- 1Department of Pediatrics and Child Health Nursing, College of Medicine and Health Science, Injibara University, Injibara, Ethiopia
- 2Department of Generic Nursing, College of Health Science and Medicine, Wolaita Sodo University, Sodo, Ethiopia
- 3Department of Midwifery, College of Health Science and Medicine, Wolaita Sodo University, Sodo, Ethiopia
- 4Department of Adult Health Nursing, Debre Tabor University, Debra Tabor, Ethiopia
- 5Department of Pediatrics and Child Health Nursing, Debre Berhan University, Debre Berhan, Ethiopia
- 6Department of Pediatrics and Child Health Nursing, College of Health Science, Debre Tabor University, Debra Tabor, Ethiopia
- 7Department of Epidemiology, College of Medicine and Health Science, Injibara University, Injibara, Ethiopia
- 8Department of Midwifery, College of Medicine and Health Science, Injibara University, Injibara, Ethiopia
- 9Department of Reproductive Health, School of Public Health, Wolaita Sodo University, Sodo, Ethiopia
Background: Globally, the incidence of necrotizing enterocolitis (NEC) varies between 6 and 15% of all neonates admitted to the neonatal intensive care unit (NICU). Though necrotizing enterocolitis is a multifactorial and life-threatening disease, low birth prematurity is the single cause. Therefore, determining the time to presentation and its predictors of necrotizing enterocolitis were the main goals of this investigation.
Materials and methods: An institution-based retrospective follow-up study was conducted among 747 low birth weight (LBW) neonates admitted to the neonatal intensive care unit of Felege Hiwot comprehensive specialized Hospital from 1 January 2017 to 30 December 2019. The sample size was calculated by using the STATA package. Data were entered into Epi data version 3.1 and exported to STATA version 14 for analysis. The log-rank test and the Kaplan–Meier estimator were used to display the survival probability and differences between groups. At a significance threshold of 5%, Cox proportional hazard regression was performed to determine the net independent predictors of necrotizing enterocolitis.
Result: The overall incidence rate was 0.86 per 1,000 person-days (95% CI: 0.67, 1.14) with a 6.8% (95% i: 5.2, 8.9) proportion of necrotizing enterocolitis among low birth weight neonates. Preeclampsia [adjusted hazard ratio (AHR);1.92 (95% CI: 1.03–3.58)], premature rapture of membrane [AHR; 2.36 (95%, CI: 1.19–4.69)], perinatal asphyxia [AHR; 4.05 (95%, CI: 2.04–8.60)], gestational age between 28 and 32 weeks [AHR; 3.59 (95% CI: 1.01–8.83)], and birth weigh less than 1,000 g [AHR; 5.45 (95% CI: 3.84–9.12) were the independent predictors of necrotizing enterocolitis.
Conclusion: Within the first 1–7 days of a newborn’s life, necrotizing enterocolitis was most common. It was discovered that preeclampsia, premature rupture of membrane, perinatal asphyxia, gestational age of 28–32 weeks, and birth weight less than 1,000 g were predictors of its occurrence.
Introduction
Necrotizing enterocolitis (NEC), particularly in preterm and low birth weight neonates (LBW), is a common life-threatening condition in newborns (1, 2). Its clinical course progresses quickly and has an erratic and frequently extremely speedy beginning (3). After 8–10 days of age, the problem manifests in numerous ways, such as feeding intolerance, abdominal distention, and bloody feces, and may lead to intestinal necrosis and multi-organ failure (4).
From the newborns delivered in the world, 6–15% of newborns develop necrotizing entercolitis (5). Additionally, the incidence varies depending on the newborn’s birth weight and gestational age. For instance, it occurs four times as frequently in preterm low-birth-weight neonates as in normal birth weight neonates (6). Raised rates of preterm delivery and low birth weight in particular have increased the risk of NEC in newborns (7).
Necrotizing enterocolitis remains a challenging condition for neonatologists worldwide due to the uncertain pathophysiology and potential treatment options that might raise the morbidity and fatality rate by as much as 50% (8, 9). It is a sickness that advances quickly, frequently advancing from minor symptoms to full-blown illness, and it typically causes death within 24–48 h (10) LBW, preterm, poor Apgar score, sepsis, premature rupture of membranes (PROM), surfactant treatment, and cesarean delivery were the risk factors, however, NEC has multiple risk factors (11–13). Currently, the survival of premature LBW neonates is increasing due to the NICU’s growth and efficient organization, the accessibility of qualified healthcare professionals, and the rise in community health seeking and use habits. Contrarily, NEC is increasing since LBW prematurity is the single cause of NEC (14). Due to this, it is crucial for health professionals and other stakeholders to know when necrotizing entercolitis struck, especially among LBW and its predictors, but there is limited study on this issue at the national level, and no previous study was conducted on the problem in the study area. Therefore, the purpose of this study was to estimate the time to the occurrence of NEC and its predictors among LBW neonates admitted to the neonatal intensive care unit (NICU) of Felege Hiwot Compressive Specialized Hospital (FHCSH).
Materials and methods
Study area and period
The study was conducted at the NICU of Felege Hiwot comprehensive specialized hospital (FHCSH), Amhara regional state, Northern Ethiopia, from 1 April to 1 May 2021, by retrospectively recruiting those LBW neonates, who were admitted from 1 January 2017 to 30 December 2019. Felege Hiwot comprehensive specialized hospital is found in Bahir Dar City, which is the capital city of Amhara regional state and is located 565 km from the capital of Ethiopia.
It is one of the referral hospitals in the region and provides tertiary-level neonatal services. More than 5 million people have been served annually. It is organized into different service areas, such as term, preterm, isolation, and procedure rooms with Kangaroo Mother Care and maternal waiting rooms. The NICU ward has 75 neonatal beds with an average annual admission of 3,500 neonates and 900 LBW neonates. Currently, a total of 35 nurses and 12 physicians are working in the NICU, according to the 2021 report.
Study design
An institution-based retrospective follow-up study was conducted.
Source population
All LBW neonates who were hospitalized in the NICU of FHCSH between 1 January 2017 and 31 December 2019.
Inclusion criteria
The study population included all LBW neonatal records admitted between 1 January 2017 and 31 December 2019, with a gestational age of more than 28 weeks.
Exclusion criteria
Low birth weight neonatal charts with incomplete data and not found at the time of data collection were excluded.
Sample size determination and procedure
The STATA version 14 software was used to calculate the sample size by considering the following statistical assumptions: two-sided significant level of 5%, power of 80%, survival probability in the exposed (55%) and non-exposed (45%) groups, survival probability (0.5), the proportion of withdrawal (10%), and one-to-one exposed-to-non-exposed ratio (1:1). Finally, 846 was the calculated sample size for this study.
Sampling technique and procedure
A simple random sampling technique was used to select the LBW neonates’ charts. The LBW neonatal charts were obtained from the medical record office of the FHCSH registration logbook by reviewing the 3-year data from 1 January 2017 to 30 December 2019. The total number of low birth weight neonatal admissions to the NICU over 3 years was actively courted by looking through the Federal Ministry of Registration logbook. A total of 2,716 LBW neonates charts were obtained over these years. The Medical Registration Numbers of the neonates were picked up from the charts and entered into Microsoft Excel 2010 spreadsheets. Then, a computer-generated random numbering system in excel was used to select 846 LBW neonates charts. Finally, the LBW neonate’s charts were chosen following the eligibility requirements by a simple random sampling technique.
Operational definition
Necrotizing enterocolitis: it is a condition diagnosed by clinical and radiographic findings and classified according to modified Bell’s criteria (15).
Event: low birth weight neonates diagnosed with necrotizing enterocolitis.
Censored: low birth weight neonates without necrotizing enterocolitis.
Data collection tools and procedures
The standardized national neonatal and delivery room registration book served as the inspiration for the data extraction checklist. The content of the checklist incorporates socio-demographic characteristics, predictors of maternal and neonatal, the status of LBW neonates, date of admission, and date of occurrence of an event or censored. The data were retrospectively reviewed by two BSc nurses.
