- 1Faculty of Health at the Queensland Centre for Children's Health Research, Queensland University of Technology (QUT), Brisbane, QLD, Australia
- 2Sport and Exercise Science, School of Health Sciences, Western Sydney University, Sydney, NSW, Australia
- 3UNSW Fatigue Research Program, Kirby Institute, University of New South Wales, Sydney, NSW, Australia
- 4Menzies Health Institute Queensland, Griffith University, Brisbane, QLD, Australia
- 5School of Health Sciences and Social Work, Griffith University, Nathan, QLD, Australia
- 6Cancer and Palliative Care Outcomes Centre, at Centre for Children's Health Research, Queensland University of Technology, South Brisbane, QLD, Australia
- 7School of Human Movement and Nutrition Sciences, University of Queensland, Brisbane, QLD, Australia
Background: Improved survival rates for children with solid tumors presents an ongoing challenge of how to maximize quality of survivorship and effectively manage the short- and long-term complications of disease and treatment. To gain an understanding of the extent and nature of research pertaining to therapeutic exercise interventions and identify knowledge gaps, we conducted a scoping review of exercise training studies conducted in pediatric survivors of brain cancer and other solid tumors.
Method: A systematic literature search was performed across four electronic databases. Papers were selected for full-text review if they included participants treated for brain cancer or other solid tumors, with at least 50% of participants aged ≤ 21 years, evaluated an exercise intervention ≥2-weeks in duration, and were published in an English, peer-reviewed journal. We included the following quantitative study designs; randomized controlled trials, non-randomized trials, and single-arm pre-test-post-test.
Results: Of the 7,482 citations identified, 17 papers met the inclusion criteria (presenting findings from eleven studies). Two studies were randomized controlled trials, five studies were non-randomized controlled trials, and four studies were a single-arm pre-test post-test design. Average age of participants ranged from 7.3–15.5 years, and time since diagnosis ranged from 3 to 70 months. Five studies included participants with brain tumors exclusively, three studies included other solid tumors, and three studies included a mixed sample (brain and other solid tumors). A wide range of exercise modalities were employed, including cycle ergometry, resistance training, sport, yoga, and active gaming. The length of the exercise program ranged from 3–40 weeks and frequency from 3–11 sessions per week. Exercise session duration ranged from 15–180 min, with most studies reporting 30–90-min sessions. Adherence ranged from 77 to 100%, with none of the studies reporting adverse events. Studies reported improvements in cardiorespiratory fitness, functional strength, physical activity, and quality of life.
Conclusions: A small number of mostly low methodological quality studies have examined the effects of therapeutic exercise in pediatric survivors of solid tumors. Although limited, the extant literature supports the feasibility and safety of therapeutic exercise interventions for pediatric survivors of brain cancer and other solid tumors.
Introduction
Solid tumors account for approximately 40–60% of cancer diagnoses in children and adolescents (aged 0–21 years) worldwide (1–3). Solid tumor types include tumors of the central nervous system, neuroblastomas, Wilms tumor, Ewing's tumor, rhabdomyosarcoma, soft tissue sarcomas, germ cell tumor and melanomas, and unlike adult cancers, childhood cancers are characterized by their cell of origin, not by their location (4). With advances in surgical intervention, radiotherapy, chemotherapy, and stem cell transplantation, the survival rates of children with solid tumors have increased dramatically over the past several decades, with the current overall 5-year survival rates ranging from 74 to 99% (2, 3). Improved survival rates present an ongoing challenge in survivorship of how to effectively manage the short- and long-term complications acquired from the disease and treatment. Further, the prevention of disabling secondary chronic health conditions, such as cardiovascular disease, metabolic disorders and secondary malignancies is an important factor of care post treatment. In this review, a “survivor” is considered any individual diagnosed and treated for cancer (5).
Pediatric survivors of solid tumors experience a myriad of short- and long-term complications following treatment, as well as late effects following treatment. Common late effects include reduced strength, poor cardiorespiratory fitness, increased fatigue, lowered executive function, and increased pain, consequently making home, school, and recreation activities more challenging (6–11). Quality of life in survivorship is a major concern for patients, families, and clinicians, with survivors of childhood solid tumors reporting a more severe impact of cancer and treatment into adulthood, compared to other cancers (12, 13). With more children than ever surviving solid tumors and living with late effects from the disease and its treatment, there is an urgent need for effective therapies to improve patient outcomes. Physical activity is beneficial for outcomes in both healthy and disease-burdened populations (14). Previous reviews have summarized the research literature on exercise training for mixed pediatric cancer diagnoses (15–18), the combination of adolescent and young adult cancer groups (19, 20), and exercise interventions during the treatment phase (21–23). The results indicate that therapeutic exercise training of sufficient frequency, intensity, and duration can improve cardiorespiratory fitness, muscular strength, fatigue and cognitive functioning (15–17). Additional benefits include improved immune function, reduced days of hospitalization and reduced risk of infection (22). However, the bulk of studies included in these reviews have been conducted in survivors of blood cancers and there is a dearth of research evidence on the efficacy of therapeutic exercise training among pediatric survivors of solid tumors (16, 24). Children with solid tumors differ in their clinical presentation, can receive more intensive treatment combinations of surgery, radiotherapy, and chemotherapy, and thus are likely to respond differently to therapeutic exercise (25–27). To gain a better understanding of the extent and nature of research pertaining to therapeutic exercise programs we conducted a scoping review of exercise training studies conducted in pediatric survivors of brain cancer and other solid tumors.
Methods
Search strategy
A systematic literature search was performed across four electronic databases: Embase, CINAHL, PubMed and Scopus in June 2020, and updated in April 2022. Search terms were developed in consultation with a librarian and based on previously conducted systematic reviews (18, 20, 28). Search terms were deliberately kept broad to ensure the full scope of the research was identified. No limits on publication date were applied. The search strategy contained four key topics: (1) pediatrics and adolescents, (2) solid tumors, (3) physical activity or exercise, and (4) study design (e.g., randomized controlled trials). Details of the search strategies can be found in the Supplementary material 1. The review is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) (29).
