- Department of Child Health Care, Children’s Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Child Health and Nutrition, Chongqing, China
Objective: Family history of atopic diseases (FHA) contributes to food allergy (FA). But little is known whether FHA primarily increases IgE–mediated, non–IgE–mediated FA, or both. And the trends in the contributions of FHA to food sensitization (FS) and FA remain unclear. We aim to clarify the associations among FHA, FS and FA and to understand the trends in the contributions of FHA to FS and FA.
Methods: We used chi–square test and mediating effect model to analyze the associations among FHA, FS and FA through comparisons between two cross–sectional investigations on FA in children under 2 years old in 2009 and 2019.
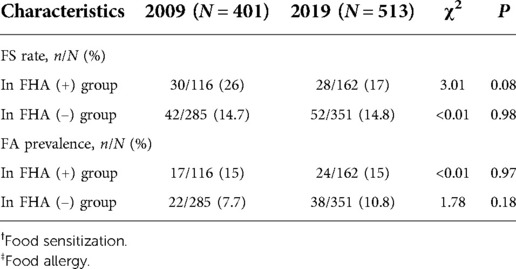
Results: In 2009 and 2019, the positive FHA proportion tended to be increasing without significance (28.9% to 31.6%, P = 0.39). Subgroup analysis showed the FS rate in FA group decreased significantly (37/39 to 44/62, P = 0.003). In 2009, the FS rate and FA prevalence were higher in FHA (+) group than in FHA (–) group (26% vs. 14.7%, P = 0.008 and 15% vs. 7.7%, P = 0.03), and FS had a complete mediating effect on the association between FHA and FA (Z = 2.54, P = 0.011), but the results lost significance in 2019.
Conclusions: The association between FHA and FA was completely mediated by FS, which means FHA mainly increases IgE–mediated FA. And the contributions of FHA to FS and FA tended to be stabilized or even diminished, which means FHA alone could no longer be enough to screen high–risk children.
Introduction
Food allergy (FA) means abnormal immune responses and symptoms caused by food antigens, and is more complicated than other atopic diseases as it includes IgE–mediated, non–IgE–mediated and mixed–mediated types. In recent years, the reported incidence of FA has increased (1), and FA has become an important global public health issue and has brought heavy burdens to families and societies (2, 3).
Like other atopic diseases, genetic factors contribute to FA (4). Many genes are involved in FA, such as the nonsense mutation of the filaggrin gene increases the risk of FA (5). Meanwhile, clinical studies have confirmed that family history of atopic diseases (FHA) is also involved in FA. For example, peanut allergy had a strong genetic susceptibility and the estimated heritability (the proportion of variation that is due to hereditary factors) (6) was approximately 81.6% (7). Maternal asthma was significantly associated with cow's milk allergy in children with atopic dermatitis (AD) (OR = 10.9, 95% CI 1.6–73) (8). And the risk of FA increased to 2.6–fold (95% CI 1.2–5.6) in children with FA siblings (9). Therefore, inquiring about the family history is essential (10), and FHA is often used as the indicator of genetic factors to screen children at high risk of FA in clinical practice.
However, little is known about whether FHA primarily increases IgE–mediated, non–IgE–mediated FA, or both. In other words, whether FHA is associated with the development of FA directly or indirectly. It is known that FHA has an impact on IgE production and infants with FHA have increased Th2 cytokines (IL–4, IL–13, etc.) (11, 12), which are the key cytokines in the initiation and chronicity of Th2 immune responses (13, 14). As a consequence, FHA can promote the differentiation of immune system towards Th2 type in children, which may lead to atopic diseases. Moreover, parental atopic respiratory disease increased the risk significantly more for IgE–associated AD (15), and FHA children with food sensitization (FS) might be a susceptible subgroup to FA (16). Thus, we speculate that FS could mediate the association between FHA and FA, that means FHA may mainly increase the risk of FS, and then FA.
