
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr. , 20 September 2022
Sec. Pediatric Immunology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.962585
 Zhaoling Wang1†
Zhaoling Wang1† Qi Zheng1†
Qi Zheng1† Wenjie Xuan2
Wenjie Xuan2 Xisheng Xu1
Xisheng Xu1 Meiping Lu1*
Meiping Lu1* Jianqiang Wu1
Jianqiang Wu1 Lixia Zou1
Lixia Zou1 Yiping Xu1
Yiping Xu1 Xuefeng Xu1
Xuefeng Xu1Objective: To determine the short-term effectiveness safety of baricitinib in children with refractory and/or severe juvenile dermatomyositis (rsJDM) in a real-world setting.
Methods: This was a single-center retrospective study, including 20 children with rsJDM. They were all treated using baricitinib combined with steroids and other immunosuppressive agents. The childhood myositis assessment scale (CMAS) and PRINTO remission criteria were used to evaluate the disease severity and treatment outcome at 0, 4, 12, and 24 weeks after initiation of baricitinib.
Results: The skin rash improved in 95% of patients (19/20) at week 24, with a significant decrease of skin-DAS at weeks 12 (6.0 vs. 2.0, p < 0.05] and week 24 [6.0 vs. 1.0, p < 0.05) by median statistics. The CMAS score increased significantly at week 12 (41.0 [29.0, 44.0] vs. 46.0 [42.0, 52.0], p < 0.05) and week 24 (41.0 [29.0, 44.0] vs. 50.0 [45.0, 52.0], p < 0.05), as did the manual muscle testing (MMT)-8 score at week 24 (73.0 [610, 76.0] vs. 79.0 [77.0, 80.0], p < 0.05). At 24 weeks, the complete response (CR) and partial response (PR) were achieved in 75% (15/20) and 15% (3/20), respectively. The dose of corticosteroids (CS) decreased by 37% from the baseline (0.53 [0.42, 1.00] mg/kg) to week 12 (0.33 [0.18, 0.40] mg/kg) (p < 0.05), and by 49% at week 24 (p < 0.05). No serious side effects were observed.
Conclusion: Baricitinib combined with traditional immunosuppressants treatment was efficacious in rsJDM. Add-on therapy of baricitinib was helpful for tapering CS dose. No serious side effects were observed in this study.
Juvenile dermatomyositis (JDM) is an autoimmune disease characterized by inflammation of both the skin and muscles. The typical skin signs of JDM are Gottron papules, heliotrope rashes, and nailfold capillary changes (1). Other organs such as the lungs, the heart, and the gastrointestinal tract can also be involved, which would result in progression to severe JDM (sJDM). Although the prognosis of JDM has been remarkably improved by traditional drugs including corticosteroids (CS) and other immunosuppressive agents, 20% of cases still develop refractory JDM (rJDM) or even become unresponsive to the above therapy (2). Other therapies such as immune support and biologic therapies have yielded some promising results, but disease relapse, difficulty in tapering CS dose, and adverse effects (AEs) due to long-term CS use are still hindrances in the treatment course (3–6). Recent studies have found that interferons (IFNs) play a key role in dermatomyositis (DM) pathogenesis (7, 8). Elevated signatures of types I and II IFNs have been found in the tissues and cells of patients with DM (9–11), with signal transduction reliant on the Janus kinase (JAK)-signal transducers and activators of the transcription pathway. Given the presence of IFN dysregulation in the JDM, IFN-targeting therapies have been attempted. Janus kinase inhibitors such as tofacitinib (JAK1/3 inhibitor), ruxolitinib (JAK1/JAK2 inhibitor), and baricitinib (JAK1/2 inhibitor) were studied in patients with JDM (12–19). However, there are insufficient data about the effectiveness and safety of baricitinib in JDM, with only eight cases reported worldwide: three by Voyer et al. (15), four by Kim et al. (17), and one by Papadopoulou et al. (18). Here, we report on 20 cases with refractory and/or severe juvenile DM (rsJDM) that received baricitinib combined with tradition drugs in a real-world setting to evaluate its effectiveness and safety in a real-world setting.
