- 1Department of Gastroenterology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2School of Medicine, South China University of Technology, Guangzhou, China
- 3School of Bioscience and Bioengineering, South China University of Technology, Guangzhou, China
- 4Shantou University Medical College, Shantou, China
- 5Department of General Medicine, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
Necrotizing enterocolitis (NEC) is one of the most prevalent neonatal gastrointestinal disorders. Despite ongoing breakthroughs in its treatment and prevention, the incidence and mortality associated with NEC remain high. New therapeutic approaches, such as breast milk composition administration, stem cell therapy, immunotherapy, and fecal microbiota transplantation (FMT) have recently evolved the prevention and the treatment of NEC. This study investigated the most recent advances in NEC therapeutic approaches and discussed their applicability to bring new insight to NEC treatment.
Introduction
Necrotizing enterocolitis (NEC) is an inflammatory bowel disease that is particularly dangerous in premature or low-birth-weight babies (1). Despite tremendous advancements in NEC treatment and neonatal care over the past few decades, the current state of treatment remains unsatisfactory, and mortality and morbidity remain high (2). Short bowel syndrome and intestinal failure are possible outcomes of surgical resection of the necrotic part of the intestine. Patients who survive NEC have a higher risk of developing long-term complications, such as neurodevelopmental delay (3, 4).
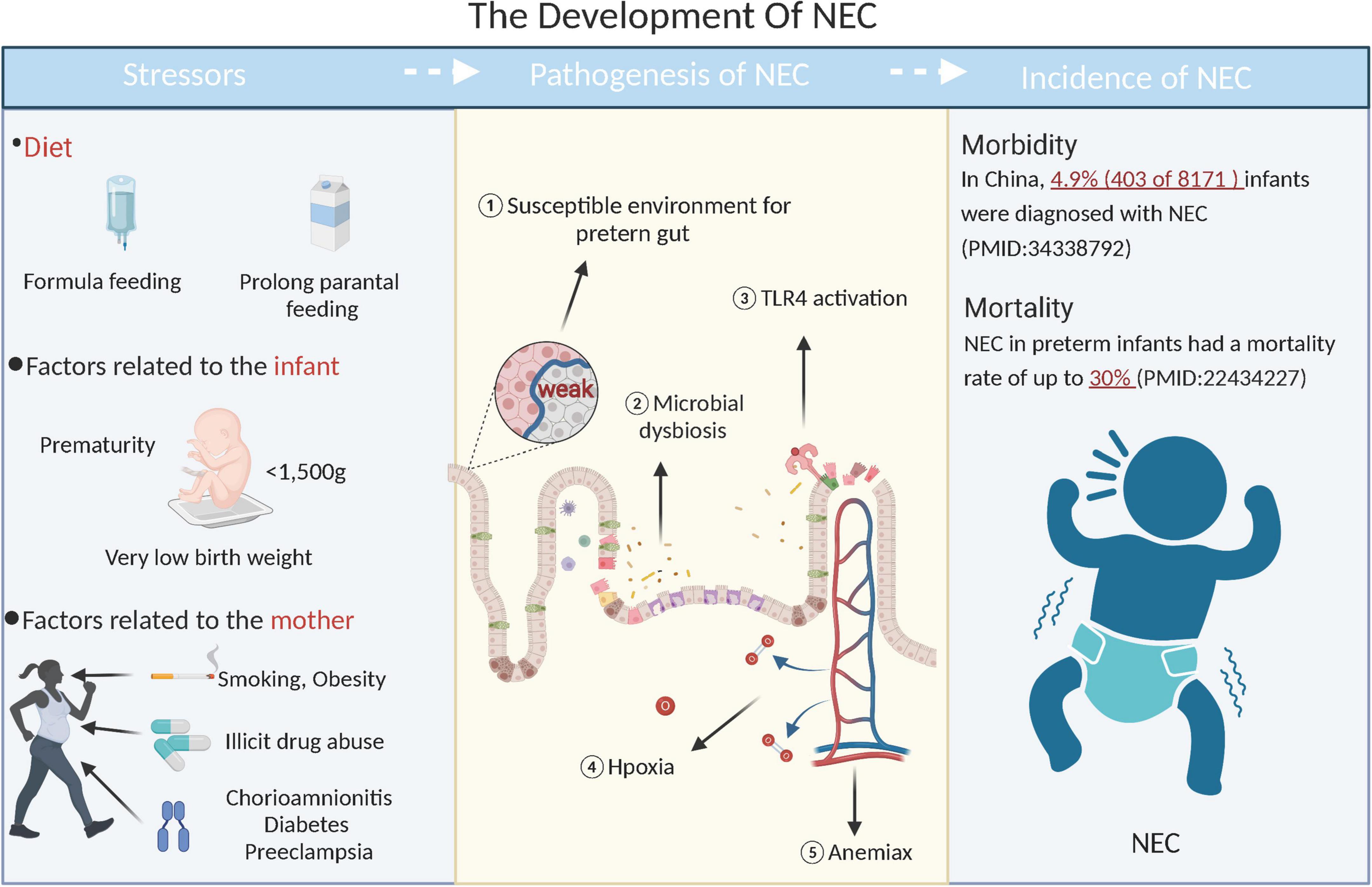
Prevalence and development of NEC are extraordinarily complex. Low birth weight, prolonged parenteral feedings, and short gestation periods are all risk factors of preterm birth. Additionally, mother’s lifestyle (such as smoking and obesity), the prevalence of associated disorders (such as diabetes mellitus, preeclampsia, and chorioamnionitis), and prenatal medications (such as antibiotics and corticosteroids) are risk factors for NEC (Figure 1) (5–10). There are multiple factors involved in developing NEC, including genetic susceptibility, immature intestinal host defense, abnormal microbiota colonization, hypoxia, ischemia, hyperresponsiveness of the intestinal mucosa (11, 12). Despite the study of NEC from various angles, the mechanisms that cause the disease are still largely unknown, which impedes its development into a specific treatment. Prevailing treatment strategies for NEC include antibiotics, surgery, and advanced life support, but their effect is limited. Therefore, a more effective approach to treating NEC is necessary.