Data processing and analysis
The data were checked for completeness and consistency before being coded and entered into Epi-data version 3.1, and then exported to STATA version 14 for cleaning and analysis. Continuous data were reported either as mean [standard deviation (SD)] or median [interquartile range (IQR)] after checking for the normality distribution, by the Shapiro–Wilk test, and categorical data were described with frequency and proportion. The outcomes of each participant were dichotomized into failure or censored and the incidence density rate was calculated for the entire study period. The Kaplan–Meier survival curve was used to estimate survival time for those neonates with necrotizing enterocolitis and log-rank tests were employed to evaluate the survival curves of a different categorical explanatory variable. The Schoenfeld residual test and graphical techniques were used to test the necessary assumption of the Cox-proportional hazard regression model. In addition, the Cox-Snell residuals and global fit tests were used to evaluate the overall model adequacy and fineness, respectively. After checking the multi-collinearity and interaction term, each independent predictor variable with a p < 0.25 in the bivariate analysis was included in the multivariate Cox proportional hazard regression. The survival time was computed by subtracting the first date of admission from the last date of occurrence of necrotizing enterocolitis (event) or censoring. Finally, the adjusted hazard ratio (AHR) with a 95% confidence interval and p < 0.05 was used to measure the strength of association and identify statistical significance.
Data quality management
A standardized data collection checklist was used. For the purpose of reorganizing the checklist per registration system in FHCSH, 5% of LBW neonatal charts were updated before data collection. The supervisor and the data collectors received the 1-day training. Five percent of the charts underwent the pre-test. Finally, the researchers have examined all the information gathered to ensure its accuracy and consistency.
Results
Maternal and neonatal socio-demographic characteristics
From the total 846 LBW neonatal charts reviewed, 84.5% fulfill the enrollment criteria of the study and were considered in the final analysis. Out of the total charts, 409 (54.8%) were male charts. The median weight of the LBW neonates was 1,800 g with an interquartile range (IQR) of 650 g. A majority, 571 (67.4%) of LBW neonates at admissions were less than 24 h of age, and a median gestational age of all LBW neonates was 34 weeks with IQR of 5 weeks. Most (85.8%) of mothers were aged between 18 and 35 years and 442 (59.2%) were residing outside Bahir Dar city (Table 1).
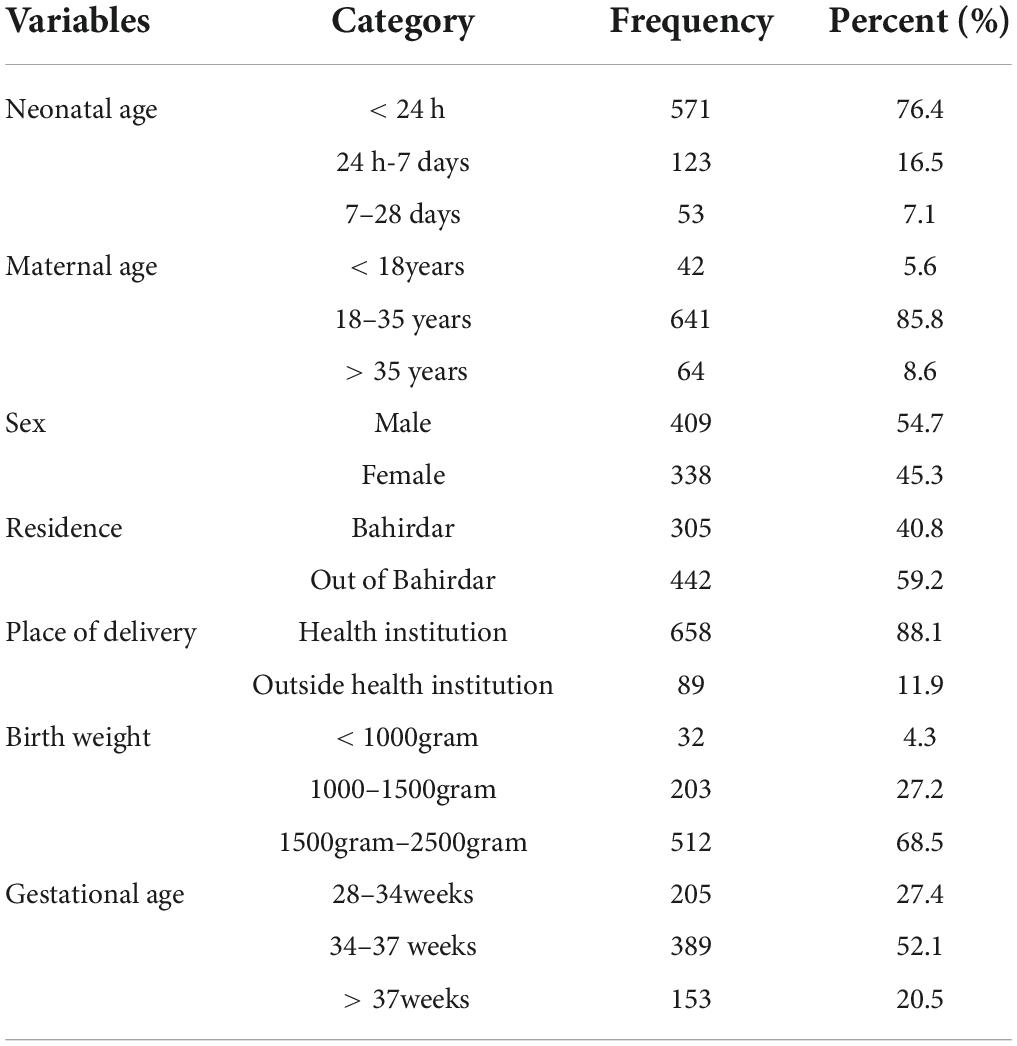
Table 1. Sociodemographic characteristics of mothers and neonates admitted to the neonatal intensive care unit (NICU) of Felege Hiwot Compressive Specialized Hospital (FHCSH), Ethiopia from 1 January 2015 to 30 December 2019.
Neonatal additional medical characteristics
From all the reviewed carts, 692 (92.6%) were diagnosed with an additional medical case; the most common one includes sepsis (66.7%), hypothermia (43.2%), respiratory distress syndrome (30.1%), jaundice (11.9%), perinatal asphyxia (PNA) (9.1), NEC (6.8), congenital heart disease (3.1%), and others (12,8%) (Figure 1).