We retrospectively analyzed 20 cases with rsJDM who received baricitinib for 12–24 weeks combined with CS and immunosuppressive agents between January 2019 and January 2022. The diagnosis of JDM was based on the Bohan and Peter classification (20). The study was approved by the Ethics Committee of Children’s Hospital of the Zhejiang University School of Medicine (IRB approval no. 2019-IRB-154). In total, twenty-two patients treated with baricitinib were included, and two patients were excluded. In total, one case was excluded due to incomplete clinical data, and the other was due to the loss of follow-up. Of the remaining patients, the median [interquartile range, IQR] value of the baricitinib dose was 0.05 [0.04, 0.09] mg/kg/day. The median time [IQR] from diagnostic to baricitinib onset was 16.5 [8.8, 35.3] months. Baricitinib was administered to 17 cases for 24 weeks, and to the other 3 for 12 weeks. The subjects were mean aged 7.8 ± 4.0 y, and their women-to-men ratio was 3:1. Clinical and laboratory data were analyzed at the baseline (week 0) and at weeks 4, 12, and 24 after baricitinib administration.
The inclusion criteria were: (i) diagnosis of JDM according to the Bohan and Peter classification and meeting the diagnostic criteria for rJDM and/or sJDM, (ii) all the patients with JDM received baricitinib (at least 12 weeks), (iii) follow-up at least 24 weeks after the initiation of baricitinib.
The exclusion criteria were: (i) myositis overlapping with other autoimmune diseases, (ii) evidence of any other acute or chronic infectious disease, (iii) history of malignancy in any organ system, (iv) incomplete clinical and laboratory data, and (v) history of hypersensitivity to baricitinib.
Patients who met the diagnostic criteria for rJDM and/or sJDM, rJDM were defined by inadequate response to glucocorticoids and at least one other first-line immunosuppressive agent (e.g., azathioprine, methotrexate, mycophenolate mofetil, cyclosporine, tacrolimus, cyclophosphamide, leflunomide, or IVIg) (6). The sJDM was referring to young-onset (age < 1 year), severe muscle involvement (CMAS score < 15 or MMT-8 score < 30) or severe skin involvement (ulcerative skin disease), or involvement of other organs and systems, such as gastrointestinal and cardiac manifestations, interstitial lung disease, development of calcinosis, or need intensive care unit management (21). The ILD was defined based on the findings of the chest radiography and the chest CT scans that were evaluated by radiologists (22, 23).
Skin disease activity was assessed using the skin disease activity score (skin-DAS, which ranges from 0 to 9) (24). Muscle strength was assessed using the childhood myositis assessment scale (CMAS, score range 0–52), and the Manual Muscle Testing-8 scale (MMT-8, score range 0–80). A complete response (CR) was defined by both the Paediatric Rheumatology International Trials Organisation (PRINTO) remission criteria and inactive skin-DAS (15). The PRINTO remission comprised meeting at least three of the four following criteria: creatine kinase ≤ 150 U/L, CMAS score ≥ 48, MMT-8 score ≥ 78, and physicians’ global activity of ≤2 cm on a visual analog scale ranging from 1 to 10 cm (20). Inactive skin-DAS was defined by a score ≤ 1/9 without cutaneous ulcerations or erythema. A partial response (PR) was defined as improvements in CMAS/MMT-8 score and skin-DAS, allowing CS dosage tapering of at least 50% from the initial dosage, without combining with a new immunosuppressive agent (25).
The anti-myositis antibody spectrum Ig-G detection kit (Lot No.: DL1530-1601-4G) was applied to detect myositis antibody (Omon Company of Germany). The detection kit contains 15 anti-myositis antibodies (Mi-2α, Mi-2β, TIF1γ, MDA5, NXP2, SAE1, Ku, PMScl100, PM-Scl75, Jo-1, SRP, PL-7, PL-12, EJ, and OJ, Ro-52). Test results on the EUROBlotMaster II. Staining intensity > 15 is positive (EURO Line Scan).
Statistical analyses were performed using IBM SPSS Statistics (version 25) and GraphPad Prism (version 8.0.1) software. The data were analyzed using the Friedman test. The continuous variables were presented as medians with interquartile ranges or means with standard deviations. The categorical variables were presented as the number of patients and percentages. A probability value of p < 0.05 was considered significant.