Inclusion and exclusion criteria
Papers were selected for full text review based on the following criteria: (1) included participants were treated for a solid tumor including tumors of the central nervous system, neuroblastomas, retinoblastomas, renal tumors, hepatic tumors, malignant bone tumors, soft tissue sarcomas, germ cell tumor and/or melanomas; (2) results were reported separately for solid tumors where studies included blood cancers; (3) at least 50% of participants were aged ≤ 21 years; (4) evaluated the effects of a physical activity or exercise intervention with a minimum duration of 2 weeks; and (5) published in an English-language, peer-reviewed journal. Papers were excluded if they evaluated an exercise program which was delivered in combination with other therapies (e.g., cognitive behavioral therapy, diet, and nutrition interventions) or if the record was a conference abstract, unpublished theses, commentary, newsletter, protocol, or case study.
Selection of included papers
Search results were exported into EndNote (Version X9), duplicates were removed, and citations (title and abstract) uploaded to online systematic review software (Covidence). Each citation was screened against the inclusion and exclusion criteria by at least two independent reviewers (BK, and either EB or CS) in two stages: (1) title and abstract screening and (2) full-text screening. Discrepancies were resolved by a third reviewer (EB or CS). In addition, a hand search of references lists of included papers was undertaken.
Data extraction
A data extraction table was developed by all authors to collate relevant information about the study design, sample size, tumor type(s), exercise training (location, supervision, frequency, intensity, duration and modes), and reported outcomes. Data extraction for one paper were completed collectively by all authors before one author (BK) completed data extraction for all remaining studies. Methodological quality was assessed independently by two authors (BK, and either EB or CS) using the Physiotherapy Evidence Database Scale (PEDro) (30). Any discrepancies were resolved through consensus. Scores of <4 were considered “poor”, 4 to 5 were considered “fair”, 6 to 8 were considered “good” and 9 to 10 were considered “excellent” (31).
Results
Search results
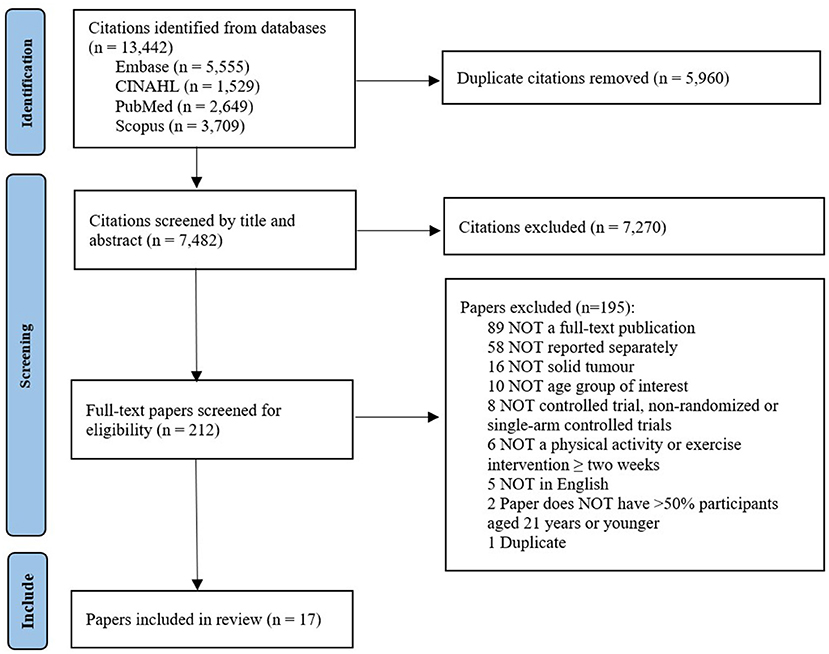
After removal of duplicates, the search identified a total of 7,482 papers. Following title and abstract screening, 212 were selected for full-text review. Of this number, 17 papers presenting findings from 11 studies met the eligibility criteria and were included for data extraction (see Figure 1).
Study characteristics
Supplementary material 2 provides a detailed summary of the 17 papers. All papers were published between 2014 and 2021. Five of the eleven studies were conducted in North America (32–39), five conducted in Europe (40–47), and one conducted in Asia (48). Two studies were randomized controlled trials (40–42, 47), five studies were non-randomized trials with a control condition (32–36, 39, 45, 46), and four studies were single-arm pre-test post-test studies (37, 38, 43, 44, 48). Five studies conducted assessments immediately post-intervention only (37, 38, 44, 46, 48), five studies conducted follow-ups at 2–6-months post-intervention (32–36, 39–42, 47), and one study conducted follow-up assessments at 12-months post-intervention (43, 45). In single-arm pre-test post-test studies, the sample size ranged between nine (38) and 88 participants (44). In studies with a control group, total sample size ranged between 13 (41, 42) and 57 (39), with control group sample sizes ranging between seven (41, 42) and 35 (32). The average participant age ranged from 7.3 years (38) to 15.5 years (45). Time since diagnosis ranged from 3 months (45) to 70 months (33–36). Five studies included participants with brain cancer exclusively (33–38, 41, 42, 48), three studies included other solid tumors (32, 39, 40, 47), and three studies included mixed sample with brain cancer and other solid tumors (43–46). Specific tumor types included astrocytoma, medulloblastoma, ependymoma, juvenile pilocytic astrocytoma, craniopharyngioma, germ cell tumor, retinoblastoma, choroid plexus carcinoma, glioma, osteosarcoma, Ewing's sarcoma, chondroblastoma, soft tissue sarcomas, neuroblastoma, and Wilms tumor. Based on the PEDro scoring, four papers were rated as “poor” (37, 39, 43, 44), eight papers as “fair” (33–36, 38, 45, 46, 48), four papers as “good” (32, 41, 42, 47), and one paper as “excellent” (40).
