream infections (PLABSI) are limited (21–24) and do not indicate any preference for either PIVC or epicutaneo-caval catheter (ECC) (25, 26). Outcomes from a 2015 Cochrane review to determine the effects of infusion of parenteral nutrition via percutaneous central venous catheters vs. peripheral cannulae concluded that there was no evidence suggesting that percutaneous central venous catheter use increased the risks of adverse events, particularly invasive infection (27).
Extrinsic modifiable factors may influence successes and failures, highlighting quality clinician training, established competencies, clinical experience, choices regarding device type, patient assessment and insertion techniques, including care and maintenance bundles, device stabilization and securement, and finally dressing protocols (3, 8, 26–28). The application of a transparent dressing plays an important role in reducing the secondary dislodgement of central and peripheral VADs. However, more recently published evidence in the neonatal population has demonstrated the use of cyanoacrylate glue for sutureless securement of epicutaneo-caval catheters might play a significant role in neonates (28). It is well known that each venous access device has its indications, technique of insertion, dwell time, contraindications, and complications. However, evidence from larger-scale studies in these patient populations regarding factors influencing VAD outcomes is still lacking. This study intends to explore the associations between risk factors and different VAD complications in the neonatal setting, and use the evidence to develop an algorithm based on observational data.
In this retrospective, single-center cohort study, anonymized intravenous device data from January 2016 to December 2018 was evaluated. The main study outcome was the occurrence of any venous access device-related complication with the use of the different types of VAD utilized. The study was performed in a large NICU (112 beds) of the Women's Wellness and Research Centre (WWRC) of Hamad Medical Corporation (HMC), Doha, Qatar. The study protocol (MRC-01-19-166) was approved by the above facility's Institutional Review Board (IRB). Because the data source was anonymized, the local IRB deemed that participant consent was not required and determined the study as a “quality chart review.” The project aims to Assess the situation and patients Better Before initiating venous Access (acronym, ABBA), potentially reducing unwarranted venous device-related complications.
Considering the retrospective design, neither study participants nor parents were involved in the design, conduct or reporting of this study.
All term and preterm neonates who were admitted to the NICU and required intravenous therapy during the NICU stay through a PIVC, ED-PIVC, ECC, or umbilical venous catheters (UVC), were included in the study. Participants were excluded from the study if their data was incomplete or if the data only related to the use of an arterial device, e.g., umbilical or peripheral arterial catheters.
In the facility, intravenous cannulation is routinely performed by the neonatal venous access team (NeoVAT). The NeoVAT group is defined as a group of clinicians (nurses and doctors) whose primary role is the assessment of IV access device needs, insertion, oversight of care and use, the management of complications, and collection of venous access device-related data (29, 30). Two notable team delineations are the nurse-led team for peripheral including extended dwell-peripheral IV catheters (ED-PIVC) venous access alone, and a more experienced mixed professional group providing all responsibility for central venous access devices (CVADs). The neonatal ED-PIVC catheter is a short single-lumen catheter that is manufactured in 6 or 8 cm lengths. It is designed to be administered intravenously for up to 29 days. During the patient assessment stage, the team follows a locally developed mnemonic, the “5 Rights for Venous Access,” which stands for the Right device, for the Right vein, with the Right therapy, for the Right duration, for the Right patient, as described in a similar concept by Steere et al. (25) (Figure 1).
Both peripheral and central venous cannulation is performed strictly under the guidance of the local hospital policy and in accordance with the current international standards of practice (27). The selection of suitable veins is performed using the near infrared (NIR) technology for vein visualization, with the saphenous and antecubital veins generally being avoided for peripheral IV cannulation. In this facility's practice, the choice of appropriate venous access device is based on the duration of required therapies; fluid characteristics [pH (5–9) and osmolarity]; patient characteristics, including body weight; and a known history of difficult intravenous access. The design of the venous access device selection flowchart is based on the current infusion standards of practice (27, 31) and local contexts such as product compatibility, hospital purchasing decisions, and practitioner consensus.