All patients (n = 20) had skin involvement, namely, recurrent or refractory skin rashes (n = 19, 95%) and calcinosis (n = 3). The median skin-DAS was 6.0 [4.0, 7.3]. In total, seven patients had muscle involvement. The median CMAS score was 41.0 [29.0, 44.0], while the median MMT-8 score was 73.0 [61.0, 76.0] (Table 1, Supplementary material). The median creatine kinase was 93.5 [52.5, 178.0] U/L. In total, five patients had interstitial lung disease (ILD), and one patient who received baricitinib at the initial onset of JDM (P9) had complicated macrophage activation syndrome (MAS) (Table 2). At the baseline, patients received CS (n = 20), intravenous methylprednisolone (IVMP, n = 1), intravenous immune globulin (IVIG, n = 9), methotrexate (MTX, n = 15), hydroxychloroquine (HCQ, n = 2), tacrolimus (n = 2), mycophenolate mofetil (MMF, n = 1), cyclosporine A (CsA, n = 1), thalidomide (n = 1), infliximab (n = 1), and tocilizumab (n = 1).
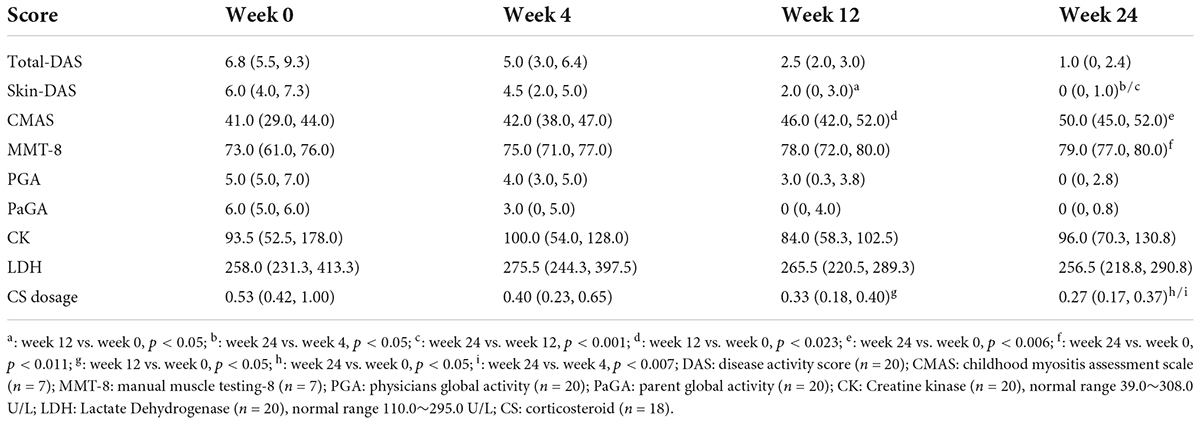
Table 1. Effectivenessand daily dose of corticosteroids (CS) in patients with baricitinib treatment.
The skin rash improved in 9 patients (45%) at week 4, in 15 (75%) at week 12, and in 19 (95%) at week 24. Compared with the baseline, skin-DAS was significantly decreased at week 12 [2.0 (0, 3.0) vs. 6.0 (4.0, 7.3), p < 0.05] and week 24 [0 (0, 1.0) vs. 6.0 (4.0, 7.3), p < 0.05]. Compared with the baseline, the CMAS score was significantly increased at week 12 [46.0 (42.0, 52.0) vs. 41.0 (29.0, 44.0), p < 0.023] and week 24 [50.0 (45.0, 52.0) vs. 41.0 (29.0, 44.0), p < 0.006], as was the MMT-8 score at week 24 [79.0 (77.0, 80.0) vs. 73.0 (61.0, 76.0), p < 0.011].
The CR and PR were achieved in 19 of 20 patients at 24 weeks after baricitinib therapy, including 15 (79%) with CR (Figure 1). The X-rays indicated that calcinosis was improved in one patient (P3) and stabilized in two (P2 and P12). ILD was improved in four patients (P4, P5, P9, and P17) and stabilized in one (P14). MAS was resolved in P9.