Exercise interventions
Five studies evaluated exercise programs post-treatment (33–38, 41–44) and six studies evaluated exercise programs during treatment (32, 39, 40, 45–48). Six studies examined the effects of a hospital inpatient program (39, 40, 43–48), two examined combined outpatient and home-based programs (33–36, 38), two examined home-based programs (37, 41, 42), and one study examined the effects of a combined hospital inpatient and outpatient program (32). Nine studies evaluated individual exercise programs delivered one-on-one (32, 37–42, 45–48), with the remaining two studies evaluating programs that combined individual and group-based exercise (33–36, 43, 44). In all eleven studies, the exercise program was supervised or remotely coached by a trained health professional (e.g., nurse, physiotherapist, exercise physiologist or trained exercise leader).
Multiple modes or types of exercise were reported across the eleven studies, including aerobic exercise (cycle ergometry, sports, active games) (32–36, 40, 45–47), resistance training (32, 40, 45–47), stretching (32, 45), yoga (48), and active video gaming (41, 42). One study evaluated the efficacy of physical activity goal setting and self-monitoring with an activity tracker (37). One study employed constraint-induced movement therapy (CIMT), which required participants to wear a removable cast on the unaffected arm and engage in task-specific training using the affected arm (38). One study utilized a staged coaching program during routine clinic visits to identify barriers to physical activity and prescribe physical activity and resources accordingly (39). Seven studies reported utilizing adult-like exercise programs (e.g., treadmills, cycle ergometry, resistance training) and/or competitive games/sports (e.g., basketball, relay running, dodgeball) (32–36, 39, 40, 45–48).
The length of the exercise program ranged from 3 weeks (38) to 40 weeks (45), with four studies opting for 10–12 week intervention periods (32–37, 41, 42). Exercise frequency ranged from three (32–36, 40, 45–48) to 11 (43, 44) sessions per week. Two studies did not report frequency (37, 39). The duration of the exercise program ranged from 15 min (45) to 180 min (38), with most studies reporting 30- to 90-min sessions (32–36, 40–48). Seven studies did not report exercise intensity (37–39, 41–44, 46, 48). Studies that reported intensity prescribed moderate-to-high intensity exercise determined by percentage of peak heart rate (33–36), age-predicted heart rate maximum (32, 40, 47), or Borg ratings of perceived exertion (RPE) (45). Resistance training comprised 1–3 sets of 6–15 repetitions for major muscle groups (32, 40, 45).
Adherence was operationally defined differently across studies. Six studies defined adherence as the number of sessions attended divided by the number prescribed, adherence ranged from 77 to 100% (32–36, 38, 41, 42, 45, 48). One study defined adherence as the number of prescribed exercises completed, where 68% of participants completed >90% of prescribed exercises (40, 47). One study defined adherence as the number of weeks the participant met or exceeded their goal, reported to be 69% (37). Three studies did not report adherence (43, 44, 46). Six of the eleven studies monitored adverse events (32–37, 40, 45, 48), with no adverse events reported.
Outcomes
A total of nine different outcomes were measured across the 17 papers. The most commonly measured outcomes were physical activity (n = 6) (37, 39–41, 43, 45), QoL (n = 6) (37–40, 43, 46), motor performance (n = 5) (35, 38, 41, 42, 44), cardiorespiratory fitness (n = 4) (34, 35, 37, 40), and brain structure and function (n = 4) (33, 34, 36, 42). There were multiple measures used for each outcome. Physical activity was measured by wearable devices, including step counters (37) and accelerometers (39, 40, 43–45, 47); and/or by self-report questionnaires, including the Godin-Leisure-Time Exercise Questionnaire (37, 39). Motor performance was measured by the Bruininks-Osterestsky Test of Motor Performance (35, 41), the Assessment of Motor and Process Skills (42), gait analysis (44), balance (44), and three upper limb function measures (38). Cardiorespiratory fitness was measured by the Six-Minute Walk Test (34, 37) and/or by a graded exercise test with spirometry (35, 40). According to the International Classification of Functioning, Disability and Health (ICF), studies assessed impairment- or activity-related outcomes; no specific participation-related outcomes were reported, except for QoL (49, 50).
Exercise programs that involved a combination of aerobic and resistance training (n = 5) (32, 40, 43–47) resulted in improvements in strength (40), functional mobility (32, 40), physical activity (43, 45), QoL (43), balance (44), immune function (47) and bone mass (45). Exercise programs that involved aerobic training only (n = 1) (33–36) resulted in improvements in cardiorespiratory fitness (34, 35), brain structure and function (33, 34, 36), and motor performance (35, 41). Active gaming (n = 1) (41, 42) resulted in improvements in physical activity (41), cognitive function (42), coordination (41), and activities of daily living to a score above the cut-off for independent living (42). Goal setting and self-monitoring daily step counts (n = 1) (37) resulted in improvements in cardiorespiratory fitness, QoL and fatigue. CIMT (n = 1) (38) resulted in improvements in the amount and quality of hemiplegic arm use, general fatigue, and sleep/rest fatigue sub-domain scores. Yoga (n = 1) (48) resulted in improvement in parent-reported child symptoms (appetite, pain, headache, sleep, physical activity, fatigue). A staged nurse-led coaching program resulted in no changes to physical activity, and results showed a significant increase in fatigue at 6 months (39).
Discussion
Compared to other cancer types, only a small number of studies have examined the effects of therapeutic exercise in pediatric survivors of solid tumors. Studies conducted to date vary considerably in methodological quality, exercise modalities, program duration, and clinical endpoints. The available evidence, although limited, supports the feasibility and safety of therapeutic exercise in this patient group, but well-designed trials are needed to assess the efficacy of such programs.
This review identified a small number of studies of mostly low methodological quality. Over a third (36%) of studies did not have a control group, and only five papers (29%) scored good-excellent quality on the PEDro Scale. Furthermore, over half of the studies (55%) evaluated small samples (n = <30), thus limiting statistical power. Brain cancer and other solid tumor diagnoses are relatively rare and the recruitment process is time- and resource-intensive, making it difficult to recruit the sample sizes required for adequately powered studies (51). Most studies did not include extended follow-up periods (>3 months) thus, the long-term effectiveness of the interventions, remains unclear. To address these methodological limitations, adequately powered multi-site studies employing rigorous study designs and long-term follow-up are needed. Alternative study design and analytic approaches also need to be explored to address limitations of small sample sizes (i.e., single-subject study designs) (52, 53).