More importantly, the FS rate among infants with FHA has remained stable in Australia since the 1990s (17). But the incidence of both IgE–mediated and non–IgE–mediated FA is increasing, which is beyond the range that could be explained by genetic variation (18, 19). This discrepancy could be due to increased FA in the non–FHA population, increased conversion of FS to FA, or increased number of high–risk infants (17). In fact, limited evidence suggests that FHA is even not enough to predict FS or FA in children (20–23), two or more allergic family members or FHA combined with environmental factors may be more effective in screening children at high risk for FA (24, 25), Therefore, the contributions of genetic and environmental factors to the development of FS and FA could have changed over time, suggesting that it is time for allergists to re–evaluate the role of FHA in FS and FA. In this article, we aim to clarify the associations among FHA, FS and FA, and to understand the trends in the contributions of FHA to FS and FA.
Participants and methods
Study design
This article was complementary to our previously published study (26). In a word, we conducted three cross–sectional investigations on FA in the Department of Child Health Care, Children's Hospital of Chongqing Medical University (CHCMU) in 1999, 2009, and 2019. As previously described, we had already discussed the FS rate and FA prevalence in the past 20 years (the most common food allergens were cow's milk and egg). Here, we grouped the participants based on FHA into FHA (+) group and FHA (–) group, and analyzed the differences of FS rate and FA prevalence between the two groups to better understand the possible changes in the contributions of FHA to FS and FA. Notably, the data from 2009 to 2019 were included, and the data from 1999 were excluded due to unclear definition of FHA. The article followed the STROBE Statement.
Participants
All healthy children under 2 years old attending routine physical examinations at the Department of Child Health Care were recruited in the two investigations without selection. All children recruited came from the main nine districts of Chongqing, because they might be recalled for oral challenge test (OFC). Informed consent was obtained upon initial contact. According to the National Health Commission of the People's Republic of China, children should attend at least 6 routine physical examinations within the first 2 years after birth (27), with a community management rate of more than 80% (28). In addition, the Department of Child Health Care, CHCMU is the largest department that carries children's routine physical examinations in Chongqing, with an average annual outpatient volume of 110,000 in the past three years. Therefore, although this study was conducted through a hospital based convenient sample, it still had good community representation (29).
The sample sizes were calculated using a single proportion sample size estimating algorithm (30). The reference value for FA prevalence in 2009 was 3.5% from our 1999 survey, with an allowable error of 2.0%. The reference value in 2019 was 7.7% from our 2009 survey, with an allowable error of 2.5% (26). The confidence level was set as 95%, and the drop–out rate was predicted to be 10%. The calculations provided 362 and 485 as the minimum sample sizes for the two investigations, respectively.
Investigation and diagnosis procedure
The same survey design was adopted in 2009 and 2019. The diagnosis of FA was performed by questionnaire, skin prick test (SPT), food avoidance test and OFC in sequence. The entire procedure was carried out by trained pediatricians as previously described (26). In a word, SPT was performed in all eligible children after obtaining medical histories. Then, 2-weeks food elimination tests were carried out for those with positive histories or SPT (the mean wheal diameter was at least 3 mm greater than the negative control at 15 min). OFC was performed in the hospital by a trained pediatrician to diagnose FA in children who benefited from food elimination tests. The diagnosis procedure was in accordance with the EAACI guidelines (31). In this article, FHA was defined as any first–degree relative diagnosed with AD, FA, allergic asthma, or atopic rhinitis. And FS and FA were determined by positive SPT and OFC, respectively.
Data processing and statistical analysis
All data were analyzed by SPSS 26.0 (IBM Corp). The chi–square test was used to analyze the demographic characteristics (age, sex, and positive FHA proportion), FS rate and FA prevalence between the two investigations. The associations among FHA, FS and FA were analyzed by binary Logistic regression according to the mediating effect model of the categorical variables (32, 33). The standard tri–variate mediation model (Figure 1) was adopted to build three binary Logistic regression models: (1) regression of the independent variable (X) on the dependent variable (Y) (Model 1), (2) regression of X on the mediator (M) (Model 2), and (3) regression of X and M on Y (Model 3). We applied the method proposed by Sobel (34) to calculate the Z value of the mediation model, and the P value was obtained from the Z table. Statistical significance was defined using a two–sided significance level of α = 0.05, and a P value < 0.05 or an absolute Z value >1.96 was considered statistically significant.