All patient demographics and baseline data included gender, gestational age at birth (in weeks and days), birth weight, and current body weight in grams. Data regarding the procedure for intravenous cannulation were date and time, the number of attempts needed to successful cannulation, cannulation laterality (left, right, or middle), extremity and site/vessel on the extremity (e.g., dorsum of the hand, wrist or forearm, antecubital fossa and upper arm, foot, ankle and lower leg, or knee and upper leg), device type and size (PIVC, ED-PIVC, ECC, UVC), indication for intravenous treatment (intravenous fluids, medication therapy, total parenteral nutrition, blood and/or blood product administration, blood sampling, or procedural, i.e., for power/contrast injection), date and time of device removal, total dwell time in hours (calculated as the removal date and time minus the insertion date and time), reason for removal [therapy completed/discontinuation of therapy warranting (elective) device removal], PIVIE, phlebitis, CLABSI, occlusion, dislodgement and accidental removal, discoloration, and administrative censoring (patient transferred or expired). The data were collected and validated by the authors based from Hamad Medical Corporation.
Descriptive statistics were used to summarize the outcomes, which included a mean and its standard deviation or median and an interquartile range for continuous variables regarding normal distribution, and absolute numbers with percentages for discrete variables. The assumption of normal distribution was proved with Kolmogorov–Smirnov testing. Differences regarding outcomes and measurements were demonstrated by using the χ2 test, Mann–Whitney U test, or unpaired samples t-test, as appropriate. A Kaplan–Meier curve was plotted for device dwell time stratified by device type, including its numbers at risk. Hereafter, Cox regression analyses were performed to detect any relationship between independent variables with the outcome of interest. Variables with significance (P < 0.05) were included in the multivariable model (main analyses). Using a backward selection process based on the highest Wald score and lowest P-value, the smallest set of variables was identified. The hazard ratio (HR) with its 95% confidence interval (CI) was identified in these analyses. Throughout this study, a P < 0.05 was denoted to be statistically significant. SPSS (Version 25.0; SPSS Inc., Chicago, IL, United States) was used for statistical analysis.
During the study period, the authors recorded a total of 23,858 VADs inserted during the study period. In total, 51,179 catheter days were registered, with 2.17 catheter days reported per patient. Of these, 21,313 (89%) were PIVCs, 689 (3%) were ED-PIVC, 1,335 (6%) were ECCs, and 521 (2%) were umbilical venous catheters. Insertion of a VAD was successful in 21,594 patients, resulting in a 90% overall success rate. Individual success rates were observed after the insertion of PIVC (90%), ED-PIVC (92%), ECC (88%), and UVC (96%). In 41% of cases, the VAD was removed after the successful completion of therapy. A complication that may have led to premature VAD removal occurred in 48% of all cases. The study showed an overall complication incidence rate of 260/1,000 catheter days. The most frequently reported failure of therapy was in the PIVC group (411/1,000 catheter days) and the least frequent in the ECC group (10/1,000 catheter days). The risk of therapy failure had four significant variables: the type of VAD, reason or indication for intravenous therapy, dwell time of the VAD (in days), and weight of the patient at the insertion of the VAD. An incidence rate of peripheral line-associated bloodstream infection (PLABSI) was 1.19/1,000 catheter days observed for all peripheral intravenous access devices (PIVC and ED-PIVC), whereas a CLABSI incidence rate of 0.34/1,000 catheter days was observed after CVAD insertion (P < 0.001). No other specific complications related to the extended dwell time in CVAD were observed.
A summary of patient characteristics is shown in Table 1. Overall, there was an equal distribution of patients’ gender across the type of devices inserted. The mean gestational age at birth of the total cohort was 34.4 ± 4.8 weeks, and the median age of the patients in days at the moment of insertion of the VAD was 2 (±4) days. A higher number of preterm infants underwent CVAD placement (ECC or UVC), with a mean gestational age at birth of 30.4 ± 4.6 weeks, compared with infants who received a peripheral device (PIVC or ED-PIVC) with a mean gestational age of 34.7 ± 4.6 weeks. Patients who received a peripheral device were slightly older, with a median age of 8 (±5) days, when compared with all infants receiving a CVAD, with a median age of 6 (±4) days. This difference was not statistically significant. Regarding a patient's weight at the time of VAD insertion, those who underwent insertion of a peripheral device had a mean weight of 2,430 ± 928 g and patients who received a CVAD had a mean weight of 1,566 ± 923 g, demonstrating statistical significance. The participants in PIVC and ED-PIVC were, in general, older and had an increased body weight at birth and insertion when compared with other groups; participants in UVC were much younger when compared with other groups, with the oldest participants in PIVC and ECC groups.