The age of participants, tumor type, and time since diagnosis varied considerably across studies. Participants were, however, mostly “school-aged” (i.e., 5–18 years), highlighting an absence of studies involving young children aged 0 to 5 years. This is despite almost half (47%) of all childhood cancers diagnosed between the ages of 0 to 4 years (2). Early childhood is a crucial period of growth and development, where minimizing the impact of impairments, optimizing neuroplasticity, and enhancing rehabilitation is critical (54, 55). More research is needed to investigate the effects of therapeutic exercise in children with solid tumors during early childhood.
Children with brain cancer (e.g., medulloblastoma) were more frequently studied than other solid tumors, likely due the higher prevalence rates and high levels of treatment complications and long-term impairments (13). Challenges in crossing the blood-brain barrier mean that treatment for CNS tumors differ to that of other solid tumors, with brain cancer treated with more surgery and radiation-focused treatments compared to other solid tumors, which are more often treated with a rigorous regimen of surgery and chemotherapy (56, 57). Children with bone tumors in particular, (e.g., osteosarcoma) have their own unique experiences and needs, often effected by amputations, disability and poorer QoL (7, 58). Currently there is a near-absence of evidence investigating the role exercise may play in the survivorship phase of these children. As this group of children present different clinically, and undergo different treatment regimes, they may also respond differently to exercise. Regardless of the diagnoses, providing supportive care throughout the course of a child's acute treatment and survivorship is critical in achieving optimal outcomes.
The exercise programs varied by setting, level of supervision, length, and exercise modalities. Most studies delivered the exercise program in hospital-based settings, with few utilizing home-based programs. No studies delivered the exercise program in a community-based setting such as recreational halls, and parks. Whilst hospitals may be rich in resources (e.g., qualified staff, specific equipment), they also place additional challenges on children and their families. Hospitals can be inconvenient and expensive for travel, and for those children in the post-treatment phase, returning to hospital can induce fear and anxiety (59). The appropriate exercise setting is therefore crucial and may influence adherence, enjoyment, and long-term participation. To better meet the needs of children with solid tumors and their families, future research should investigate the effectiveness of patient-centered therapeutic exercise programs delivered in community-based settings.
Most programs included in this review involved face-to-face supervision by a trained exercise professional. Some patients and their families prefer flexible and convenient modes of intervention delivery (e.g., face-to-face at home, telehealth, mobile-health apps), which may enhance exercise adherence and long-term sustainability of program outcomes (59, 60). While supervised exercise may be initially required for monitoring safety and ensuring program fidelity, these remote delivery modes warrant investigation to delineate what is the most feasible and effective for children and their families who desire alternative delivery modes (e.g., children living in remote communities).
Studies in this review predominantly used adult-based exercise modalities and prescription closely aligned with generic American College of Sports Medicine guidelines (61). Few studies employed play- or game-based exercise, which is more likely to be engaging and motivating for children (62). Children are not little adults, and exercise programs for young children should be designed and implemented in a developmentally appropriate manner. Exercise interventions will likely be more effective and result in sustainable improvements in habitual physical activity, movement competence, and functional capacity if they are play- or game-based. Evaluations of developmentally appropriate play-based exercise programs in pediatric survivors of brain and other solid tumors are urgently needed to determine its effectiveness in this clinically unique patient group.
There was a notable lack of studies evaluating patient-centered, personalized exercise programs (63, 64), with multiple programs (n = 5) assessing traditional ‘impairment-based' exercise interventions (32–36, 40, 45–47, 50). Programs of this type are based on the expectation that remediating impairments will lead to improved participation in their activities of choice. However, such interventions typically report poor participation outcomes in pediatric cohorts (65, 66). Intrinsically-motivated behavior (e.g., regular physical activity) is theorized to be influenced by three basic psychological needs; autonomy, perceived competence, and relatedness (67, 68). Autonomy-supportive environments encourage intrinsic motivation for sustainable behavior change through fulfillment of these basic psychological needs (69, 70). An example of fostering an autonomy-supportive environment is goal setting, whereby goals are set collaboratively by the patients and their families to be meaningful, individualized, and sensitive to the patient's clinical presentation and preferences. There is evidence supporting the effectiveness of goal-directed interventions to improve motor performance, physical activity and QoL in other pediatric groups (e.g., children with cerebral palsy, muscular dystrophy, or intellectual disability), which may be more conducive to long-term improvements (71–73). There are a small number of published study protocols advocating for the use of goal-directed interventions (74–76).
Although some outcomes were assessed in multiple studies, the measures used to assess these outcomes varied considerably. This finding is consistent with the conclusions of recent reviews on assessment of physical function in pediatric cancer patients (77–79). Outcomes measures were predominantly impairment-focused and lacked participation-level outcomes, based on the ICF framework. To improve the consistency, comparability, and transparency of study findings, future studies should adopt a more standardized approach to outcome selection and reporting. Future studies should also include participation-based outcomes such as the Participation and Environment Measure for Children and Youth (80) or participation-focused goal-directed measures, including the Canadian Occupational Performance Measure (81) or the Goal Attainment Scale (82).
Considering the relatively small number of studies and the wide range of outcomes and measures utilized across studies, it was not feasible to conduct a quantitative synthesis of the results. Nevertheless, across studies, therapeutic exercise was associated with improvements in cardiorespiratory fitness, habitual physical activity, muscle strength, functional mobility, motor performance, body composition and QoL. There were no reported adverse events from participation in therapeutic exercise. This suggests that therapeutic exercise is a potentially safe and feasible intervention for improving the QoL and wellbeing in pediatric survivors of brain cancer and other solid tumors; however, higher grade evidence is needed to make firm conclusions about the effectiveness of therapeutic exercise in this patient group and formal clinical recommendations.