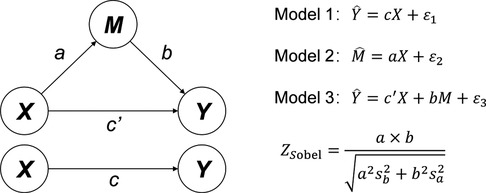
Figure 1. Standard tri–variate mediating effect model and Logistic regression models. X: Independent variable. Y: Dependent variable. M: Mediator.
Participants who failed to follow the entire diagnostic procedure were regarded as drop–outs (all drop–outs in these two investigations did not perform OFC). The demographic characteristics were compared between participants following the diagnostic procedure and drop–outs. The FA prevalence has been reported referring to the intention–to–treat principle (35) as previously described (26). To further discuss the associations among FHA, FS and FA, multiple imputations were performed to create five complete datasets in this study. Each dataset was analyzed separately, and the results were the average of the five datasets. Additionally, the results before and after imputation were compared.
Results
Demographic characteristics
In 2009 and 2019, 401 and 513 participants were included with 19 (4.7%) and 10 (1.9%) participants dropping out for personal reasons, respectively. The proportion of participants under 1 year of age in the drop–outs was lower in 2009 (11/19 vs. 306/382, P = 0.04), while sex and positive FHA proportion in 2009 and the demographic characteristics in 2019 were not significantly different between these two groups. In addition, the results before and after multiple imputation were not significantly different, and the conclusions were consistent (results not shown).
Table 1 shows the demographic characteristics of the two investigations. In 2009 and 2019, 54.4% (218/401) and 52.2% (268/513) of the participants were male, and 79.1% (317/401) and 73.9% (379/513) of the participants were under 1 year of age, respectively. The age and sex in the two investigations were not significantly different (P = 0.52 and 0.07). From 2009 to 2019, the positive FHA proportion was 28.9% (116/401) in 2009, and 31.6% (162/513) in 2019, which did not change significantly in different groups, respectively (P > 0.05) (Figure 2A).
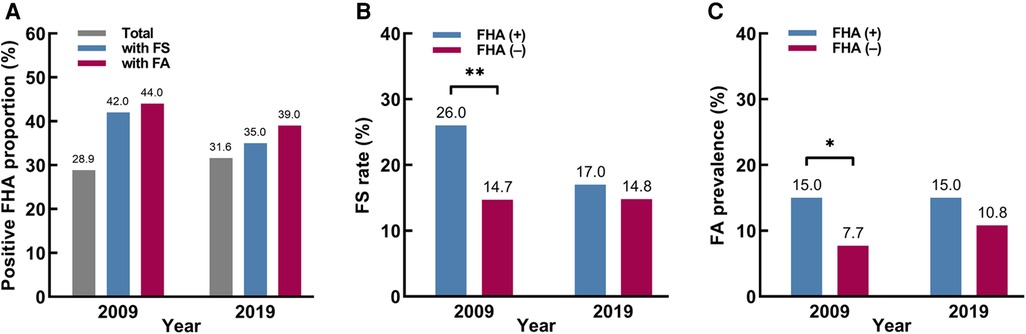
Figure 2. The proportions with a positive family history of atopic diseases (FHA), food sensitization (FS) rates and food allergy (FA) prevalence among different participants. (A) The positive FHA proportions among different participants. (B) The FS rates based on FHA. (C) The FA prevalence based on FHA. * P < 0.05. ** P < 0.01.