The primary reason for VAD insertion was to provide continuous infusion for IV therapies and/or medication administration (91%). In general, a median of one attempt was required to obtain successful venous access. See Table 2.
Data regarding VAD removal are presented in Table 3. The complication rate was highest in the patient who got a PIVC inserted (411/1,000 catheter days), and the lowest complication rate was registered after the insertion of an ECC (10/1,000 catheter days). After the insertion of a peripheral device, a complication occurred in 11,177 (51%) patients, which occurred only in 221 (12%) patients where a CVAD was inserted. In general, for patients who received a peripheral catheter, the complication rate was significantly higher when compared with infants receiving a CVAD (407/1,000 and 13/1,000 catheter days, respectively). Data from 2,713 (11%) patients were denoted as missing values, due to administrative censoring (17%) or a lack of administration (83%).
An overall mean dwell time of 49 ± 78 h was recorded throughout the total cohort of included patients. Peripheral device dwell times were significantly lower when compared with central venous catheter devices (P < 0.001), with mean dwell times of 22 days ± 23 h and 236 days ± 183 h. Catheter dwell times differed between the types of devices inserted, as shown in Figure 2 (P < 0.001).
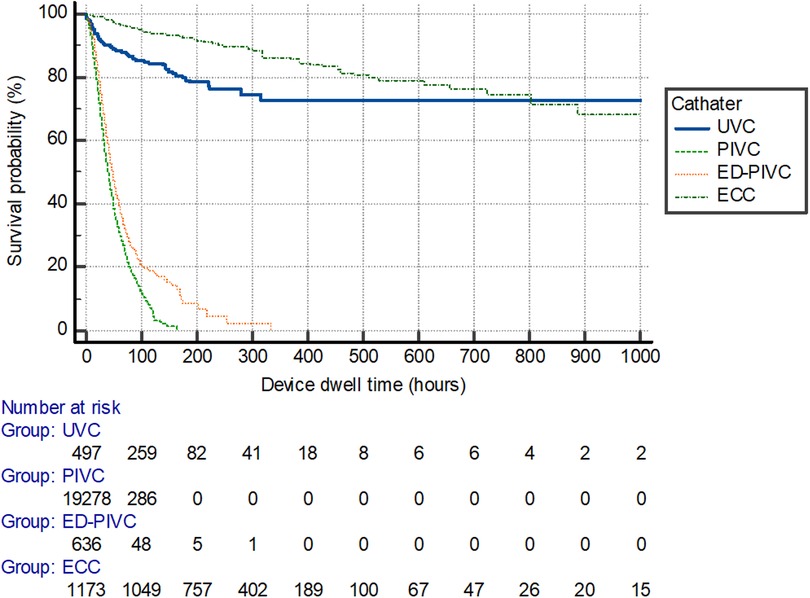
Figure 2. Survival curve per catheter device type for dwell time duration. UVC, umbilical venous catheter; PIVC, peripheral intravenous catheter; ED-PIVC, extended dwell-peripheral intravenous catheter; ECC, epicutaneo-caval catheter.
Univariable Cox regression analyses identified eight variables demonstrating significant relationships to the outcomes of interest: gestational age at birth, age at device insertion, weight at device insertion, indication for intravenous therapy, type of VAD inserted, chosen site of insertion, side of cannulation, and successful insertion on the first attempt. These variables were all entered for multivariable Cox regression analysis, of which three had a significant relationship with outcomes: the type of device inserted (P < 0.001, HR = 0.52, 95% CI: 0.50–0.54), reason/indication for intravenous therapy (P < 0.001, HR = 0.85, 95% CI: 0.82–0.87) and side of the insertion of the device (P < 0.001, HR = 1.25, 95% CI: 1.24–1.27). Multivariable Cox regression analyses regarding the type of intravenous catheter are represented in Table 4.
Neonates are an extremely vulnerable and challenging patient population. VADs provide the only gate of all parenterally administered medications and fluids. The incidence of VAD therapy failure may negatively impact clinical therapies and outcomes which are dependent on appropriate, safe, and reliable venous access. Carr et al. (28) determined failure of VADs, resulting in premature device removal, occurred in 48% of participants, with a complication incidence rate of 260/1,000 device days.