This review has several strengths. It is the first scoping review of exercise training studies conducted in pediatric survivors of brain cancer and other solid tumors. The review was completed according to the PRISMA_ScR guidelines. An extensive search for articles was conducted across four databases, with no limitation on publication date. To supplement this search, an extensive manual search of all included articles was conducted. The review and extraction processes were rigorous. Titles and abstracts and full -text citations were reviewed by two independent authors and all citations were independently checked for accuracy after extraction. Opposing these strengths were some limitations. It is possible that not all relevant publications were identified through the systematic search and cross-reference searches. For example, papers published in languages other than English or in the gray literature were not included in the review. Despite most participants being “school-aged”, a small of number of studies included participants who were 18 years or over.
Conclusion
This scoping review provides the first synthesis on the extent and nature of research pertaining to therapeutic exercise programs conducted in pediatric survivors of brain cancer and other solid tumors. Compared to blood cancer types, a small number of studies have examined the effects of therapeutic exercise in pediatric survivors of solid tumors. The methodological quality of studies conducted to date has been low (i.e., non-randomized study designs, no control group, small sample sizes) and have limited follow-up (e.g., greater than 6 months). Most of the research has been conducted in brain cancer survivors, with the bulk of studies evaluating highly structured supervised exercise programs delivered in hospital settings. The role of therapeutic exercise in children with other solid tumors, such as osteosarcoma and Ewing's sarcomas, has received limited research attention, particularly in relation to QoL in survivorship. Few studies employed play- or game-based exercise programs, instead utilizing adult-based modalities and prescription. There were multiple measures used for each outcome, with a paucity of standardized outcomes measures administered across papers. Although limited, the extant research supports the feasibility and safety of therapeutic exercise for children with solid tumors before, during and after treatment. Nonetheless, significant knowledge gaps were identified. Future research should address the major gaps in the literature, including the evaluation of developmentally appropriate play-based physical activity interventions for children aged 5 years and under; the feasibility, acceptability, and potential efficacy of exercise programs delivered in of different settings (e.g., home and community-based settings) and delivery channels (e.g., telehealth and apps). To improve the quality of evidence, collaborative, multi-site studies are needed to ensure that trials are adequately powered to detect clinically meaningful changes in outcomes.
Author contributions
ST, CS, EB, and BK were responsible for the conceptualization and design of the study. CS, EB, and BK screened citations and critically appraised included papers. BK completed data extraction and drafted the manuscript, which was critically reviewed by ST, CS, EB, and NB. All authors read and approved the final manuscript.
Funding
This review is supported by a project grant from the Children's Brain Cancer Centre through the Children's Hospital Foundation (CCABCR010). The funders had no role in the design of the study or preparation of the manuscript, and will remain separate for data collection, analysis, and interpretation of the data.
Acknowledgments
We would like to acknowledge the contributions of the library staff at Queensland University of Technology for their assistance with search strategy development. CS is supported by Cancer Institute NSW Early Career Fellowship (2021/ECF1310).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.979292/full#supplementary-material
References
1. Ljungman G, Jakobson A, Behrendtz M, Ek T, Friberg LG, Hjalmars U, et al. Incidence and survival analyses in children with solid tumours diagnosed in Sweden between 1983 and 2007. Acta Paediatr. (2011) 100:750–7. doi: 10.1111/j.1651-2227.2010.02122.x
2. Youlden D, Aitken J. Childhood Cancer in Australia 1983–2015. Brisbane, Australia: Cancer Council Australia. (2019).
3. Lacour B, Guyot-Goubin A, Guissou S, Bellec S, Désandes E, Clavel J. Incidence of childhood cancer in France: National Children Cancer Registries, 2000–2004. Eur J Cancer Prevent. (2010) 19:173–81. doi: 10.1097/CEJ.0b013e32833876c0
4. Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer, third edition. Cancer. (2005) 103:1457–67. doi: 10.1002/cncr.20910
5. Denlinger CS, Carlson RW, Are M, Baker KS, Davis E, Edge SB, et al. Survivorship: introduction and definition. JNCCN. (2014) 12:34–45. doi: 10.6004/jnccn.2014.0005
6. Ness KK, Mertens AC, Hudson MM, Wall MM, Leisenring WM, Oeffinger KC, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. (2005) 143:639–47. doi: 10.7326/0003-4819-143-9-200511010-00007
7. Bekkering WP, Vliet Vlieland TP, Koopman HM, Schaap GR, Bart Schreuder HW, Beishuizen A, et al. Functional ability and physical activity in children and young adults after limb-salvage or ablative surgery for lower extremity bone tumors. J Surg Oncol. (2011) 103:276–82. doi: 10.1002/jso.21828
8. Riggs L, Bouffet E, Laughlin S, Laperriere N, Liu F, Skocic J, et al. Changes to memory structures in children treated for posterior fossa tumors. J Int Neuropsychol Soc. (2014) 20:168–80. doi: 10.1017/S135561771300129X