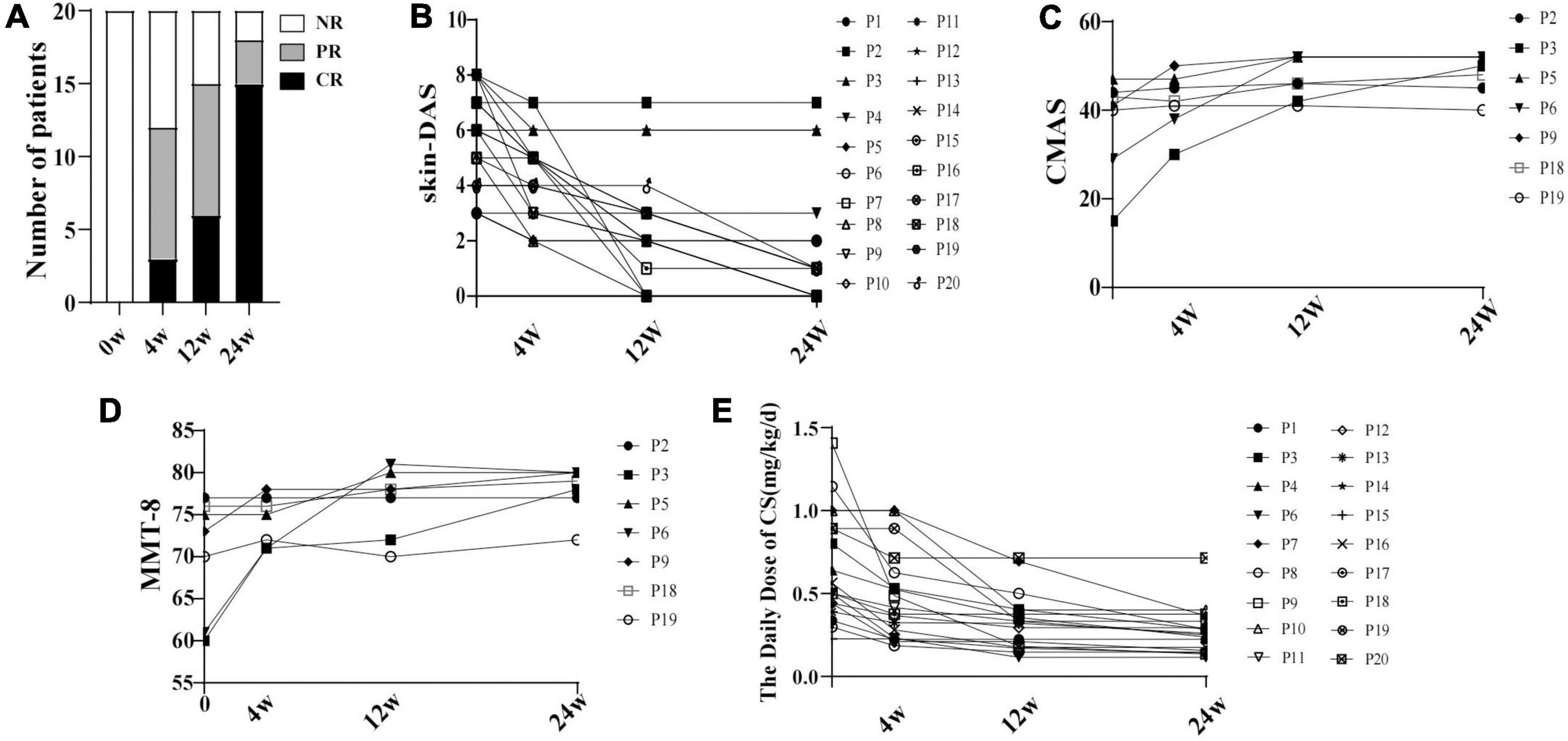
Figure 1. The disease activity changing in 20 patients with JDM with baricitinib treatment. (A) Proportion of the 20 patients with JDM achieving CR and/or PR within 24 weeks at different follow-up times with baricitinib treatment. (B) Decrease of skin-DAS of 20 patients. (C) Increase of CMAS of 7 patients. (D) Increase of MMT of 7 patients. (E) Decrease of the daily dose of CS. NR, non-response; CR, complete response; PR, partial response; DAS, disease activity score; p, patient; CMAS, childhood myositis assessment scale.
Compared with the baseline, the daily CS dose was decreased by 37% at week 12 [0.33 (0.18, 0.40) vs. 0.53 (0.42, 1.0), P < 0.05], and by 49% at week 24 [0.27 (0.17, 0.37) vs. 0.53 (0.42, 1.0), P < 0.05] in patients who achieved CR and PR. Comparing to the week 4, the CS dosage was decreased significantly at week 24 [0.27 (0.17, 0.37) vs. 0.40 (0.23, 0.65), P < 0.007] (Figure 1).