While this study provides recent clinical evidence on various risks of therapy failure with intravenous devices in neonates, it has also been reported in previous publications (2, 5, 30). To the best of the authors’ knowledge, a study of this sample size including both peripheral and central catheters, with a focus on device-related failures in neonates, has not been previously published.
To obtain venous access during hospitalization, PIVCs are often sourced as the primary and most inserted devices (5). It is difficult to give an unambiguous clarification for this, although the pattern of complications, and their relative incidences, do correlate.
Results from a small retrospective study performed in a level III NICU stated that the use of an ED-PIVC may be a useful option in providing venous access choices and reducing costs in NICU patients (2, 3). This research compared complications of ECC, ED-PIVC, and PIVC, and when compared with standard PIVCs alone, ED-PIVCs demonstrated advantages in overall device dwell time (ranging from 1 to 29 days), despite that 28% of devices were removed before the completion of therapy. ED-PIVCs also indicated a lower rate of infiltration/extravasation-related episodes when compared with standard PIVCs (1% vs. 4%). The authors concluded at ED-PIVCs offer significant benefits over standard PIVC and moderate financial savings when compared with ECC use and frequent PIVC replacement.
In this study, the use of an ECC reported the least number of therapy failures and longest dwell times. In the study by Chenoweth et al., ECCs had similar success rates compared with peripheral catheters (PIVC and ED-PIVC), when regarding the completion of therapy (P = 0.001) (4). Another recent study evaluated the clinical efficacy and safety of ultrasound-guided, subcutaneously tunneled, femorally inserted ECC in the NICU (29). No insertion-related or post-insertion complications were reported, and all patients completed the prescribed therapy with one catheter. However, the device dwell and the device burden on the newborn remain important to mention in this context.
In most NICUs, UVCs are not used for more than 5–7 days, due to increased incidence of late-onset sepsis (LOS) related to UVC longevity. A previous study by Konstantinidi et al. shows the incidence of complications associated with the use of UVCs and ECCs does not significantly differ (30, 32). This current research also confirms these results and shows that ECCs have the benefits of the longest extended dwell times and the lowest incidence of related therapy failures.
There is a significant difference between peripheral and central venous access, hence the knowledge to understand the different causes of failure is essential. Blood flow, vein diameter, type of device materials, and even stabilization and securement (dressing), differ per type of VAD. Facilitating the use of the Right device, for the Right vein, with the Right therapy, for the Right duration, for the Right patient is considered an essential aspect of patient care. However, consistency with international consensus has currently not been achieved.
To the authors’ understanding, this is the first and largest study of this kind to examine the use of VADs and their effect on therapy failures in the neonatal population in this geographical region. All eligible neonates were included, and the sample size was large and representative of the neonatal population requiring VADs. This increased the statistical power of the study's findings, helping to minimize selection bias and increase the generalizability of the findings to similar settings.
Despite these strengths, there are some limitations to this research. This study was conducted in a single center, with a retrospectively collected dataset, and in contrast to randomized studies, this method creates a risk for selection bias. In this study, every infant with a successfully inserted ECC was included to minimize the risk of selection bias. Interrater variability may have affected the results; however, the facility's use of a standardized education program and limiting venous access procedures to members of dedicated venous access team (neoVAT) may have helped reduce data variability. Data outcomes that were not available for neonates (death or were transferred out of the facility) were deemed as “administrative censoring” (in Table 3). Although this population was relatively small, patients lost to follow-up may have different outcomes than those who completed the study. Nonetheless, future research should focus on the introduction of new and clinically beneficial strategies to help improve successful therapy outcomes, no matter the type of VAD.
In general, many neonatal patients experienced venous access-related complications, resulting in therapy failure. Several variables demonstrated the benefit of the use of an ECC, resulting in the successful completion of prescribed infusion therapies for patients admitted to the NICU.
Four different types of VADs (PIVC, ED-PIVC, ECC, and UVC) were identified, and divided into two groups (central and peripheral), each device with unique characteristics affecting therapy and dwell time in patients admitted to the NICU. Four variables had a significant relationship with therapy failure. Weight (birth and current weight), cannulation site, type of device, and the indication for intravenous treatment all affected the risk for failure. This study demonstrated the highest complication rates were amongst the peripheral VADs when compared with CVADs, including CLABSI. It appears that when a VAD is inserted, it is of utmost importance for the clinician to choose the most appropriate device for the patient and the required therapy. The most frequently observed therapy failure in this neonatal population was with the standard peripheral VAD group. Consequently, the authors concur that peripheral VADs should be used judiciously, and careful thought should be given before their use, particularly when there are alternate means of central access devices that may be utilized more appropriately.