9. Piscione PJ, Bouffet E, Mabbott DJ, Shams I, Kulkarni AV. Physical functioning in pediatric survivors of childhood posterior fossa brain tumors. Neuro Oncol. (2014) 16:147–55. doi: 10.1093/neuonc/not138
10. Marina N, Hudson MM, Jones KE, Mulrooney DA, Avedian R, Donaldson SS, et al. Changes in health status among aging survivors of pediatric upper and lower extremity sarcoma: a report from the childhood cancer survivor study. Arch Phys Med Rehabil. (2013) 94:1062–73. doi: 10.1016/j.apmr.2013.01.013
11. Fernandez-Pineda I, Hudson MM, Pappo AS, Bishop MW, Klosky JL, Brinkman TM, et al. Long-term functional outcomes and quality of life in adult survivors of childhood extremity sarcomas: a report from the St.Jude Lifetime Cohort Study. J Cancer Survivorship. (2017) 11:1–12. doi: 10.1007/s11764-016-0556-1
12. Ness KK, Leisenring WM, Huang S, Hudson MM, Gurney JG, Whelan K, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer. Cancer. (2009) 115:1984–94. doi: 10.1002/cncr.24209
13. Ness KK, Hudson MM, Ginsberg JP, Nagarajan R, Kaste SC, Marina N, et al. Physical performance limitations in the Childhood Cancer Survivor Study cohort. J Clin Oncol. (2009) 27:2382. doi: 10.1200/JCO.2008.21.1482
14. Strong WB, Malina RM, Blimkie CJR, Daniels SR, Dishman RK, Gutin B, et al. Evidence based physical activity for school-age youth. J Pediatr. (2005) 146:732–7. doi: 10.1016/j.jpeds.2005.01.055
15. Morales J, Valenzuela PL, Olivares AM, Baño-Rodrigo A, Castillo-García A, Rincon C, et al. Exercise Interventions and Cardiovascular Health in Childhood Cancer: a Meta-Analysis. International J Sports Medicine. 2020;41. doi: 10.1055/a-1073-8104
16. Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochr Datab System Rev. (2016) 3:CD008796. doi: 10.1002/14651858.CD008796.pub3
17. Wurz A, McLaughlin E, Lategan C, Chamorro Viña C, Grimshaw SL, Hamari L, et al. The international Pediatric Oncology Exercise Guidelines (iPOEG). Transl Behav Med. (2021) 11:1915–22. doi: 10.1093/tbm/ibab028
18. Ospina PA, McNeely ML. Scoping Review of Physical Therapy Interventions for Childhood Cancers. Physiother Canada. (2019) 71:287–96. doi: 10.3138/ptc.2018-13.pp
19. Caru M, Levesque A, Rao P, Dandekar S, Terry C, Brown V, et al. A scoping review to map the evidence of physical activity interventions in post-treatment adolescent and young adult cancer survivors. Crit Rev Oncol Hematol. (2022) 171:103620. doi: 10.1016/j.critrevonc.2022.103620
20. Brunet J, Wurz A, Shallwani SM. scoping review of studies exploring physical activity among adolescents and young adults diagnosed with cancer. Psychooncology. (2018) 27:1875–88. doi: 10.1002/pon.4743
21. Rustler V, Hagerty M, Daeggelmann J, Marjerrison S, Bloch W, Baumann FT. Exercise interventions for patients with pediatric cancer during inpatient acute care: A systematic review of literature. Pediatric Blood Cancer. (2017) 64:e26567. doi: 10.1002/pbc.26567
22. Beller R, Bennstein SB, Götte M. Effects of exercise interventions on immune function in children and adolescents with cancer and HSCT recipients - a systematic review. Front Immunol. (2021) 12:746171. doi: 10.3389/fimmu.2021.746171
23. Santos SDS, Moussalle LD, Heinzmann-Filho JP. Effects of physical exercise during hospitalization in children and adolescents with cancer: a systematic review. Rev Paulista de Pediatria. (2020) 39:e2019313. doi: 10.1590/1984-0462/2021/39/2019313
24. Donnan BM, Webster T, Wakefield CE, Dalla-Pozza L, Alvaro F, Lavoipierre J, et al. What about school? Educational challenges for children and adolescents with cancer the educational and developmental. Psychologist. (2015) 32:23–40. doi: 10.1017/edp.2015.9
25. Casey DL, Cheung N-KV. Immunotherapy of pediatric solid tumors: treatments at a crossroads, with an emphasis on antibodies. Cancer Immunol Res. (2020) 8:161–6. doi: 10.1158/2326-6066.CIR-19-0692
26. Merchant TE, Kortmann R-D. Pediatric Radiation Oncology. 1st ed Cham: Springer International Publishing. (2018). doi: 10.1007/978-3-319-43545-9_1
27. Hayat MA. Pediatric Cancer, Volume 2 Teratoid/Rhabdoid, Brain Tumors, and Glioma. 1st ed. 2012. ed. Dordrecht: Springer Netherlands. (2012). doi: 10.1007/978-94-007-2957-5
28. Gmelig Meyling C, Verschuren O, Rentinck IR, Engelbert RHH, Gorter JW. Physical rehabilitation interventions in children with acquired brain injury: a scoping review. Dev Med Child Neurol. (2021). doi: 10.1111/dmcn.14997
29. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
30. Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro). Austr J Physiother. (2002) 48:43–9. doi: 10.1016/S0004-9514(14)60281-6
31. Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J Physiother. (2020) 66:59. doi: 10.1016/j.jphys.2019.08.005
32. Corr AM, Liu W, Bishop M, Pappo A, Srivastava DK, Neel M, et al. Feasibility and functional outcomes of children and adolescents undergoing preoperative chemotherapy prior to a limb-sparing procedure or amputation. Rehabil Oncol. (2017) 35:38–45. doi: 10.1097/01.REO.0000000000000050
33. Cox E, Bells S, Timmons BW, Laughlin S, Bouffet E, de Medeiros C, et al. A controlled clinical crossover trial of exercise training to improve cognition and neural communication in pediatric brain tumor survivors. Clin Neurophysiol. (2020) 131:1533–47. doi: 10.1016/j.clinph.2020.03.027
34. Riggs L, Piscione J, Laughlin S, Cunningham T, Timmons BW, Courneya KS, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro Oncol. (2017) 19:440–50. doi: 10.1093/neuonc/now177
35. Piscione PJ, Bouffet E, Timmons B, Courneya KS, Tetzlaff D, Schneiderman JE, et al. Exercise training improves physical function and fitness in long-term paediatric brain tumour survivors treated with cranial irradiation. Eur J Cancer. (2017) 80:63–72. doi: 10.1016/j.ejca.2017.04.020