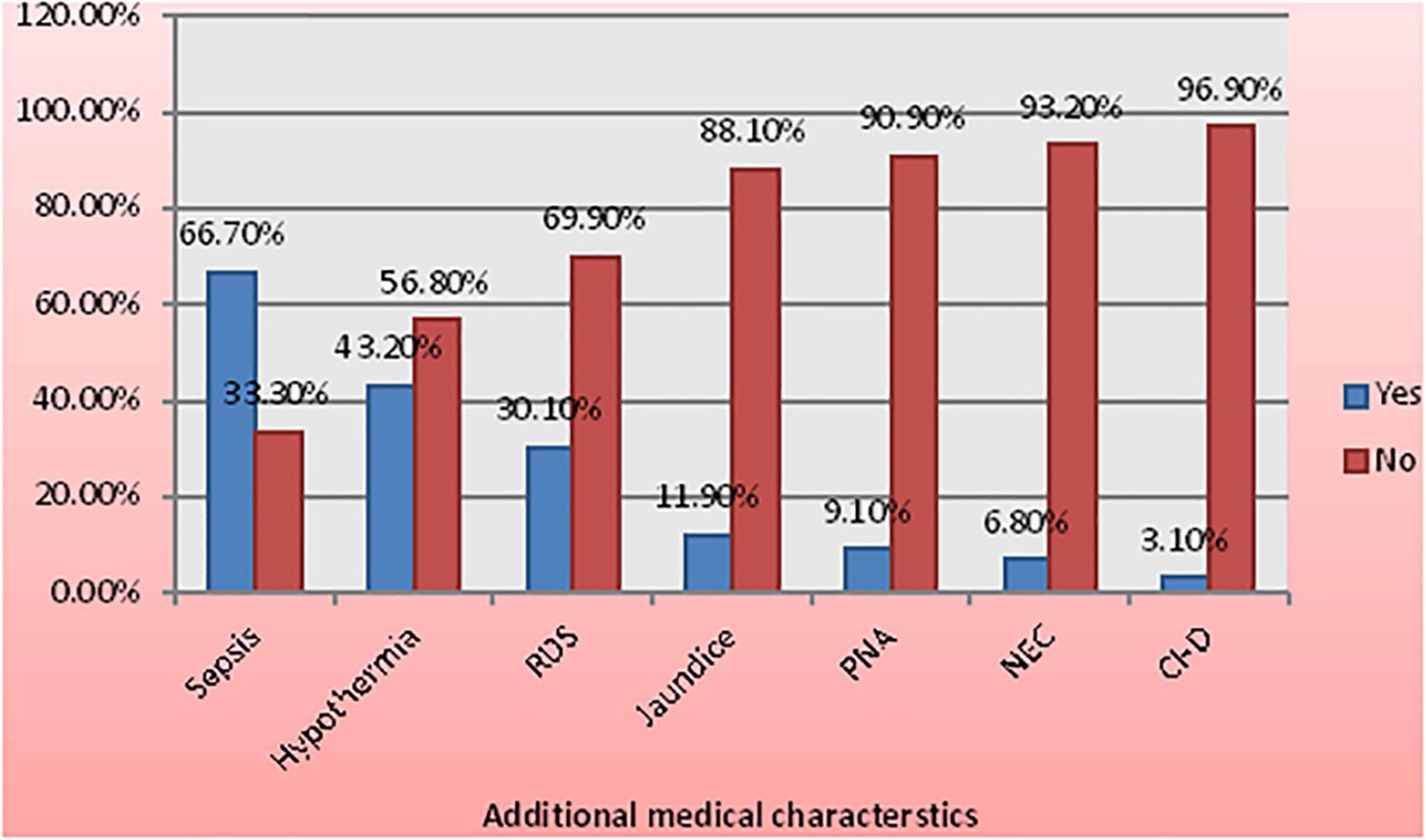
Figure 1. Medical characteristics among low birth weight (LBW) neonates admitted to the neonatal intensive care unit (NICU) of Felege Hiwot Compressive Specialized Hospital (FHCSH), Ethiopia from 1 January 2015 to 30 December 2019.
Maternal obstetric and medical characteristics
The majority of 456 (61%) women gave birth through spontaneous vaginal delivery. Most of the women had antenatal care (ANC) follow-up at nearby health institutions 661 (88.5%). Of all the mothers enrolled in the study, 15.3, 11, and 5.2% were diagnosed with the obstetric problems preeclampsia, pre-mature rupture of membranes (PROM), and antepartum hemorrhage (APH), respectively. Less than 10% of the mothers were also diagnosed with medical problems, such as HIV, anemia, and chronic hypertension. In this study, 460 (61.6%) women had ≥ 2 pregnancies and 456 (61%) gave birth (Table 2).
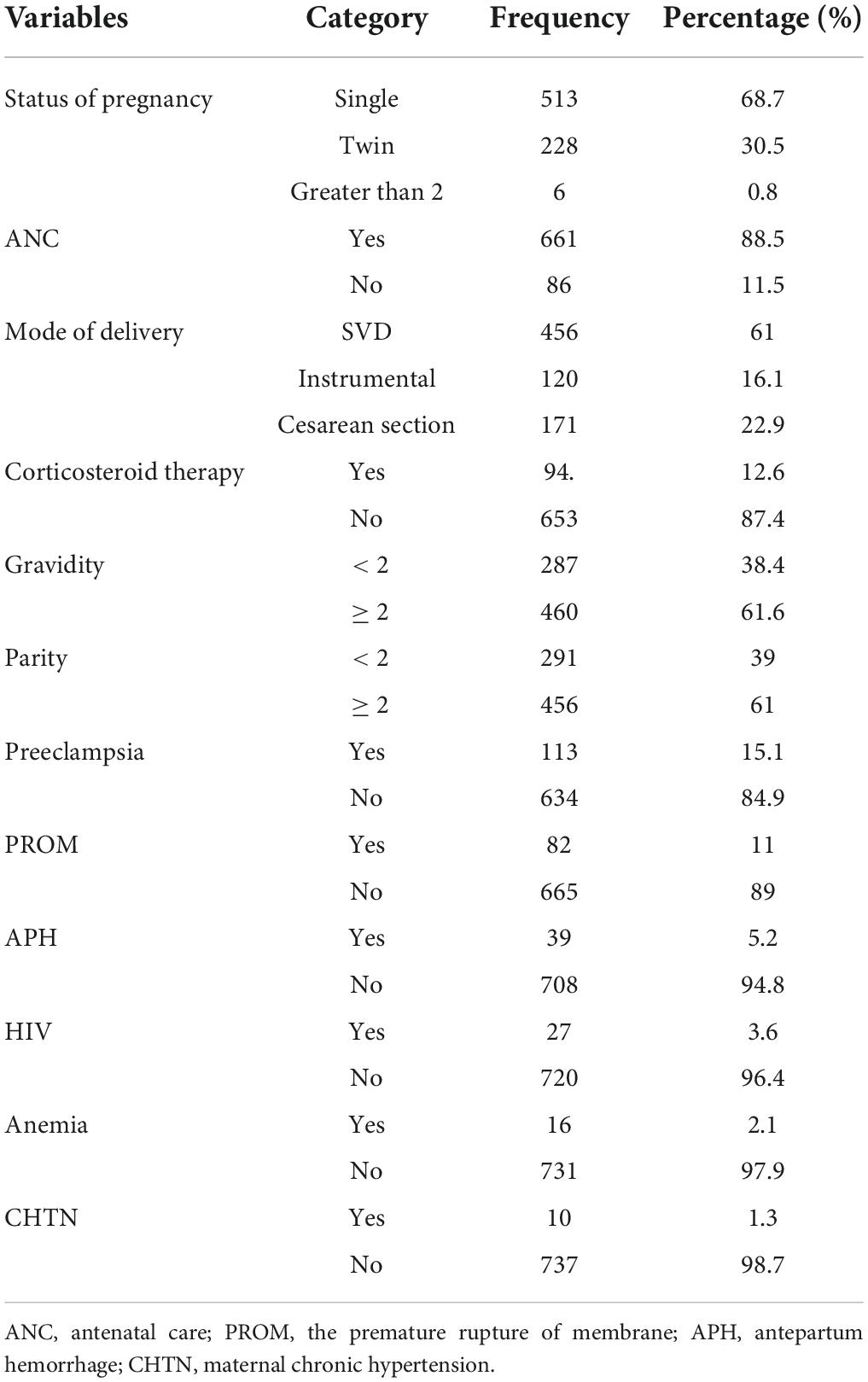
Table 2. Maternal obstetric characteristics among low birth weight (LBW) neonates admitted to the NICU of FHCSH, Ethiopia from 1 January 2015 to 30 December 2019.
The overall proportion and incidence rate of necrotizing enterocolitis
The overall proportion of necrotizing enterocolitis among LBW neonates was found to be 51 (6.8%) (95% CI: 5.2, 8.9). In this study, a total of 747 LBW neonates were retrospectively followed for 5,905.7 person-days with an overall incidence rate of 0.86/1,000 person-days (95% CI: 0.67, 1.14) (Figure 2).