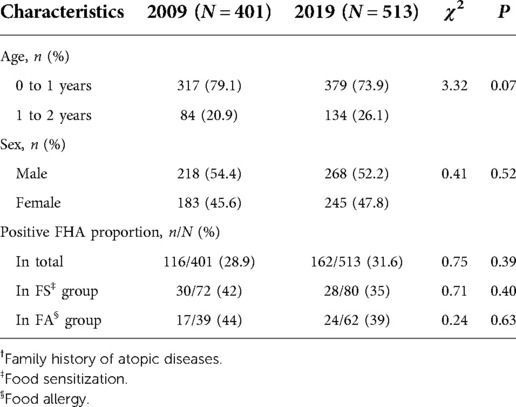
Table 1. Demographic characteristics, and the positive FHA† proportions in different participants in 2009 and 2019.
FS rate and FA prevalence
Table 2 shows the FS rates and FA prevalence based on FHA in 2009 and 2019. The FS rate tended to be decreasing in FHA (+) group (26% to 17%, P = 0.08) but stabilized in FHA (–) group (14.7% to 14.8%, P = 0.98). And the FA prevalence tended to be increasing in FHA (–) group (7.7% to 10.8%, P = 0.18) but stabilized in FHA (+) group (15% to 15%, P = 0.97). Then, the stratified analysis by year showed that in 2009, the FS rate and FA prevalence were higher in FHA (+) group than in FHA (–) group (FS rate: 26% vs. 14.7%, P = 0.008 and FA prevalence: 15% vs. 7.7%, P = 0.03). However, in 2019, the same trends still existed but lost significance (Figures 2B,C).
In addition, the FS rate in participants with FA decreased significantly (37/39 to 44/62, P = 0.003). Notably, all the allergens which caused symptoms were tested by SPT with negative results.
The associations among FHA, FS and FA
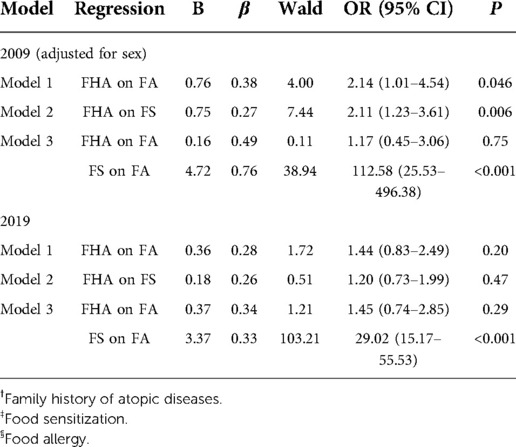
FHA, FS and FA were included in the standard tri–variate mediation model as X, M and Y, respectively. Before analysis, age and sex were examined between different FHA groups to determine whether to be included in the model for adjustment (Supplementary Table S1). Model 1 and Model 2 showed that in 2009, FHA had significant associations with FA and FS (P = 0.046, P = 0.006), respectively. However, the association between FHA and FA in Model 1 lost significance after adding FS into the model (Model 3). The Z value was 2.54 with a P value of 0.011, indicating that FS had a complete mediating effect on the association between FHA and FA in 2009; in other words, the association between FHA and FA was mediated by FS. Interestingly, in 2019, Model 1 and Model 2 showed that the associations between FHA and FA or FS lost significance (Table 3).
Discussion
Principle findings
Epidemiological studies on atopic diseases show that the overall incidence has been increasing in the past 20 to 30 years (36, 37), which will increase the proportion of FHA–positive children. According to our data, infants with positive FHA accounted for 28.9% in 2009 and 31.6% in 2019. It seemed that the positive FHA proportion in China was lower than in Western countries (58.6% to 69.0%) (24, 38). But the proportion could be lower than the actual level due to the lack of allergists and insufficient knowledge about atopic diseases in general pediatricians and parents in China (39), which may lead to missed diagnoses of atopic diseases. Interestingly, although without significance, the increasing trends in positive FHA proportion appeared to be inconsistent with the decreasing trends in FS rate. It suggested that in some ways, the contributions of FHA to FS and FA may have changed over time.