36. Szulc-Lerch KU, Timmons BW, Bouffet E, Laughlin S, de Medeiros CB, Skocic J, et al. Repairing the brain with physical exercise: Cortical thickness and brain volume increases in long-term pediatric brain tumor survivors in response to a structured exercise intervention. Neuroimage-Clin. (2018) 18:972–85. doi: 10.1016/j.nicl.2018.02.021
37. Ovans J, Hooke MC, Bendel AE, Tanner L. Physical therapist coaching to improve physical activity in children with brain tumors: a pilot study. Pediatr Phys Ther. (2018) 30:310–7. doi: 10.1097/PEP.0000000000000531
38. Sparrow J, Zhu L, Gajjar A, Mandrell BN, Ness KK. Constraint-induced movement therapy for children with brain tumors. Pediat Phys Ther. (2017) 29:55–61. doi: 10.1097/PEP.0000000000000331
39. Hooke MC, Hoelscher A, Tanner LR, Langevin M, Bronas UG, Maciej A, et al. Kids are moving: a physical activity program for children with cancer. J Pediatr Oncol Nursing. (2019) 36:379–89. doi: 10.1177/1043454219858607
40. Fiuza-Luces C, Padilla JR, Soares-Miranda L, Santana-Sosa E, Quiroga JV, Santos-Lozano A, et al. Exercise intervention in pediatric patients with solid tumors: the physical activity in pediatric cancer trial. Med Sci Sports Exerc. (2017) 49:223–30. doi: 10.1249/MSS.0000000000001094
41. Sabel M, Sjolund A, Broeren J, Arvidsson D, Saury JM, Blomgren K, et al. Active video gaming improves body coordination in survivors of childhood brain tumours. Disabil Rehabil. (2016) 38:2073–84. doi: 10.3109/09638288.2015.1116619
42. Sabel M, Sjolund A, Broeren J, Arvidsson D, Saury JM, Gillenstrand J, et al. Effects of physically active video gaming on cognition and activities of daily living in childhood brain tumor survivors: a randomized pilot study. Neuro-Oncol Pract. (2017) 4:98–110. doi: 10.1093/nop/npw020
43. Müller C, Krauth K, Gerß J, Rosenbaum D. Physical activity and health-related quality of life in pediatric cancer patients following a 4-week inpatient rehabilitation program. Suppor Care Cancer. (2016) 24:3793–802. doi: 10.1007/s00520-016-3198-y
44. Müller C, Rosenbaum D, Krauth KA. Prospective evaluation of postural control and gait in pediatric patients with cancer after a 4-week inpatient rehabilitation program. Am J Phys Med Rehabil. (2017) 96:646–53. doi: 10.1097/PHM.0000000000000729
45. Müller C, Winter C, Boos J, Gosheger G, Hardes J, Vieth V, et al. Effects of an exercise intervention on bone mass in pediatric bone tumor patients. Int J Sports Med. (2014) 35:696–703. doi: 10.1055/s-0033-1358475
46. Spreafico F, Barretta F, Murelli M, Chisari M, Gattuso G, Terenziani M, et al. Positive impact of organized physical exercise on quality of life and fatigue in children and adolescents with cancer. Front Pediatr. (2021) 9:627876. doi: 10.3389/fped.2021.627876
47. Fiuza-Luces RC, Padilla SJ, Valentín JJ, Santana-Sosa JE, Santos-Lozano JA, Sanchis-Gomar JF, et al. Effects of exercise on the immune function of pediatric patients with solid tumors: insights from the PAPEC randomized trial. Am J Phys Med Rehabil. (2017) 96:831–7. doi: 10.1097/PHM.0000000000000757
48. Govardhan HB, Nelson N, Khaleel IA, Kumar A, Roy M, Divyashree S, et al. Effect of yoga on the symptomps response in pediatric brain tumor in-patients undergoing chemo and radiotherapy. Oncol Radiother. (2019) 1:034–8.
49. McDougall J, Wright V, Rosenbaum P. The ICF model of functioning and disability: incorporating quality of life and human development. Dev Neurorehabil. (2010) 13:204–11. doi: 10.3109/17518421003620525
50. International Classification of Functioning. Disability and Health Children and Youth Version ICF-CY. Albany: World Health Organization. (2006).
51. Bradford N, Cashion C, Condon P, Rumble S, Bowers A. Recruitment principles and strategies for supportive care research in pediatric oncology. BMC Med Res Methodol. (2021) 21:178. doi: 10.1186/s12874-021-01371-1
52. Kazdin AE. Single-Case Research Designs: Methods for Clinical and Applied Settings. 2nd ed New York: Oxford University Press. (2011).
53. Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. (2009) 338:b2393. doi: 10.1136/bmj.b2393
54. Novak I, Morgan C. High-risk follow-up: Early intervention and rehabilitation. Handb Clin Neurol. (2019) 162:483–510. doi: 10.1016/B978-0-444-64029-1.00023-0
55. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech, Language, Hear Res. (2008) 51:S225–S39. doi: 10.1044/1092-4388(2008/018)
56. Power EA, Rechberger JS, Gupta S, Schwartz JD, Daniels DJ, Khatua S. Drug delivery across the blood-brain barrier for the treatment of pediatric brain tumors – An update. Adv Drug Deliv Rev. (2022) 185:114303. doi: 10.1016/j.addr.2022.114303
57. Reed DR, Hayashi M, Wagner L, Binitie O, Steppan DA, Brohl AS, et al. Treatment pathway of bone sarcoma in children, adolescents, and young adults. Cancer. (2017) 123:2206–18. doi: 10.1002/cncr.30589
58. Bekkering WP, Vliet Vlieland TP, Koopman HM, Schaap GR, Schreuder HW, Beishuizen A, et al. Quality of life in young patients after bone tumor surgery around the knee joint and comparison with healthy controls. Pediatric Blood Cancer. (2010) 54:738–45. doi: 10.1002/pbc.22439
59. Ross WL, Le A, Zheng DJ, Mitchell H-R, Rotatori J, Li F, et al. Physical activity barriers, preferences, and beliefs in childhood cancer patients. Support Care Cancer. (2018) 26:2177–84. doi: 10.1007/s00520-017-4041-9
60. Wright M. Physical activity participation and preferences: developmental and oncology-related transitions in adolescents treated for cancer. Physiother Canada. (2015) 67:292–9. doi: 10.3138/ptc.2014-25LHC
61. Liguori G, Feito Y, Fountaine C, Roy B. ACSM's Guidelines for Exercise Testing and Prescription. Eleventh ed Philadelphia: Wolters Kluwer. (2022).