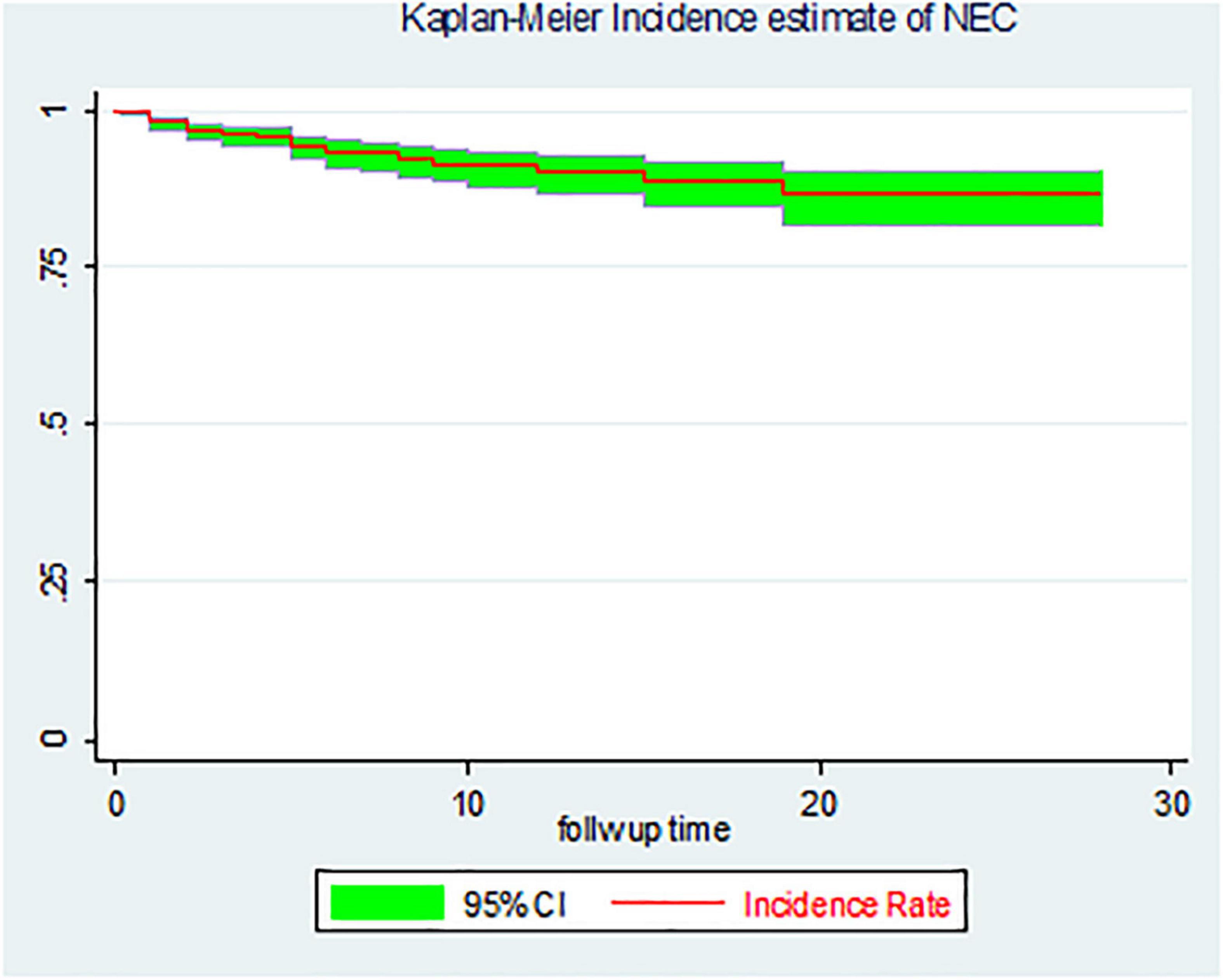
Figure 2. The overall Kaplan–Meier incidence estimate of necrotizing enterocolitis (NEC) among LBW neonates admitted to the NICU of FHCSH, Ethiopia from 1 January 2015 to 30 December 2019.
Incidence rate by neonatal age and gestational age
In the current study, the incidence rate of NEC among LBW neonates within the first 24 h was 0.83/1,000 person-days and 1–7 days accounted for 1.48/1,000 person-days observations. Similarly, the incidence rate of NEC for LBW neonates with gestational ages of 28–32, 32–37, and ≥ 37 weeks was 1.91, 0.46, and 0.33/1000 person-days observation, respectively (Table 3).
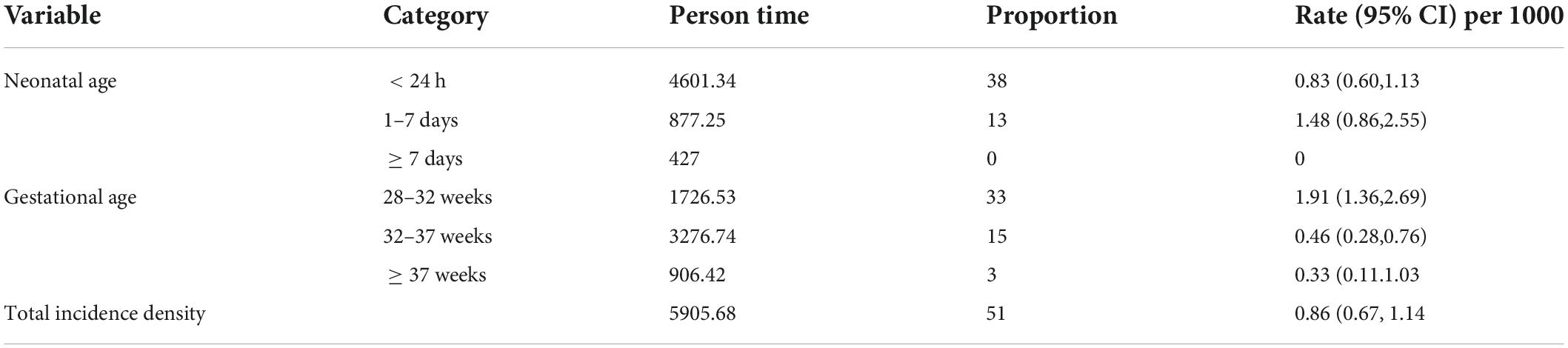
Table 3. Overall incidence rate and incidence rate for neonatal age and gestational among LBW neonates admitted to the NICU of FHCSH, Ethiopia from 1 January 2015 to 30 December 2019.
Survival ship function among cohorts
The findings of this study showed that LBW neonates delivered by preeclamptic women had a mean survival time of 22.41 days (95% CI: 20.01, 24.81) with SD ± 1.23; while, those LBW neonates from non-preeclamptic women had a mean survival time of 25.91 days (95% CI: 25.19, 26.62) with SD ± 0.36 and the difference was statistically significant at the log-rank test (p-value = 0.000) (Figure 3A).