Innovations and clinical advancements in the world of neonatology and VADs with the least chance for therapy failures play an important role in improving patient and therapy-related outcomes. There is clear superiority in using vein visualization technologies like NIR for peripheral venous access and training on simulators and virtual reality for central access devices (32).
The choice of the most appropriate venous device while utilizing the latest technology is an important step for clinicians to place and care for VADs, as well as for patients depending on a reliable venous access device for their required therapy. It is recommended to Assess the patient Better Before inserting the venous Access device (ABBA) for proper venous access device selection using the VAD algorithm together. Using the “5 Rights for Venous Access” that is, the Right device, for the Right vein, with the Right therapy, for the Right duration, for the Right patient will provide better outcomes and prevent unnecessary catheter-related complications in NICU.
The datasets generated for this study are available on reasonable request to the corresponding author.
The study protocol (MRC-01-19-166) was approved by the IRB of the Women's Wellness and Research Center, Hamad Medical Corporation. All methods were carried out in accordance with relevant guidelines and regulations. As the data source was anonymized, the Ethics Committee/IRB deemed that participant consent was not required and determined the study a “quality review”. Study participants and parents were not involved in the design, conduct or reporting of this study. The local IRB made funds available to support any Open Access publication of this article after acceptance. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and institutional requirements.
MFPTvR and MAAB were the main investigators who conceptualized and designed the study, coordinated and supervised data collection, drafted the initial manuscript, and reviewed and revised the subsequent manuscript versions. AvdH and TRS provided a critical review and edited the manuscript for important intellectual, statistical, and academic content. ALVF and IJC designed the data collection instruments, collected data, and reviewed and revised the manuscript. FHJvL carried out statistical analyses, critically reviewed the manuscript for important intellectual content, and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors would like to acknowledge all members of the neoVAT, Binsy Padinharayil, and the nursing and medical staff of the NICU at WWRC for their support during the study period.
TRS was employed by company Global Vascular Access, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
VADs, venous access devices; NICU, neonatal intensive care unit; ABBA, Assess Better Before Access; PIVC, peripheral intravenous catheter; ED-PIVC, extended dwell-peripheral intravenous catheters; ECC, epicutaneo-caval catheter; UVC, umbilical venous catheters; IV, intravenous; PIVIE, peripheral intravenous infiltration/extravasation; IRB, institutional review board; CVAD, central venous access device; CLABSI, central line-associated bloodstream infection; PLABSI, peripheral line-associated bloodstream infection
1. Legemaat M, Carr PJ, van Rens RM, van Dijk M, Poslawsky IE, van den Hoogen A. Peripheral intravenous cannulation: complication rates in the neonatal population: a multicenter observational study. J Vasc Access. (2016) 17(4):360–5. doi: 10.5301/jva.5000558
2. Odom B, Lowe L, Yates C. Peripheral infiltration and extravasation injury methodology: a retrospective study. J Infus Nurs. (2018) 41(4):247–52. doi: 10.1097/NAN.0000000000000287
3. van Rens MFPT, Hugill K, Mahmah MA, van Rens MF, Hugill K, Mahmah MA, et al. Evaluation of unmodifiable and potentially modifiable factors affecting peripheral intravenous device-related complications in neonates: a retrospective observational study. BMJ Open. (2021) 11(9):e047788. doi: 10.1136/bmjopen-2020-047788
4. Chenoweth KB, Guo J-W, Chan B. The extended dwell peripheral intravenous catheter is an alternative method of NICU intravenous access. Adv Neonatal Care. (2018) 18(4):295–301. doi: 10.1097/ANC.0000000000000515
5. Taylor JE, McDonald SJ, Tan K. A survey of central venous catheter practices in Australian and New Zealand tertiary neonatal units. Aust Crit Care. (2014) 27(1):36–42. doi: 10.1016/j.aucc.2013.11.002
6. Sharpe E, Pettit J, Ellsbury DL. A national survey of neonatal peripherally inserted central catheter (PICC) practices. Adv Neonatal Care. (2013) 13(1):55–74. doi: 10.1097/ANC.0b013e318278b907