More importantly, we found that the association between FHA and FA was completely mediated by FS, and the contributions of FHA to FS and FA tended to be stabilized or even diminished. The role of FHA in FS and FA is already confirmed (7, 8), but the associations among FHA, FS and FA remain unclear. Thus, we conducted a mediating effect analysis to further examine the associations from a population perspective. As we speculated, the results showed that FS had a complete mediating effect on the association between FHA and FA in 2009, which means FHA mainly increases IgE–mediated FA. It is consistent with a few other studies (15, 16), which can be explained by the fact that FHA can promote the differentiation of immune system towards Th2 type (12), culminating in the production of IgE (40).
Interestingly, the associations between FHA and FS or FA lost significance in 2019, suggesting that the contributions of FHA to FS and FA could have changed over time. Moreover, our 2009 data showed that the FS rate and FA prevalence in FHA (+) group were significantly higher than in FHA (–) group, but the differences also lost significance in 2019 (Figures 2B,C). This seemed to be due to the decreasing trends in FS rate in FHA (+) group and increasing trends in FA prevalence in FHA (–) group. These results suggest that there is a need to re–evaluate the role of FHA in the development of FS and FA. In recent years, some studies have already focused on the effect of FHA on FS and FA. In 2013, Goldberg et al (20). reported that parental atopy (positive SPT) was not a risk factor for persistent IgE–mediated cow's milk allergy in children. Then, in 2015, Nissen et al. (21) found that isolated FHA was not a strong predictor for sensitization and allergic symptoms from childhood until early adulthood. Later, Gupta et al. (22) showed that in FA families, only a small proportion of siblings were both sensitized and symptomatic to food (13.6%), while asymptomatic sensitization was more common (53.0%). Most recently, Keet et al. (23) indicated that family history of peanut allergy did not confer substantial risk in the absence of moderate–severe eczema (only 1% of infants with FHA and no eczema had peanut allergy). All these studies suggest that FHA alone is not sufficient to predict FS or FA in children, which is basically consistent with our study. Moreover, our results further suggested it may not be that FHA could not predict FA, but that the contributions changed to no longer sufficient for prediction. Therefore, it is reasonable to assume that the contributions of FHA to FS and FA could have stabilized or even diminished.
Furthermore, our results showed that FA was significantly associated with FS both in 2009 and 2019 (Table 3), suggesting that the change in the contributions of FHA was mainly between FHA and FS rather than between FS and FA. The change could be absolute (the contributions of genetic factors decreased) or relative (the contributions of environmental factors increased). That means some robust environmental factors could have influenced the production of IgE, and concealed the contribution of genetic factors. For example, urbanization has large–scale effects on the human microbiota (41), and some bacteria (staphylococcus aureus, etc.) do release virulence factors as superantigens to promote IgE production (42), which will undoubtedly cause sensitization and allergy (43–45). Our results showed that the FS rate in FA group decreased significantly, and given the continuous increase in FA incidence and the effects of environmental factors (18, 46), we prefer that the change is more likely to be a relative change caused by environmental factors. Therefore, the contributions of environmental factors will be interesting to elucidate.
Limitations
This study had some limitations. First, this study did not conduct stratified analysis on FHA by different relatives or atopic diseases, which could bring some bias in using FHA as an indicator of genetic factors. Second, although selection bias may be not significant as the children recruited were attending for a routine physical examination, it could not be ruled out because CHCMU is the largest children's medical center in western China, which may attract children from families with better economic conditions in the community. Third, the sample size of our study was relatively small, and the possibility of a type II error could not be definitely excluded. Finally, the environment of the children may have changed in the past ten years, resulting in potential bias.