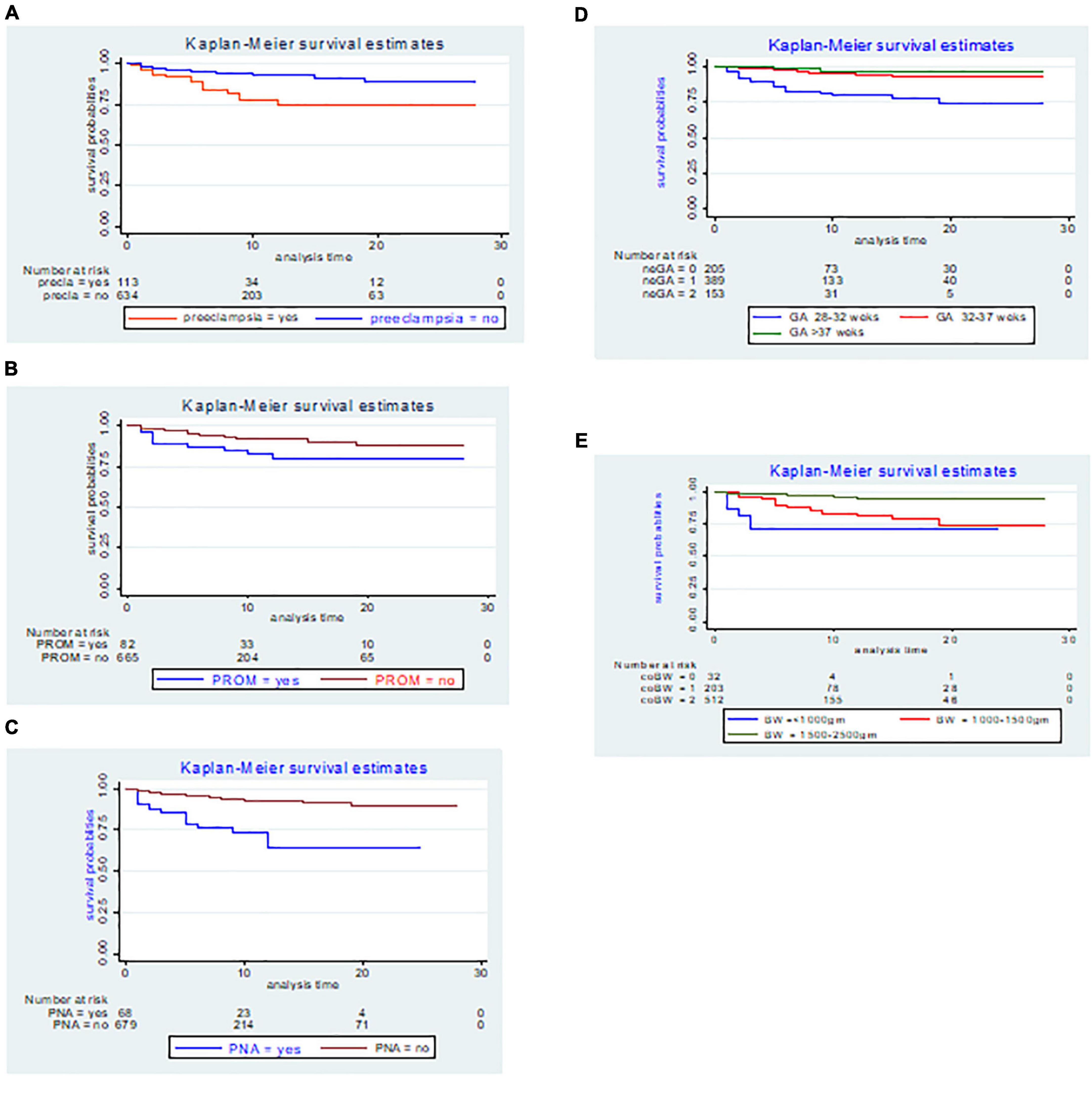
Figure 3. The Kaplan–Meier incidence estimate of NEC with respect to (A) preeclampsia, (B) premature rupture of membranes (PROM), (C) perinatal asphyxia (PNA), (D) gestational age, and (E) birth weight LBW neonates admitted to the NICU of FHCSH, Ethiopia from 1 January 2015 to 30 December 2019.
In the present study, the Kaplan–Meier graph of those LBW neonates delivered by women who had faced PROM with a mean survival time of 23.28 days (95% CI: 20.84, 25.71) with SD ± 1.24, laid below the Kaplan–Meier graph of those LBW neonates delivered by women who did not face PROM with a mean survival time of 25.66 days (95% CI: 24.92, 26.39) with SD ± 0.38, and the difference was statistically significant at the log-rank test (p = 0.008) (Figure 3B).
The Kaplan–Meier estimate showed that LBW neonates with PNA had a lower survival rate, with an overall survival rate of 64.48% (95% CI: 46.45, 77.79) than its counterpart with a survival rate of 89.19% (95% CI: 83.90, 92.81). The mean survival time for neonates with PNA was 18.13 days (95% CI: 15.28, 20.98) with SD ± 1.45 and the difference was statistically significant at the log-rank test (p = 0.000) (Figure 3C).
The mean survival time among LBW neonates delivered at 28–32, 32–37, and above 37 weeks of gestation was significantly different at the log-rank test 0.000 and the mean survival time for each category was 22.44 days (95% CI: 20.74, 24.14) with SD ± 0.87, 26.46 days (95% CI: 25.68, 27.25) with SD ± 0.40, and 27.05 days (95% CI: 25.93, 28.18) with SD ± 0.57, respectively (Figure 3D).
The overall survival probability of NEC within the cohort birth weight (1,500–2,500 g) was 94.78% (95% CI: 90.99, 96.99) SD ± 0.01, 74.47% (95% CI: 63.55, 82.56) SD ± 0.05 for birth weight (1,000–1,500 g), and 71.29% (95% CI: I0.48, 85.68) SD ± 0.10 for birth weight less than (1,000 g) respectively with the Kaplan–Meier graph difference was statistically significant at the log-rank test (p = 0.000) (Figure 3E).
Bivariate and multivariate cox proportional hazard regression
In the bivariate Cox regression, the sex of the neonate, preeclampsia, PROM, APH, maternal anemia, sepsis, jaundice, Respiratory distress syndrome, PNA, Congenital heart disease, maternal age, gestational age, and birth weight were found to be significant predictors. While in the adjusted final Cox regression preeclampsia, PROM, PNA, gestational age, and birth weight had an association with NEC.
This study showed that the hazard of developing NEC in the LBW neonates delivered by preeclamptic mothers was 1.92 times higher risk than that of LBW neonates delivered by non-preeclamptic mothers [AHR; 1.92 (95% CI: 1.03–3.58)]. Low birth weight neonates delivered by PROM mothers were 2.36 [AHR; 2.36 (95% CI: 1.19–4.69)] times more likely to have NEC at any time than LBW neonates delivered by non-PROM mothers. At any time, the risk of developing NEC in LBW neonates diagnosed with PNA was 4.05 [AHR; 4.05 (95% CI: 2.04–8.60)] times more likely to have NEC than those LBW neonates without PNA. Low birth weight neonates with a gestational age between 28 and 32 weeks were 3.59 times as likely to have NEC at any time as LBW neonates with a gestational age > 37 weeks [AHR; 3.59 (95% CI:1.01–8.83)]. In addition, low birth weight neonates born with birth weights of less than 1,000 g were 5.45 times as likely to have NEC at any time as LBW neonates weighing between 1,500 and 2,500 g [AHR; 5.45 (95%, CI: 3.84–9.12)] (Table 4).
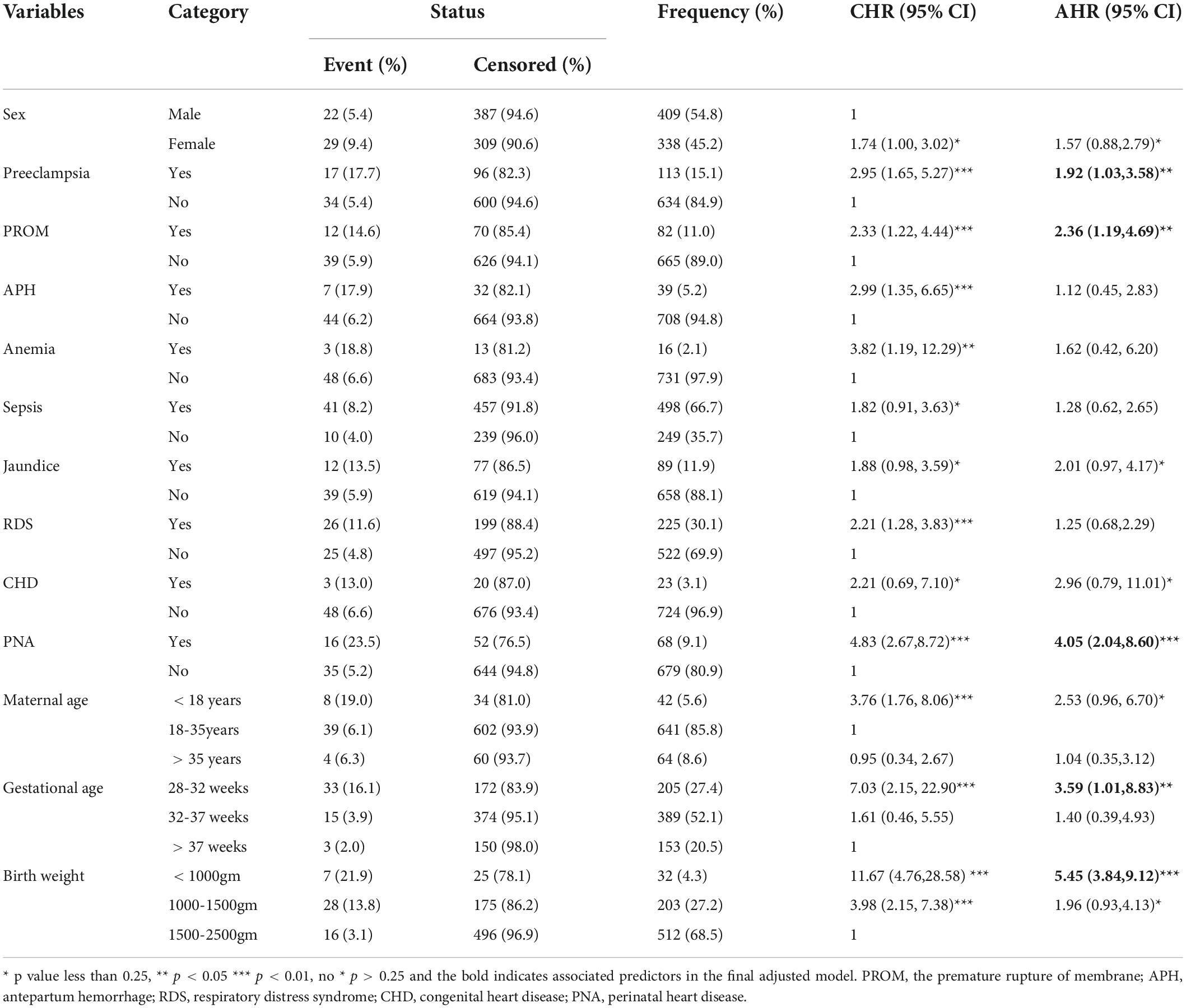
Table 4. The bivariate and multivariate Cox proportional hazard regression output among LBW neonates admitted to the NICU of FHCSH, Ethiopia from 1 January 2015 to 30 December 2019.