In this review, breast milk composition, stem cells, immunotherapy, and fecal microbiota transplantation (FMT) were considered the most recent developments in NEC treatment (Figure 2). In addition, their applications to the NEC treatment were evaluated to illuminate the limitations and challenge of the NEC treatment.
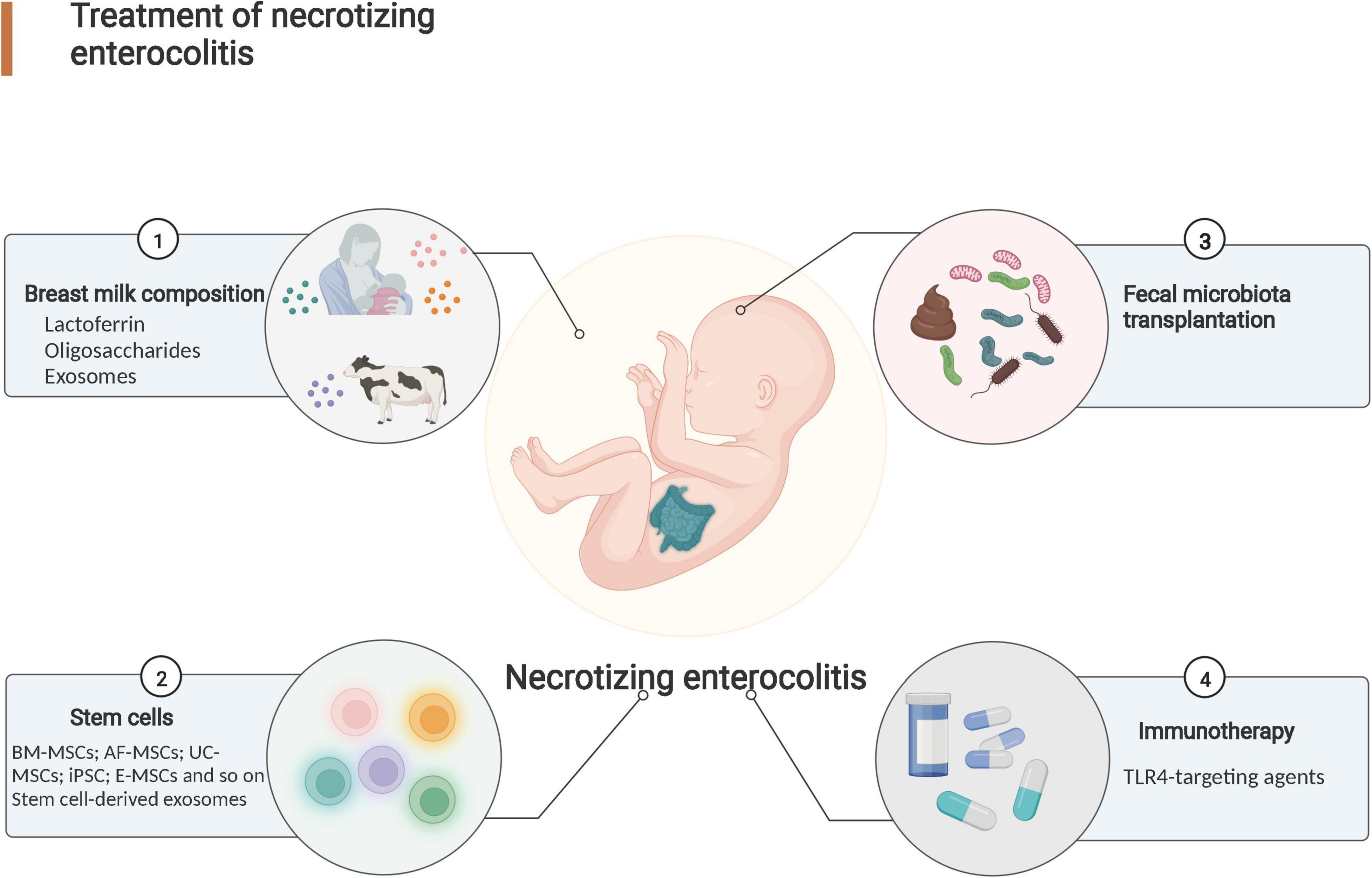
Figure 2. Research progress in necrotizing enterocolitis treatment. Effective treatments for NEC include breast milk composition administration, stem cell therapy, fecal microbiota transplantation (FMT) and immunotherapy.
Necrotizing enterocolitis treatment strategy
Therapy with breast milk composition
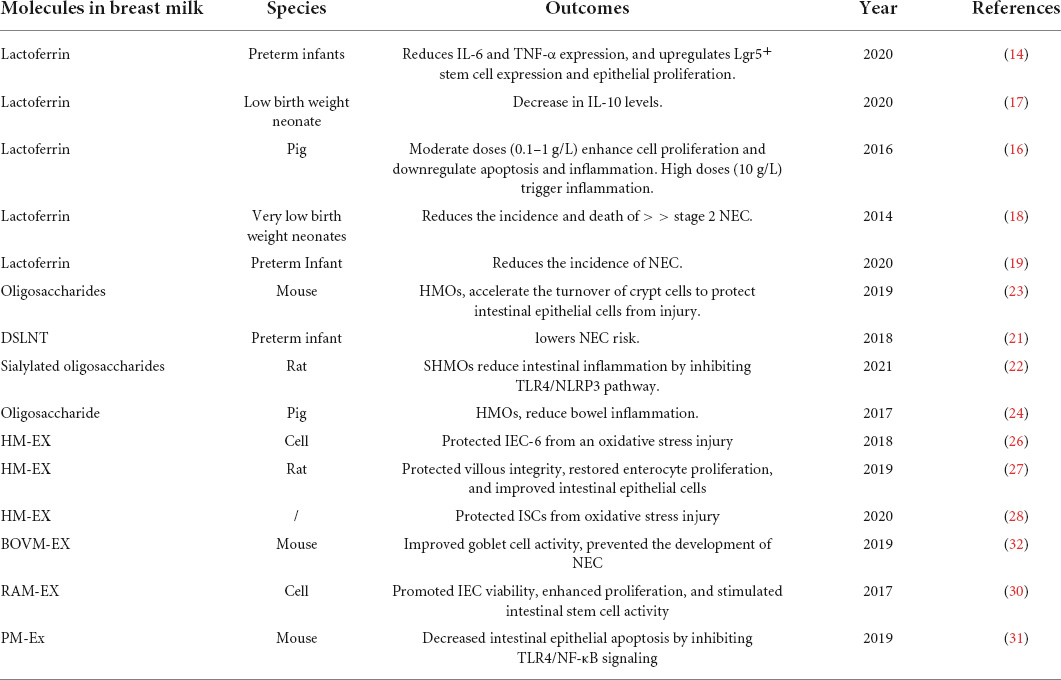
Multiple studies have demonstrated that Breast milk composition, including lactoferrin, oligosaccharides, breast milk-derived exosome and so on, is one of the most effective methods for preventing and treating NEC (13, 14). In this section, We focused mainly on lactoferrin, oligosaccharides and breast milk-derived exosome based on their potential applications in NEC prevention and treatment (Table 1).