7. Sharma PK, Singh K. Venous access in neonates: our experience. Int J Contemp Pediatr. (2018) 5(4):1571–5. doi: 10.18203/2349-3291.ijcp20182567
8. Unbeck M, Förberg U, Ygge B-M, Ehrenberg A, Petzold M, Johansson E. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr. (2015) 104(6):566–74. doi: 10.1111/apa.12963
9. Hugill K. Vascular access in neonatal care settings: selecting the appropriate device. Br J Nurs. (2016) 25(3):171–6. doi: 10.12968/bjon.2016.25.3.171
10. Wu J, Mu D. Vascular catheter-related complications in newborns. J Paediatr Child Health. (2012) 48(2):E91–5. doi: 10.1111/j.1440-1754.2010.01934.x
11. Worth LJ, Daley AJ, Spelman T, Bull AL, Brett JA, Richards MJ. Central and peripheral line-associated bloodstream infections in Australian neonatal and paediatric intensive care units: findings from a Comprehensive Victorian Surveillance Network, 2008-2016. J Hosp Infect. (2018) 99(1):55–61. doi: 10.1016/j.jhin.2017.11.021
12. Maximiano C, Cunha C, Silva A, Pereira A. Catheter-related bloodstream infection in neonatal intensive care unit: prospective surveillance study. Gaz Med. (2021) 8(1):11–6. doi: 10.29315/gm.v8i1.389
13. Wallis MC, McGrail M, Webster J, Marsh N, Gowardman J, Playford EG, et al. Risk factors for peripheral intravenous catheter failure: a multivariable analysis of data from a randomized controlled trial. Infect Control Hosp Epidemiol. (2014) 35:63–8. doi: 10.1086/674398
14. Alexandrou E, Ray-Barruel G, Carr PJ, Frost SA, Inwood S, Higgins N, et al. Use of short peripheral intravenous catheters: characteristics, management, and outcomes worldwide. J Hosp Med. (2018) 13(5):E1–7. doi: 10.12788/jhm.3039
15. Kovacs CS, Fatica C, Butler R, Gordon SM, Fraser TG. Hospital-acquired Staphylococcus aureus primary bloodstream infection: a comparison of events that do and do not meet the central line-associated bloodstream infection definition. Am J Infect Control. (2016) 44(11):1252–5. doi: 10.1016/j.ajic.2016.03.038
16. Wójkowska-Mach J, Chmielarczyk A, Strus M, Lauterbach R, Heczko P. Neonate bloodstream infections in organization for economic cooperation and development countries: an update on epidemiology and prevention. J Clin Med. (2019) 8:1750. doi: 10.3390/jcm8101750
17. Barone G, Pittiruti M. Epicutaneo-caval catheters in neonates: new insights and new suggestions from the recent literature. J Vasc Access. (2020) 21(6):805–9. doi: 10.1177/1129729819891546
18. Ainsworth S, McGuire W. Percutaneous central venous catheters versus peripheral cannulae for delivery of parenteral nutrition in neonates. Cochrane Database Syst Rev. (2015) (10):CD004219. doi: 10.1002/14651858.CD004219.pub4
19. Atay S, Sen S, Cukurlu D. Incidence of infiltration/extravasation in newborns using peripheral venous catheter and affecting factors. Rev Esc Enferm USP. (2018) 52:e03360. doi: 10.1590/S1980-220X2017040103360
20. Rickard CM, Marsh NM, Webster J, Gavin NC, McGrail MR, Larsen E, et al. Intravascular device administration sets: replacement after standard versus prolonged use in hospitalised patients—a study protocol for a randomised controlled trial (the RSVP trial). BMJ Open. (2015) 5(2):e007257. doi: 10.1136/bmjopen-2014-007257
21. van Rens M, Nimeri AMA, Spencer TR, Hugill K, Francia ALV, Olukade TO, et al. Cyanoacrylate securement in neonatal PICC use: a 4-year observational study. Adv Neonatal Care. (2022) 22(3):270–9. doi: 10.1097/ANC.0000000000000963
22. D’Andrea V, Pezza L, Barone G, Prontera G, Pittiruti M, Vento G. Use of cyanoacrylate glue for the sutureless securement of epicutaneo-caval catheters in neonates. J Vas Access. (2022) 23(5):801–4. doi: 10.1177/11297298211008103
23. Bayoumi MA, Van Rens MF, Chandra P, Francia AL, D’Souza S, George M, et al. Effect of implementing an epicutaneo-caval catheter team in neonatal intensive care unit. J Vas Access. (2021) 22(2):243–53. doi: 10.1177/1129729820928182
24. van Rens M, Hugill K, Gaffari MA, Francia AV, Ramkumar T, Garcia KL, et al. Outcomes of establishing a neonatal peripheral vascular access team. Arch Dis Child Fetal Neonatal Ed. (2021) 0:F1. doi: 10.1136/archdischild-2021-322764. [Epub ahead of print]
25. Steere L, Ficara C, Davis M, Moureau N. Reaching one peripheral intravenous catheter (PIVC) per patient visit with lean multimodal strategy: the PIV5Rights TM bundle. J Assoc Vasc Access. (2019) 24(3):31–43. doi: 10.2309/j.java.2019.003.004
26. Wyckoff MM, Sharpe EL. Peripherally inserted central catheters: Guideline for practice. 3rd ed. IL, Chicago: National Association of Neonatal Nurses (2015).