Proportional hazard assumption test
The proportional hazard assumption was tested by the graphical method of the log-log plot curves test and the scaled Schoenfeld residual test. The scaled Schoenfeld residual test was done for individual covariates and the whole covariates. According to Table 5 below, for each covariate (p > 0.05) and all of covariates simultaneously (global for Cox proportional hazard p = 0.8861, i.e., >0.05). This showed that the proportional hazard assumption met the criteria (Table 5).
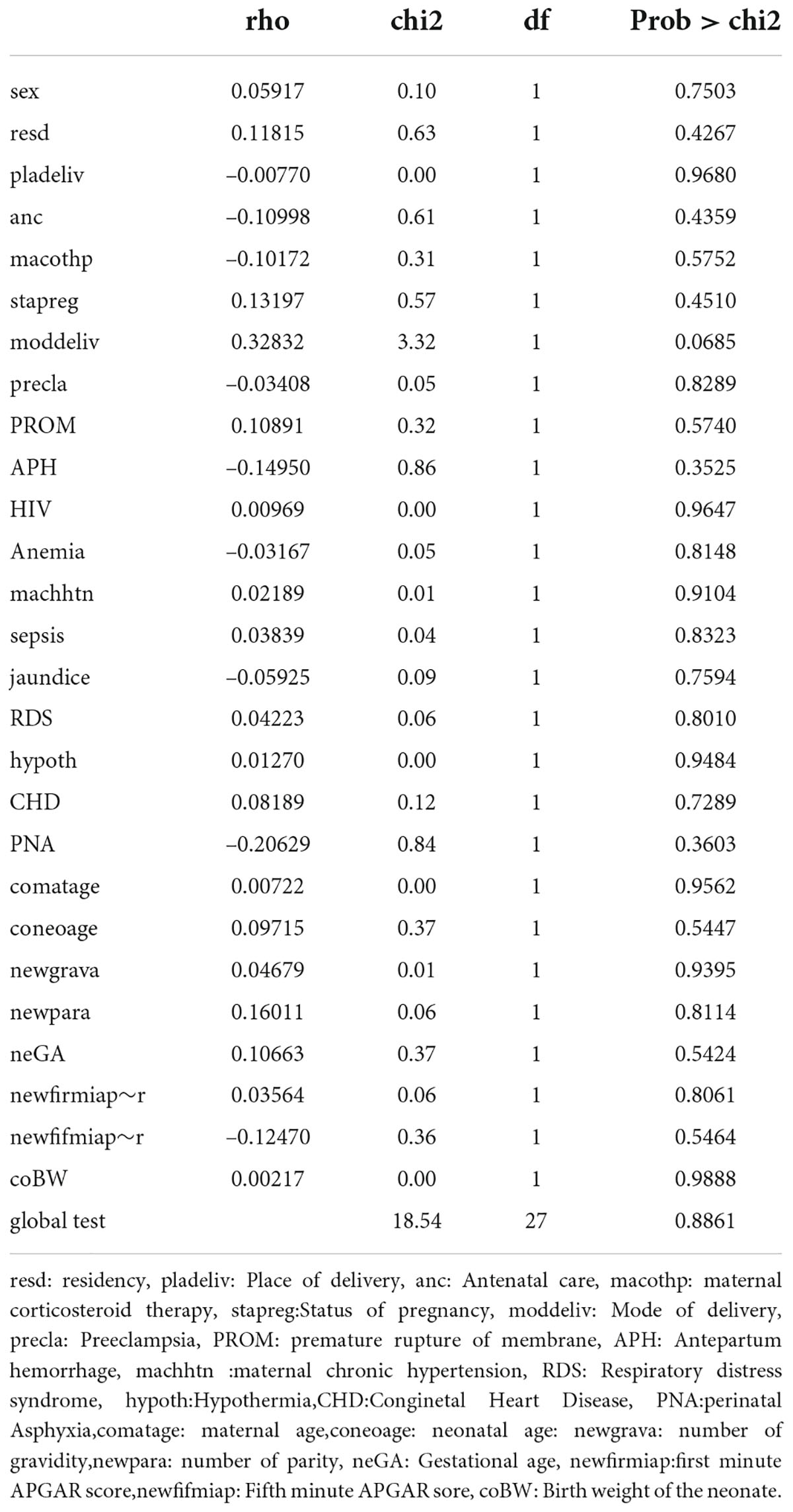
Table 5. The scaled Schoenfeld residual test of proportional hazard assumption among LBW neonates admitted to the NICU of FCSH, Ethiopia from 1 January 2015 to 30 December 2019.
Overall model fitness test
The Cox-Snell residual test was used to check the model’s goodness of fit. The residuals have a typical censored exponential distribution with a hazard ratio, as shown in the figure below, which illustrates how the Cox regression model fits the data. The hazard function closely adheres to the 45-degree line (Figure 4).