There were 26 AEs in 10 patients (Table 3), most of which manifested as mild upper airway infections. The P13 had a slight increase in liver enzymes (ALT from 57 to 85 U/L) after taking the baricitinib without the addition of other medications, or with other muscle enzymes increasing, or clinical disease activity. The P1 experienced increased creatinine after baricitinib initiation (creatinine from 60 to 71 U/L), but not yet to the point of renal dysfunction. Hospitalization and temporary discontinuation of baricitinib were required in only one patient (P6, who had herpes zoster infection). There were no venous thrombosis, malignancy, and fatalities recorded in this study.
Our study included the largest number of JDM cases treated with baricitinib to date. This study included 19 cases with a recurrent or refractory rashes that are dependent on CS therapy. Due to the expense and the lack of medical insurance, most patients in our study received doses of baricitinib that were lower than those in previous studies (17, 26). Our results showed skin rash was improved in 95% of the patients and all patients who had muscle weakness at the entry were improved at week 24. The skin-DAS and CMAS were significantly changed at week 12 and week 24, the MMT-8 was significantly improved at week 24. The CR and PR were achieved in 90% of the patients at week 24 after the baricitinb therapy.
Voyer et al. found that a new-onset JDM case with severe skin ulcerations improved to CR after 1.7 months of baricitinib initiation (15). Another previous study reported that skin rash was improved in four cases with refractory JDM after 4 weeks or later (17). Almost half of our cases were improved at week 4, while significant improvement in skin rash was noted at week 12 in skin-DAS (P < 0.05). These observations indicate that the baricitinib is clinically beneficial for skin lesions.
Our case series included seven patients with muscle involvement: six with mild muscle weakness and one (P3) with severe muscle weakness. The reason for a low proportion of muscle involvement may be the result of a long period of traditional treatment. In addition, P11 was diagnosed as clinically amyopathic dermatomyositis (CADM) based on Sontheimer criteria (27). A previous case report found that a JAK inhibitor was effective for muscle weakness in four patients who received baricitinib (15, 16, 28). We found significant differences in CMAS and MMT-8 scores after treatment. Our results indicated that baricitinib improved muscle weakness and P3 was markedly improved at 4 weeks after baricitinib treatment.
A previous study proposed that baricitinib may be helpful for tapering the CS dose. In some case reports, a JAK inhibitor may have even more beneficial when used alone (12). In this study, the daily median CS dosage decreased from 0.53 to 0.27 mg/kg/d (P < 0.05) after treatment in patients who got a response. The findings suggest that the baricitinib was effective, was fast acting, and was helpful in tapering CS dosage.
Calcinosis is a therapeutic challenge in JDM. There are previous case reports of calcinosis improving or stabilizing in four patients who received a JAK inhibitor, including two who were also treated with baricitinib (13, 15, 18). Our study found that two of three cases improved, while the third stabilized; baricitinib was effective for recurrent rash, and even calcinosis.
In recent years, some case reports have indicated that a JAK inhibitor is highly effective in treating JDM-associated ILD, with a combined effect of IFN signaling down-regulation and reduced expressions of proinflammatory cytokines (13, 16, 28–32). Five of our cases were complicated with ILD, stabilized (n = 2), improved (n = 2), or were resolved (n = 1) after baricitinib therapy, which manifested by HRCT or clinical improvement. While baricitinib might be an effective therapy for ILD, the number of cases was small and so further research is needed.
Interference with IFN α/β expression could control catastrophic hyper inflammation in MAS (33). There was no use of the baricitinib in patients with JDM with MAS, but given the successful application in hemophagocytic lymphohistiocytosis (HLH) (34, 35), we decided to apply it in JDM-related MAS. Comprehensive treatment that included IVMP, IVIG, tocilizumab, and baricitinib led to disease remission in one patient (P9) who experienced the cytokine storm, which suggests that baricitinib is an effective treatment for MAS in the real world.
Baricitinib was found to be well tolerated and safe in previous studies (18, 29). The most common AE in our study was a mild respiratory infection. One patient infected with herpes zoster had to temporarily suspend baricitinib use.