27. Gorski LA, Hadaway L, Hagle ME, Broadhurst D, Clare S, Kleidon T, et al. Infusion therapy standards of practice. 8th ed. J Infus Nurs. (2021) 44(1S Suppl 1):S1–224. doi: 10.1097/NAN.0000000000000396
28. Carr PJ, Higgins NS, Cooke ML, Mihala G, Rickard CM. Vascular access specialist teams for device insertion and prevention of failure. Cochrane Database Syst Rev. (2018) (3):CD011429. doi: 10.1002/14651858.CD011429.pub2
29. Ostroff M, Zauk A, Chowdhury S, Moureau N, Mobley C. A retrospective analysis of the clinical effectiveness of subcutaneously tunneled femoral vein cannulations at the bedside: a low risk central venous access approach in the neonatal intensive care unit. J Vas Access. (2021) 22(6):926–34. doi: 10.1177/2F1129729820969291
30. Konstantinidi A, Sokou R, Panagiotounakou P, Lampridou M, Parastatidou S, Tsantila K, et al. Umbilical venous catheters and peripherally inserted central catheters: are they equally safe in VLBW infants? A non-randomized single center study. Medicina (B Aires). (2019) 55(8):442. doi: 10.3390/medicina55080442
31. D'Andrea V, Prontera G, Rubortone SA, Pezza L, Pinna G, Barone G, et al. Umbilical venous catheter update: a narrative review including ultrasound and training. Front Pediatr. (2021) 9:1–9. doi: 10.3389/fped.2021.774705
Keywords: neonate, NICU, epicutaneo-caval catheter, peripherally inserted central catheter, peripheral intravenous devices, central venous catheters, intravenous therapy, device-related complications
Citation: van Rens MFPT, Bayoumi MAA, van de Hoogen A, Francia ALV, Cabanillas IJ, van Loon FHJ and Spencer TR (2022) The ABBA project (Assess Better Before Access): A retrospective cohort study of neonatal intravascular device outcomes. Front. Pediatr. 10:980725. doi: 10.3389/fped.2022.980725
Received: 28 June 2022; Accepted: 30 September 2022;
Published: 3 November 2022.
Edited by:
Fiammetta Piersigilli, Cliniques Universitaires Saint-Luc, BelgiumReviewed by:
Vito D'Andrea, Agostino Gemelli University Polyclinic (IRCCS), Italy© 2022 Van Rens, Bayoumi, van den Hoogen, Francia, Cabanillas, Van Loon and Spencer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matheus F. P. T. van Rens rolandvanrens@icloud.com
†These authors have contributed equally to this work and share first authorship
‡ORCID Matheus F. P. T. van Rens orcid.org/0000-0001-9359-0895 Mohammad A. A. Bayoumi orcid.org/0000-0002-2627-4806 Agnes van de Hoogen orcid.org/0000-0003-2032-2472 Airene L. V. Francia orcid.org/0000-0003-0698-1498 Irian J. Cabanillas orcid.org/0000-0001-5608-5603 Fredericus H. J. van Loon orcid.org/0000-0003-3854-6976 Timothy R. Spencer orcid.org/0000-0002-3128-2034
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.