Conclusion
In conclusion, our data demonstrated that the association between FHA and FA was completely mediated by FS, and the contributions of FHA to FS and FA tended to be stabilized or even diminished, which is more likely to be a relative change caused by the increasing contributions of environmental factors. This is a meaningful finding that FHA alone could no longer be enough to screen infants at high risk for FS or FA. Allergists may need to re–evaluate the role of FHA in FS and FA, and combine FHA with environmental factors to screen high–risk children. Notably, the present findings were preliminary, which should be reproduced in other studies using different population before the conclusion is established.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of Children's Hospital of Chongqing Medical University (File NO. 2019-103). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
HF and ZM conceptualized and designed the study, carried out the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript. LC, RX, JW, and JC designed the data collection instruments, collected data, and reviewed and revised the manuscript. HL and YH conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors gratefully acknowledge the participants for their cooperation. We also appreciate our colleagues for their long–term attention to food allergy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.967930/full#supplementary-material.
References
1. Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. (2018) 141(1):41–58. doi: 10.1016/j.jaci.2017.11.003
2. Lieberman JA, Greenhawt M, Nowak–Wegrzyn A. The environment and food allergy. Ann Allergy Asthma Immunol. (2018) 120(5):455–7. doi: 10.1016/j.anai.2018.01.010
3. Warren CM, Jiang J, Gupta RS. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep. (2020) 20(2):6. doi: 10.1007/s11882-020-0898-7
4. Suaini NHA, Wang Y, Soriano VX, Martino DJ, Allen KJ, Ellis JA, et al. Genetic determinants of paediatric food allergy: a systematic review. Allergy. (2019) 74(9):1631–48. doi: 10.1111/all.13767
5. Hirota T, Nakayama T, Sato S, Yanagida N, Matsui T, Sugiura S, et al. Association study of childhood food allergy with genome–wide association studies–discovered loci of atopic dermatitis and eosinophilic esophagitis. J Allergy Clin Immunol. (2017) 140(6):1713–6. doi: 10.1016/j.jaci.2017.05.034
6. Visscher PM, Hill WG, Wray NR. Heritability in the genomics era–concepts and misconceptions. Nat Rev Genet. (2008) 9(4):255–66. doi: 10.1038/nrg2322
7. Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol. (2000) 106(1 Pt 1):53–6. doi: 10.1067/mai.2000.108105
8. Yuenyongviwat A, Koosakulchai V, Treepaiboon Y, Jessadapakorn W, Sangsupawanich P. Risk factors of food sensitization in young children with atopic dermatitis [published online ahead of print, 2021 jan 2]. Asian Pac J Allergy Immunol. (2021). doi: 10.12932/AP-250820-0946
9. Tsai HJ, Kumar R, Pongracic J, Liu X, Story R, Yu Y, et al. Familial aggregation of food allergy and sensitization to food allergens: a family–based study. Clin Exp Allergy. (2009) 39(1):101–9. doi: 10.1111/j.1365-2222.2008.03111.x
10. Trotter TL, Martin HM. Family history in pediatric primary care. Pediatrics. (2007) 120(Suppl 2):S60–5. doi: 10.1542/peds.2013-1032D
11. Sears MR, Chow CM, Morseth DJ. Serum total IgE in Normal subjects and the influence of a family history of allergy. Clin Allergy. (1980) 10(4):423–31. doi: 10.1111/j.1365-2222.1980.tb02125.x
12. Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen–specific T–cell memory in atopic and Normal children. Lancet. (1999) 353(9148):196–200. doi: 10.1016/S0140-6736(98)05104-6