At the 24th week, P2 and P5 were classified as “NR,” the reason may be: P2 was a severe case with refractory skin ulcerations and calcinosis, however, because the patient did not have medical insurance, baricitinib was only used for 12 weeks, short duration of use may be the reason why this patient did not respond to it; P5 entered add-on treatment with baricitinib because of a persistent facial rash, but he was not covered by health insurance, so the patient agreed to receive a relatively small dose of baricitinib at 0.04 mg/kg/d.
Our study had some limitations. It was a single-center study with a short observation period, and it was carried out in a routine clinical practice situation, meaning that the effects of basic and combined medications may have influenced the observed effectiveness of the baricitinib. And as shown in Table 2, many of the patients used substandard dose and dosing intervals of baricitinib below the recommended dose based on weight and renal function (36). Besides, the lack of some critical clinical information did not allow us to examine the American College of Rheumatology/European League Against Rheumatism response criteria in JDM (37). We also did not measure the type I IFN levels and IFN response gene signatures of the patients.
This study has indicated that baricitinib combined with CS and other immunosuppressants is effective and safe for refractory or severe patients with JDM, especially in recurrent skin rashes. Baricitinib was helpful for tapering the daily CS dose in patients. We also observed baricitinib to be effective in ILD, MAS, and calcinosis in some patients. However, a multicenter study with a longer observation period needs to be performed in the future.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Children’s Hospital of Zhejiang University School of Medicine. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
ZW was responsible for the collection of clinical information, statistical analyses, figures, data interpretation, and manuscript preparation. QZ was responsible for critical review of the statistical analyses and the manuscript. XSX, ZW, QZ, WX, ML, JW, LZ, YX, and XFX assisted in the clinical data collection. ML is the project leader of the study, involved in the conceptualization of the project, the study design, and preparation of the manuscript. All authors read and approved the final manuscript.
This work was supported by the National Science Foundation of China (No. 82070027).
We are grateful for all participating patients and their families.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.962585/full#supplementary-material
1. Barut K, Aydin POA, Adrovic A, Sahin S, Kasapcopur O. Juvenile dermatomyositis: a tertiary center experience. Clin Rheumatol. (2017) 36:361–6. doi: 10.1007/s10067-016-3530-4
2. Ruperto N, Pistorio A, Oliveira S, Zulian F, Cuttica R, Ravelli A, et al. Prednisone versus prednisone plus ciclosporin versus prednisone plus methotrexate in new-onset juvenile dermatomyositis: a randomised trial. Lancet. (2016) 387:671–8. doi: 10.1016/S0140-6736(15)01021-1
3. de Inocencio J, Enríquez-Merayo E, Casado R, González-Granado LI. Subcutaneous Immunoglobulin in refractory juvenile dermatomyositis. Pediatrics. (2016) 137:e20153537. doi: 10.1542/peds.2015-3537
4. Rouster-Stevens KA, Ferguson L, Morgan G, Huang C, Pachman LM. Pilot study of etanercept in patients with refractory juvenile dermatomyositis. Arthrit Care Res. (2014) 66:783–7. doi: 10.1002/acr.22198
5. Wang C. Successful treatment of refractory juvenile dermatomyositis with adalimumab. J Clin Rheumatol Pract Rep Rheumat Musculosk. (2017) 23:174–5. doi: 10.1097/RHU.0000000000000514
6. Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthr Rheumat. (2013) 65:314–24. doi: 10.1002/art.37754
7. Jiang J, Zhao M, Chang C, Wu H, Lu Q. Type I interferons in the pathogenesis and treatment of autoimmune diseases. Clin Rev Allerg Immu. (2020) 59:248–72. doi: 10.1007/s12016-020-08798-2