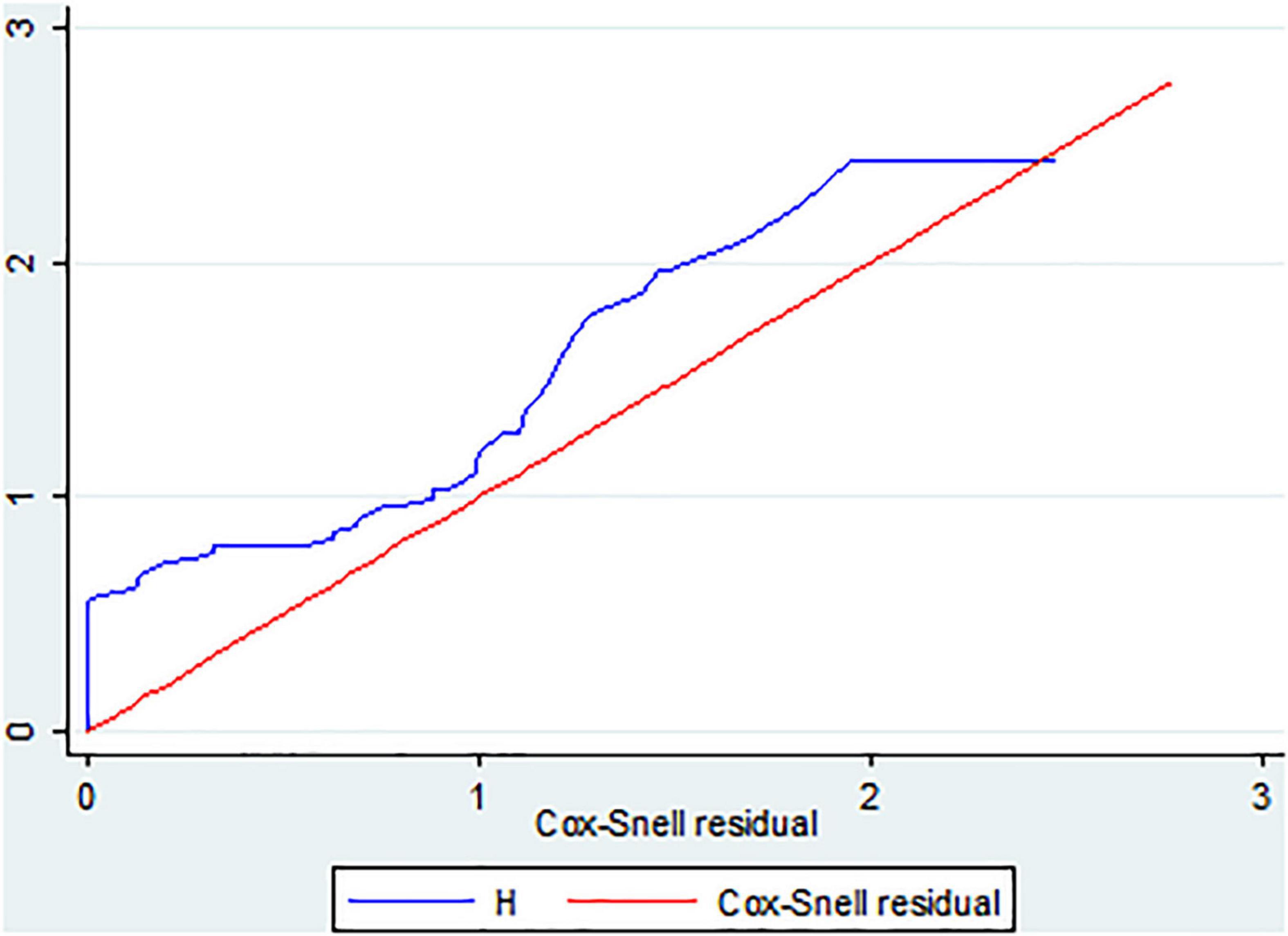
Figure 4. The Cox Snell residual overall model fitness test of LBW neonates admitted to the NICU of FHCSH, Ethiopia from 1 January 2015 to 30 December 2019.
Discussion
Based on the results of the current investigation, 6.8% of LBW neonates had NEC overall (95% CI: 5.2, 8.9). This was consistent with the findings of the United Kingdom study (7.5%) (16), and Polish (8.7%) (17). However, this finding was higher than the study conducted in multicenter Hospitals in China (2.4%) (13) and the United States (2.8%) (18). This could be because 61.2% of Ethiopian mothers think that pre-lacteal feeding is crucial for a child’s health and growth, the practice is as widespread there as it is in China and the United States (19); additionally, it may be also socio-economic, sample size, study area differences, and advanced neonatal and maternal care in China and the United States. On the other hand, this result was less than that of an observational prospective study carried out at Uludag University and a study carried out in Ethiopia (25.4%) (20, 21), and the study conducted at the National Institute of Child Health and Human Development Neonatal Research Network, which reported the prevalence of proven NEC (10.1%) (22). The variation may be in study design differences, which were cross-sectional in Ethiopia and prospective observational design in Uludag University. Moreover, the difference could be due to the sample size difference in Ethiopia (350) and Uludag University (88) participants. Additionally, the current study also prevailed that the overall incidence rate of NEC in LBW neonates was 0.86 per 1,000 person-days with a 5,905.7 risk time observation.
Based on this finding, preeclampsia, PROM, PNA, gestational age of 28–32 weeks, and birth weight less than 1,000 g remain the best predictors of NEC. The hazard of having NEC in the LBW neonates delivered by preeclamptic mothers was 1.92 times higher risk than in LBW neonates delivered by non-preeclamptic mothers. This was also similar to an observational prospective study conducted at Uludag University (21). This might be due to the presence of maternal preeclampsia, hypoxia, and increased mesenteric vascular resistance that might produce a hypoxic-ischemic state in the intestine or the mucosa in the antenatal period, and this prolonged exposure to hypoxia might provide an intestine that is more susceptible to stasis, abnormal colonization, and overgrowth bacteria during the postnatal period (23).
Furthermore, this study found that the risk of NEC in LBW neonates born by PROM mothers was 2.36 times higher than for those neonates born by non-PROM mothers. This study was also supported by a retrospective follow-up study conducted in Saudi Arabia (24) and Children’s Hospital of Illinois at OSF Saint Francis Medical Center (25). This is due to the fact that PROM increases the chance of premature labor which in turn causes immaturity of the gastrointestinal tract, both in its structure and secretory activity (26). The risk of NEC in LBW neonates diagnosed with PNA was 4.05 times higher than in LBW neonates without PNA (27). In addition, prolonged hypoxia associated with birth asphyxia causes reduced blood flow to the gut that may expose the susceptibility of neonates to gut ischemia causing necrotizing enterocolitis (28).
Furthermore, the risk of developing NEC in preterm neonates with a gestational age between 28 and 32weeks was 3.59 times higher than that of term neonates, and also those neonates with birth weight less than 1,000 g were 5.45 times riskier to have NEC at any time as LBW neonates weighing between 1,500 and 2,500 g. This also supported studies conducted in Germany and Stockholm (29, 30). The immature bowels of these babies are sensitive and prone to infection. They may have difficulty with blood and oxygen circulation and digestion, which increases their chances of developing necrotizing enterocolitis (31).
Limitations
The sample size was calculated by considering statistical assumptions in the STATA package, survival probability in the exposed group 55% and in the non-exposed group = 45%, and the survival probability = 0.5, hence the absence of a similar study in Ethiopia. Some important predictor variables were missed since the study was a chart review.
Conclusion
In this follow-up study, the highest rate of occurrence of necrotizing enterocolitis was seen within 1–7 days of neonatal life. The major predictor variables of its time to occurrence were found to be preeclampsia, PROM, PNA, gestational age of 28–32 weeks, and birth weight less than 1,000 g. Hence, stakeholders, such as the Federal Ministry of Health, Regional health Bauru, and health professionals should work on the prevention of above predictor variables to decrease the occurrence of NEC, especially in early neonatal life.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional review board (IRB) of Bahir Dar University, College of Medicine and Health Science. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Authors contributions
TA, MF, AY, AA, and AT had made substantial contributions to conceptualization, methodology, and data curation. TA, TB, GK, BW, TT, GA, KE, and YA actively participated in the write-up, formal analysis, and drafting of the article. All authors gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgments
We thank Bahir Dar University for the approval of the ethical clearance. We also warmly, thank the staff of Felege Hiwot Comprehensive Specialized Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AHR, Adjusted Hazard Ratio; ANC, Antenatal Care; APH, Antepartum hemorrhage; CHR, Crude Hazard Ratio; FHCSH, Felege Hiwot Comprehensive Specialized Hospital; LBW, Low Birth Weight; NEC, Necrotizing Enter Colitis; NICU, Neonatal Intensive Care Unit; PNA, Perinatal Asphyxia.