62. Okely AD, Ghersi D, Loughran SP, Cliff DP, Shilton T, Jones RA, et al. A collaborative approach to adopting/adapting guidelines. The Australian 24-hour movement guidelines for children (5–12 years) and young people (13–17 years): An integration of physical activity, sedentary behaviour, and sleep. Int J Behav Nutr Phys Activity. (2022) 19:1–21. doi: 10.1186/s12966-021-01236-2
63. Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. (2000) 51:1087–110. doi: 10.1016/S0277-9536(00)00098-8
64. Wijma AJ, Bletterman AN, Clark JR, Vervoort S, Beetsma A, Keizer D, et al. Patient-centeredness in physiotherapy: What does it entail? A systematic review of qualitative studies. Physiother Theory Pract. (2017) 33:825–40. doi: 10.1080/09593985.2017.1357151
65. Adair B, Ullenhag A, Keen D, Granlund M, Imms C. The effect of interventions aimed at improving participation outcomes for children with disabilities: a systematic review. Dev Med Child Neurol. (2015) 57:1093–104. doi: 10.1111/dmcn.12809
66. Reedman S, Boyd RN, Sakzewski L. The efficacy of interventions to increase physical activity participation of children with cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. (2017) 59:1011–8. doi: 10.1111/dmcn.13413
67. Deci EL, Ryan RM. Self-determination theory in health care and its relations to motivational interviewing: a few comments. Int J Behav Nutr Phys Act. (2012) 9:24. doi: 10.1186/1479-5868-9-24
68. Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. (2000) 55:68. doi: 10.1037/0003-066X.55.1.68
69. Michie S, van Stralen MM, West R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implementation Sci. (2011) 6:42. doi: 10.1186/1748-5908-6-42
70. Blackburn NE, Wilson JJ, McMullan II, Caserotti P, Giné-Garriga M, Wirth K, et al. The effectiveness and complexity of interventions targeting sedentary behaviour across the lifespan: a systematic review and meta-analysis. Int J Behav Nutr Phys Activity. (2020) 17:53. doi: 10.1186/s12966-020-00957-0
71. Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. J Develop Med Child Neurol. (2013) 55:885–910. doi: 10.1111/dmcn.12246
72. Willis C, Nyquist A, Jahnsen R, Elliott C, Ullenhag A. Enabling physical activity participation for children and youth with disabilities following a goal-directed, family-centred intervention. Res Dev Disabil. (2018) 77:30–9. doi: 10.1016/j.ridd.2018.03.010
73. Palisano RJ, Chiarello LA, King GA, Novak I, Stoner T, Fiss A. Participation-based therapy for children with physical disabilities. Disabil Rehabil. (2012) 34:1041–52. doi: 10.3109/09638288.2011.628740
74. Grimshaw S, Taylor N, Conyers R, Shields N. Promoting positive physical activity behaviours for children and adolescents with cancer: development of the canmove intervention using the behaviour change wheel. Pediatr Blood Cancer. (2021) 68:S352.
75. Kohler BE, Baque E, Sandler CX, Brookes DSK, Terranova CO, Rixon M, et al. Physical ACTivity in Survivorship (PACTS): study protocol for a randomized controlled trial evaluating a goal-directed therapeutic exercise program in pediatric posterior fossa brain tumor survivors. BMC Pediatr. (2021) 21:105. doi: 10.1186/s12887-021-02566-7
76. Brown MC, Araújo-Soares V, Skinner R, Glaser AW, Sarwar N, Saxton JM, et al. Using qualitative and co-design methods to inform the development of an intervention to support and improve physical activity in childhood cancer survivors: a study protocol for BEing Active after ChildhOod caNcer (BEACON). BMJ Open. (2020) 10:e041073. doi: 10.1136/bmjopen-2020-041073
77. Söntgerath R, Däggelmann J, Kesting SV, Rueegg CS, Wittke T-C, Reich S, et al. Physical and functional performance assessment in pediatric oncology: a systematic review. Pediatr Res. (2022) 91:743–56. doi: 10.1038/s41390-021-01523-5
78. Grimshaw SL, Taylor NF, Mechinaud F, Shields N. Assessment of physical function in children with cancer: A systematic review. Pediatr Blood Cancer. (2018) 65:e27369-n/a. doi: 10.1002/pbc.27369
79. Shank J, Chamorro-Viña C, Guilcher GMT, Langelier DM, Schulte F, Culos-Reed SN. Evaluation tools for physical activity programs for childhood cancer: a scoping review. J Pediatr Oncol Nurs. (2020) 37:163–79. doi: 10.1177/1043454219891987
80. Coster W, Bedell G, Law M, Khetani MA, Teplicky R, Liljenquist K, et al. Psychometric evaluation of the participation and environment measure for children and youth. Dev Med Child Neurol. (2011) 53:1030–7. doi: 10.1111/j.1469-8749.2011.04094.x
81. Law M, Polatajko H, Pollock N, McColl MA, Carswell A, Baptiste S. Pilot testing of the Canadian Occupational Performance Measure: clinical and measurement issues. Canad J Occup Ther. (1994) 61:191–7. doi: 10.1177/000841749406100403
Keywords: children, pediatrics, oncology, solid tumor, physical activity, therapy, survivorship
Citation: Kohler BE, Sandler CX, Baque E, Bradford NK and Trost SG (2022) Therapeutic exercise interventions in pediatric survivors of brain cancer and other solid tumors: A scoping review. Front. Pediatr. 10:979292. doi: 10.3389/fped.2022.979292
Received: 27 June 2022; Accepted: 25 August 2022;
Published: 16 September 2022.
Edited by:
Miriam Götte, Essen University Hospital, GermanyReviewed by:
Maura Massimino, Fondazione IRCCS Istituto Nazionale Tumori, ItalyRonja Beller, Essen University Hospital, Germany
Copyright © 2022 Kohler, Sandler, Baque, Bradford and Trost. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stewart G. Trost, cy50cm9zdEB1cS5lZHUuYXU=
†These authors have contributed equally to this work and share second authorship
 Brooke E. Kohler
Brooke E. Kohler Carolina X. Sandler
Carolina X. Sandler Emmah Baque
Emmah Baque Natalie K. Bradford
Natalie K. Bradford Stewart G. Trost
Stewart G. Trost