8. Kim H, Gunter-Rahman F, McGrath JA, Lee E, de Jesus AA, Targoff IN, et al. Expression of interferon-regulated genes in juvenile dermatomyositis versus Mendelian autoinflammatory interferonopathies. Arthr Res Ther. (2020) 22:69. doi: 10.1186/s13075-020-02160-9
9. Kim H. Updates on interferon in juvenile dermatomyositis: pathogenesis and therapy. Curr Opin Rheumatol. (2021) 33:371–7. doi: 10.1097/BOR.0000000000000816
10. Moneta GM, Pires Marafon D, Marasco E, Rosina S, Verardo M, Fiorillo C, et al. Muscle expression of type I and type II interferons is increased in juvenile dermatomyositis and related to clinical and histologic features. Arthr Rheumatol. (2019) 71:1011–21. doi: 10.1002/art.40800
11. Gitiaux C, Latroche C, Weiss-Gayet M, Rodero MP, Duffy D, Bader-Meunier B, et al. Myogenic progenitor cells exhibit type I interferon-driven proangiogenic properties and molecular signature during juvenile dermatomyositis. Arthr Rheumatol. (2018) 70:134–45. doi: 10.1002/art.40328
12. Yu Z, Wang L, Quan M, Zhang T, Song H. Successful management with Janus kinase inhibitor tofacitinib in refractory juvenile dermatomyositis: a pilot study and literature review. Rheumatology. (2021) 60:1700–7. doi: 10.1093/rheumatology/keaa558
13. Sabbagh S, Almeida de Jesus A, Hwang S, Kuehn HS, Kim H, Jung L, et al. Treatment of anti-MDA5 autoantibody-positive juvenile dermatomyositis using tofacitinib. Brain J Neurol. (2019) 142:e59. doi: 10.1093/brain/awz293
14. Quintana-Ortega C, Remesal A, Ruiz de Valbuena M, de la Serna O, Laplaza-González M, Álvarez-Rojas E, et al. Fatal outcome of anti-MDA5 juvenile dermatomyositis in a paediatric COVID-19 patient: a case report. Mod Rheumatol Case Rep. (2021) 5:101–7. doi: 10.1080/24725625.2020.1832755
15. Voyer TL, Gitiaux C, Authier F, Bodemer C, Melki I, Quartier P, et al. JAK inhibitors are effective in a subset of patients with juvenile dermatomyositis. Rheumatology. (2021) 60:5801–8. doi: 10.1093/rheumatology/keab116
16. Heinen A, Schnabel A, Brück N, Smitka M, Wolf C, Lucas N, et al. Interferon signature guiding therapeutic decision making: ruxolitinib as first-line therapy for severe juvenile dermatomyositis? Rheumatology. (2021) 60:e136. doi: 10.1093/rheumatology/keaa657
17. Kim H, Dill S, O’Brien M, Vian L, Li X, Manukyan M, et al. Janus kinase (JAK) inhibition with baricitinib in refractory juvenile dermatomyositis. Ann Rheum Dis. (2021) 80:406–8. doi: 10.1136/annrheumdis-2020-218690
18. Papadopoulou C, Hong Y, Omoyinmi E, Brogan PA, Eleftheriou D. Janus kinase 1/2 inhibition with baricitinib in the treatment of juvenile dermatomyositis. Brain J Neurol. (2019) 142:e8. doi: 10.1093/brain/awz005
19. Aeschlimann FA, Frémond M, Duffy D, Rice GI, Charuel J, Bondet V, et al. A child with severe juvenile dermatomyositis treated with ruxolitinib. Brain J Neurol. (2018) 141:e80. doi: 10.1093/brain/awy255
20. Lazarevic D, Pistorio A, Palmisani E, Miettunen P, Ravelli A, Pilkington C, et al. The PRINTO criteria for clinically inactive disease in juvenile dermatomyositis. Ann Rheum Dis. (2013) 72:686–93. doi: 10.1136/annrheumdis-2012-201483
21. Hinze CH, Oommen PT, Dressler F, Urban A, Weller-Heinemann F, Speth F, et al. Development of practice and consensus-based strategies including a treat-to-target approach for the management of moderate and severe juvenile dermatomyositis in Germany and Austria. Pediatr Rheumatol Online J. (2018) 16:40. doi: 10.1186/s12969-018-0257-6
22. Long K, Danoff SK. Interstitial lung disease in polymyositis and dermatomyositis. Clin Chest Med. (2019) 40:561–72. doi: 10.1016/j.ccm.2019.05.004
23. Sato S, Uejima Y, Nanbu M, Suganuma E, Takano T, Tanaka R, et al. Clinical analysis and outcome of interstitial lung disease complicated with juvenile dermatomyositis and juvenile polymyositis. Mod Rheumatol. (2017) 27:652–6. doi: 10.1080/14397595.2016.1231102
24. Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthr Rheumat. (2003) 49:7–15.
25. Ruperto N, Pistorio A, Ravelli A, Rider LG, Pilkington C, Oliveira S, et al. The Paediatric rheumatology international trials organisation provisional criteria for the evaluation of response to therapy in juvenile dermatomyositis. Arthr Care Res. (2010) 62:1533–41.