References
1. Rich BS, Dolgin SE. Necrotizing enterocolitis. Pediatr Rev. (2017) 38:552–9. doi: 10.1542/pir.2017-0002
2. Arnold M, Moore SW, Sidler D, Kirsten GF. Long-term outcome of surgically managed necrotizing enterocolitis in a developing country. Pediatr Surg Int. (2010) 26:355–60. doi: 10.1007/s00383-010-2583-8
3. Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. (2015) 372:331–40. doi: 10.1056/NEJMoa1403489
4. Tooley WH. Intensive care nursery HOUSE STAFF MANUAL the Regents of the University of California, San Francisco, CA: UCSF Benioff Children’s Hospital, 2003 Very Low and Extremely Low Birthweight Infants (2003).
5. Caplan M, Portman R Second Annual Neonatal Scientific Workshop at the EMA Report. London: International Neonatal Consortium. (2016).
6. Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: A systematic review and meta-analysis. BMC Pediatr. (2020) 20:344. doi: 10.1186/s12887-020-02231-5
7. Sylvester KG, Liu GY, Albanese CT. Necrotizing enterocolitis. Pediatric surgery. Amsterdam: Elsevier Inc (2012). p. 1187–207. doi: 10.1016/B978-0-323-07255-7.00094-5
8. Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. (2006) 368:1271–83. doi: 10.1016/S0140-6736(06)69525-1
9. Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. (2003) 6:6–23. doi: 10.1007/s10024-002-0602-z
10. Geme Joseph St., Kliegman Robert M. (0000) Nelson textbook of pediatrics. 21th ed. New York, NY: Elsevier.
11. Samuels N, van de Graaf RA, de Jonge RCJ, Reiss IKM, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: A systematic review of prognostic studies. BMC Pediatr. (2017) 17:105. doi: 10.1186/s12887-017-0847-3
13. Qian T, Zhang R, Zhu L, Shi P, Yang J, Yang CY, et al. Necrotizing enterocolitis in low birth weight infants in china: mortality risk factors expressed by birth weight categories. Pediatr Neonatol. (2017) 58:509–15. doi: 10.1016/j.pedneo.2016.10.004
14. WHO.Unicef. Survive and Thrive: Transforming care for EVERY Small and Sick Newborn. Geneva: WHO (2019).
15. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. (1986) 33:179–201. doi: 10.1016/S0031-3955(16)34975-6
16. Robertson C, Savva GM, Clapuci R, Jones J, Maimouni H, Brown E, et al. Incidence of necrotising enterocolitis before and after introducing routine prophylactic lactobacillus and bifidobacterium probiotics. Arch Dis Child Fetal Neonatal Ed. (2020) 105:380–6. doi: 10.1136/archdischild-2019-317346
17. Wójkowska-Mach J, Różańska A, Borszewska-Kornacka M, Domańska J, Gadzinowski J, Gulczyńska E, et al. Necrotising enterocolitis in preterm infants: epidemiology and antibiotic consumption in the polish neonatology network neonatal intensive care units in 2009. PLoS One. (2014) 9:e92865. doi: 10.1371/journal.pone.0092865
18. Nair J, Longendyke R, Lakshminrusimha S. Necrotizing enterocolitis in moderate preterm infants. Biomed Res Int. (2018) 2018:4126245. doi: 10.1155/2018/4126245
19. Tekaly G, Kassa M, Belete T, Tasew H, Mariye T, Teshale T. Pre-lacteal feeding practice and associated factors among mothers having children less than two years of age in aksum town, tigray, ethiopia, 2017: A cross-sectional study. BMC pediatrics. (2018) 18:310. doi: 10.1186/s12887-018-1284-7
20. Mekonnen SM, Bekele DM, Fenta FA, Wake AD. The prevalence of necrotizing enterocolitis and associated factors among enteral fed preterm and low birth weight neonates admitted in selected public hospitals in addis ababa, ethiopia: A cross-sectional study. Glob Pediatr Health. (2021) 8:2333794X211019695. doi: 10.1177/2333794X211019695
21. Cetinkaya M, Ozkan H, Koksal N. Maternal preeclampsia is associated with increased risk of necrotizing enterocolitis in preterm infants. Early Hum Dev. (2012) 88:893–8. doi: 10.1016/j.earlhumdev.2012.07.004
22. Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Phillips JB, Wright LL. National institute of child health and human development neonatal research network necrotizing enterocolitis in very low birth weight infants: Biodemographic and clinical correlates. J Pediatr. (1991) 119:630–8. doi: 10.1016/s0022-3476(05)82418-7
23. Dorling J, Kempley S, Leaf A. Feeding growth restricted preterm infants with abnormal antenatal doppler results. Arch Dis Child Fetal Neonatal Ed. (2005) 90:F359–63. doi: 10.1136/adc.2004.060350
24. Al-Alaiyan S, Abdulaziz N, Alkohlani A, Almairi SO, Al Hazzani F, Binmanee A, et al. Effects of probiotics and lactoferrin on necrotizing enterocolitis in preterm infants. Cureus. (2021) 13:e18256. doi: 10.7759/cureus.18256
25. Drenckpohl D, Knaub L, Schneider C, Mcconnell C, Wang H, Macwan K. Risk factors that may predispose premature infants to increased incidence of necrotizing enterocolitis. ICAN: Infant Child Adolesc. Nutr. (2010) 2:37–44. doi: 10.1177/1941406409359195
26. Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. (2005) 14:49–57. doi: 10.1053/j.sempedsurg.2004.10.025
27. Gamsu HR, Kempley ST. Enteral hypoxia/ischaemia and necrotizing enterocolitis. Semin Neonatol. (1997) 2:245–54. doi: 10.3310/hta25360
28. Kvietys PR, Granger DN. Relation between intestinal blood flow and oxygen uptake. Am J Physiol. (1982) 242:G202–8. doi: 10.1152/ajpgi.1982.242.3.G202
29. Mûller MJ, Paul T, Seeliger S. Necrotizing enterocolitis in premature infants and newborns. J Neonatal Perinatal Med. (2016) 9:233–42. doi: 10.3233/NPM-16915130
30. Palleri E, Aghamn I, Bexelius TS, Bartocci M, Wester T. The effect of gestational age on clinical and radiological presentation of necrotizing enterocolitis. J Pediatr Surg. (2018) 53:1660–4. doi: 10.1016/j.jpedsurg.2017.09.018
Keywords: low birth weight, necrotizing enterocolitis, incidence density, Kaplan–Meier, BahirDar, Ethiopia
Citation: Alene T, Feleke MG, Yeshambel A, Amare AT, Tigabu A, Birlie TA, Aynalem YA, Kerebeh G, Eshetu K, Tsega TD, Wassihun B, Adella GA and Chichiabellu TY (2022) Time to occurrence of necrotizing enterocolitis and its predictors among low birth weight neonates admitted at neonatal intensive care unit of felege hiwot compressive specialized hospital BahirDar, Ethiopia, 2021: A retrospective follow-up study. Front. Pediatr. 10:959631. doi: 10.3389/fped.2022.959631
Received: 01 June 2022; Accepted: 22 August 2022;
Published: 12 September 2022.
Edited by:
María L. Couce, Complejo Hospitalario Universitario de Santiago, SpainReviewed by:
Nick Zavras, University General Hospital Attikon, GreeceWojciech Górecki, Jagiellonian University, Poland
Riccardo Coletta, University of Florence, Italy
Copyright © 2022 Alene, Feleke, Yeshambel, Amare, Tigabu, Birlie, Aynalem, Kerebeh, Eshetu, Tsega, Wassihun, Adella and Chichiabellu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamiru Alene, dGFtaXJ1YWxlbmUxMjEyQGdtYWlsLmNvbQ==
 Tamiru Alene
Tamiru Alene Mulualem Gete Feleke
Mulualem Gete Feleke Addisu Yeshambel3
Addisu Yeshambel3 Abraham Tsedalu Amare
Abraham Tsedalu Amare Agimasie Tigabu
Agimasie Tigabu Tekalign Amera Birlie
Tekalign Amera Birlie Yared Asmare Aynalem
Yared Asmare Aynalem Gashaw Kerebeh
Gashaw Kerebeh Kirubel Eshetu
Kirubel Eshetu Getachew Asmare Adella
Getachew Asmare Adella Tesfaye Yitna Chichiabellu
Tesfaye Yitna Chichiabellu