13. Marone G, Granata F, Pucino V, Pecoraro A, Heffler E, Loffredo S, et al. The intriguing role of interleukin 13 in the pathophysiology of asthma. Front Pharmacol. (2019) 10:1387. doi: 10.3389/fphar.2019.01387
14. Agache I, Song Y, Rocha C, Beltran J, Posso M, Steiner C, et al. Efficacy and safety of treatment with dupilumab for severe asthma: a systematic review of the EAACI guidelines–recommendations on the use of biologicals in severe asthma. Allergy. (2020) 75(5):1058–68. doi: 10.1111/all.14268
15. Böhme M, Wickman M, Lennart Nordvall S, Svartengren M, Wahlgren CF. Family history and risk of atopic dermatitis in children up to 4 years. Clin Exp Allergy. (2003) 33(9):1226–31. doi: 10.1046/j.1365-2222.2003.01749.x
16. Schnabel E, Sausenthaler S, Schaaf B, Schäfer T, Lehmann I, Behrendt H, et al. Prospective association between food sensitization and food allergy: results of the LISA birth cohort study. Clin Exp Allergy. (2010) 40(3):450–7. doi: 10.1111/j.1365-2222.2009.03400.x
17. Peters RL, Koplin JJ, Allen KJ, Lowe AJ, Lodge CJ, Tang MLK, et al. The prevalence of food sensitization appears not to have changed between 2 Melbourne cohorts of high–risk infants recruited 15 years apart. J Allergy Clin Immunol Pract. (2018) 6(2):440–8. doi: 10.1016/j.jaip.2017.11.018
18. Carter CA, Frischmeyer–Guerrerio PA. The genetics of food allergy. Curr Allergy Asthma Rep. (2018) 18(1):2. doi: 10.1007/s11882-018-0756-z
19. Cianferoni A. Non–IgE mediated food allergy. Curr Pediatr Rev. (2020) 16(2):95–105. doi: 10.2174/1573396315666191031103714
20. Goldberg M, Eisenberg E, Elizur A, Rajuan N, Rachmiel M, Cohen A, et al. Role of parental atopy in cow's Milk allergy: a population–based study. Ann Allergy Asthma Immunol. (2013) 110(4):279–83. doi: 10.1016/j.anai.2013.01.017
21. Nissen SP, Kjaer HF, Høst A, Nielsen J, Halken S. Can family history and cord blood IgE predict sensitization and allergic diseases up to adulthood? Pediatr Allergy Immunol. (2015) 26(1):42–8. doi: 10.1111/pai.12264
22. Gupta RS, Walkner MM, Greenhawt M, Lau CH, Caruso D, Wang X, et al. Food allergy sensitization and presentation in siblings of food allergic children. J Allergy Clin Immunol Pract. (2016) 4(5):956–62. doi: 10.1016/j.jaip.2016.04.009
23. Keet C, Pistiner M, Plesa M, Szelag D, Shreffler W, Wood R, et al. Age and eczema severity, but not family history, are major risk factors for peanut allergy in infancy. J Allergy Clin Immunol. (2021) 147(3):984–91. doi: 10.1016/j.jaci.2020.11.033
24. Koplin JJ, Allen KJ, Gurrin LC, Peters RL, Lowe AJ, Tang ML, et al. The impact of family history of allergy on risk of food allergy: a population–based study of infants. Int J Environ Res Public Health. (2013) 10(11):5364–77. doi: 10.3390/ijerph10115364
25. Bo P, Xing M, Hu Y. Discussion on early prediction of high risk of food allergy in children [in Chinese]. J Clin Pediatr. (2019) 37(12):936–9. doi: 10.3969/j.issn.1000-3606.2019.12.015
26. Ma Z, Chen L, Xian R, Fang H, Wang J, Hu Y. Time trends of childhood food allergy in China: three cross–sectional surveys in 1999, 2009, and 2019. Pediatr Allergy Immunol. (2021) 32(5):1073–9. doi: 10.1111/pai.13490
27. Circular of the General Office of the Ministry of Health on printing and distributing the National Standards for Children's Health Care (trial) [in Chinese]. 2010–01–05. http://www.nhc.gov.cn/cms–search/xxgk/getManuscriptXxgk.htm?id=45453
28. Li H. The principle and practice of pediatric primary care [in Chinese]. Beijing, China: People's Medical Publishing House (2016).