26. Ohmura S, Yamabe T, Naniwa T. Successful dose escalation of tofacitinib for refractory dermatomyositis and interstitial lung disease with anti-melanoma differentiation-associated gene 5 antibodies. Mod Rheumatol Case Rep. (2021) 5:76–81. doi: 10.1080/24725625.2020.1816674
27. Sontheimer RD. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin. (2002) 20:387–408. doi: 10.1016/s0733-8635(02)00021-9
28. Paik JJ, Casciola-Rosen L, Shin JY, Albayda J, Tiniakou E, Leung DG, et al. Study of tofacitinib in refractory dermatomyositis: an open-label pilot study of ten patients. Arthr Rheumatol. (2021) 73:858–65. doi: 10.1002/art.41602
29. Wendel S, Venhoff N, Frye BC, May AM, Agarwal P, Rizzi M, et al. Successful treatment of extensive calcifications and acute pulmonary involvement in dermatomyositis with the janus-kinase inhibitor tofacitinib – a report of two cases. J Autoimmun. (2019) 100:131–6. doi: 10.1016/j.jaut.2019.03.003
30. Hosokawa Y, Oiwa H. A case of refractory interstitial lung disease in anti-MDA5-positive dermatomyositis that improved after switching to tofacitinib. J Clin Rheumatol Pract Rep Rheumat Musculoskel. (2020) 27:S661–2. doi: 10.1097/RHU.0000000000001645
31. Kurasawa K, Arai S, Namiki Y, Tanaka A, Takamura Y, Owada T, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology. (2018) 57:2114–9. doi: 10.1093/rheumatology/key188
32. Wu W, Guo L, Fu Y, Wang K, Zhang D, Xu W, et al. Interstitial lung disease in anti-MDA5 positive dermatomyositis. Clin Rev Allerg Immu. (2021) 60:293–304. doi: 10.1007/s12016-020-08822-5
33. Verweyen E, Holzinger D, Weinhage T, Hinze C, Wittkowski H, Pickkers P, et al. Synergistic signaling of TLR and IFNα/β facilitates escape of IL-18 expression from endotoxin tolerance. Am J Resp Crit Care. (2020) 201:526–39. doi: 10.1164/rccm.201903-0659OC
34. La Rosée P. Alleviating the storm: ruxolitinib in HLH. Blood. (2016) 127:1626–7. doi: 10.1182/blood-2016-02-697151
35. Ahmed A, Merrill SA, Alsawah F, Bockenstedt P, Campagnaro E, Devata S, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol. (2019) 6:e630–7. doi: 10.1016/S2352-3026(19)30156-5
36. Kim H, Brooks KM, Tang CC, Wakim P, Blake M, Brooks SR, et al. Pharmacokinetics, pharmacodynamics, and proposed dosing of the oral JAK1 and JAK2 inhibitor baricitinib in pediatric and young adult CANDLE and SAVI patients. Clin Pharmacol Ther. (2018) 104:364–73. doi: 10.1002/cpt.936
37. Rider LG, Aggarwal R, Pistorio A, Bayat N, Erman B, Feldman BM, et al. 2016 american college of rheumatology/european league against rheumatism criteria for minimal, moderate, and major clinical response in juvenile dermatomyositis: an international myositis assessment and clinical studies group/paediatric rheumatology international trials organisation collaborative initiative. Ann Rheum Dis. (2017) 76:782–91. doi: 10.1136/annrheumdis-2017-211401
Keywords: baricitinib, juvenile dermatomyositis, refractory, severe, treatment
Citation: Wang Z, Zheng Q, Xuan W, Xu X, Lu M, Wu J, Zou L, Xu Y and Xu X (2022) Short-term effectiveness of baricitinib in children with refractory and/or severe juvenile dermatomyositis. Front. Pediatr. 10:962585. doi: 10.3389/fped.2022.962585
Received: 06 June 2022; Accepted: 24 August 2022;
Published: 20 September 2022.
Edited by:
Alenka Gagro, Children’s Hospital Zagreb, CroatiaReviewed by:
Lisa G. Rider, National Institute of Environmental Health Sciences (NIH), United StatesCopyright © 2022 Wang, Zheng, Xuan, Xu, Lu, Wu, Zou, Xu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meiping Lu, bWVpcGluZ2x1QHpqdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.