29. Chen J, Hu Y, Allen KJ, Ho MH, Li H. The prevalence of food allergy in infants in Chongqing, China. Pediatr Allergy Immunol. (2011) 22(4):356–60. doi: 10.1111/j.1399-3038.2011.01139.x
30. Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. (2013) 6(1):14–7.24834239
31. Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI Food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. (2014) 69(8):1008–25. doi: 10.1111/all.12429
32. Iacobucci D. Mediation analysis and categorical variables: the final frontier. J Consum Psychol. (2012) 22(4):582–94. doi: 10.1016/j.jcps.2012.03.006
33. Jie F, Zhonglin W, Minqiang Z. Mediation analysis of categorical variables [in Chinese]. J Psychol Sci. (2017) 40(02):471–7. doi: 10.16719/j.cnki.1671-6981.20170233
34. Sobel M. Asymptotic confidence intervals for indirect effects in strucutural equation models. Sociol Methodol. (1982) 13:290. doi: 10.2307/270723
36. Genuneit J, Standl M. Epidemiology of allergy: natural course and risk factors of allergic diseases. Handb Exp Pharmacol. (2021) 268:21–7. doi: 10.1007/164_2021_507
37. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: iSAAC phases one and three repeat multicountry cross–sectional surveys [published correction appears in lancet. Lancet. (2006) 368(9537):733–43. doi: 10.1016/S0140-6736(06)69283-0
38. Gabet S, Just J, Couderc R, Seta N, Momas I. Allergic sensitisation in early childhood: patterns and related factors in PARIS birth cohort. Int J Hyg Environ Health. (2016) 219(8):792–800. doi: 10.1016/j.ijheh.2016.09.001
39. Du X, Li Y. Development history and future prospect of allergic diseases[in Chinese]. Clin Med J. (2019) 17(1):14–7. doi: 10.3969/j.issn.1672-3384.2019.01.004
40. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL–4/IL–13 pathway in atopic diseases. Expert Rev Clin Immunol. (2017) 13(5):425–37. doi: 10.1080/1744666X.2017.1298443
41. McCall LI, Callewaert C, Zhu Q, Song SJ, Bouslimani A, Minich JJ, et al. Home chemical and microbial transitions across urbanization. Nat Microbiol. (2020) 5(1):108–15. doi: 10.1038/s41564-019-0593-4
42. Bachert C, Humbert M, Hanania NA, Zhang N, Holgate S, Buhl R, et al. Staphylococcus aureus and its IgE–inducing enterotoxins in asthma: current knowledge. Eur Respir J. (2020) 55(4):1901592. doi: 10.1183/13993003.01592-2019
43. Sørensen M, Klingenberg C, Wickman M, Sollid JUE, Furberg AS, Bachert C, et al. Staphylococcus aureus enterotoxin sensitization is associated with allergic poly–sensitization and allergic multimorbidity in adolescents. Allergy. (2017) 72(10):1548–55. doi: 10.1111/all.13175
44. Murrison LB, Brandt EB, Myers JB, Hershey GKK. Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest. (2019) 129(4):1504–15. doi: 10.1172/JCI124612
45. Tsilochristou O, du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, et al. Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. J Allergy Clin Immunol. (2019) 144(2):494–503. doi: 10.1016/j.jaci.2019.04.025
Keywords: children, family history of atopic diseases (FHA), food allergy (FA), food sensitization (FS), trends
Citation: Fang H, Ma Z, Chen L, Xian R, Wang J, Chen J, Li H and Hu Y (2022) Trends in the contributions of atopic family history to pediatric food sensitization and allergy. Front. Pediatr. 10:967930. doi: 10.3389/fped.2022.967930
Received: 28 June 2022; Accepted: 8 November 2022;
Published: 7 December 2022.
Edited by:
Pedro Gutierrez-Castrellon, Hospital General Dr. Manuel Gea Gonzalez, MexicoReviewed by:
Ana Karen Gutierrez Bautista, Independent Researcher, Mexico City, MexicoCarlos Jimenez Gutierrez, Hospital General Dr. Manuel Gea Gonzalez, Mexico
© 2022 Fang, Ma, Chen, Xian, Wang, Chen, Li and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Hu aHk0MjBAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Pediatric Immunology, a section of the journal Frontiers in Pediatrics
 Heping Fang
Heping Fang Zhuoying Ma
Zhuoying Ma Lin Chen
Lin Chen