Lactoferrin
Lactoferrin is the most abundant protein in colostrum (5–6.7 g/L) and is the most important protein found in breast milk (15). Lactoferrin has been demonstrated to inhibit the release of pro-inflammatory cytokines, such as IL-6 and TNF- α, thus reducing intestinal inflammation (14). In addition to maintaining the barrier function of the gut, lactoferrin influences intestinal epithelial cell proliferation and apoptosis (16). Lactoferrin’s effectiveness in preventing and treating NEC has been demonstrated in several preclinical studies and clinical trials (17, 18). Up to now, a phase III clinical trial (ClinicalTrials.gov Identifier: NCT03431558) is currently underway to determine the health effects of lactoferrin with gradient concentration in neonates with low birth weight at the Aga Khan University Hospital, Pakistan. However, based on a systematic review and meta-analysis of nine RCTs with 3515 samples, enteral lactoferrin supplementation did not reduce late-onset sepsis incidences in NEC, all-cause mortality, sepsis-related mortality, NEC stage II or III, and other adverse outcomes (19).
Oligosaccharides
The significance of Oligosaccharides in protecting against NEC has been a developing area of research since human breast milk is a recognized protective mechanism against the development of NEC. Animals fed a formula containing DSLNT displayed a decrease in NEC severity and lower mortality in preclinical experiments using a newborn rat model of NEC (20, 21). The same study also showed sialylated oligosaccharides, similar to HMOs, but structurally different, decreased NEC incidence and pathological damage scores in rats (22). A rat model of NEC showed that supplementation with sialylated oligosaccharides reduced NEC incidence and intestinal pathology with inhibiting toll like receptor 4/NLRP3 inflammasome pathway (22).
According to a study, oligosaccharides protect intestinal epithelial cells from damage by inhibiting TLR4 expression and increasing crypt cell turnover (23). However, a model of NEC in preterm piglets receiving complex microbial blends did not show any difference in intestinal microbial diversity or protection against NEC (24).
Breast milk-derived products
Exosomes are known to include bioactive constituents such mRNA, miRNA, DNA, and proteins and to be produced by a variety of cell types (25). Breast milk-derived exosomes enhance the development of gut and exerts positive impacts on experimental NEC (Figure 3) (26–29). Breast milk-derived exosomes prevent intestinal stem cells from oxidative stress, which were regulated by the Wnt/-catenin signaling pathway (28). In addition, rat milk-derived exosomes increase intestinal stem cell activity, promote IEC viability, and boost proliferation (30). Porcine milk-derived exosomes were reported to protect the intestinal epithelium against LPS-induced injury by inhibiting excessive inflammation and preventing apoptosis through the action of exosome miRNAs (31). Exosomes isolated from bovine milk were administered to protect experimental NEC-induced bowel injury by enhancing goblet cell production and endoplasmic reticulum function (32). According to these studies, breast milk-derived exosomes may exert potential protective effects against NEC.
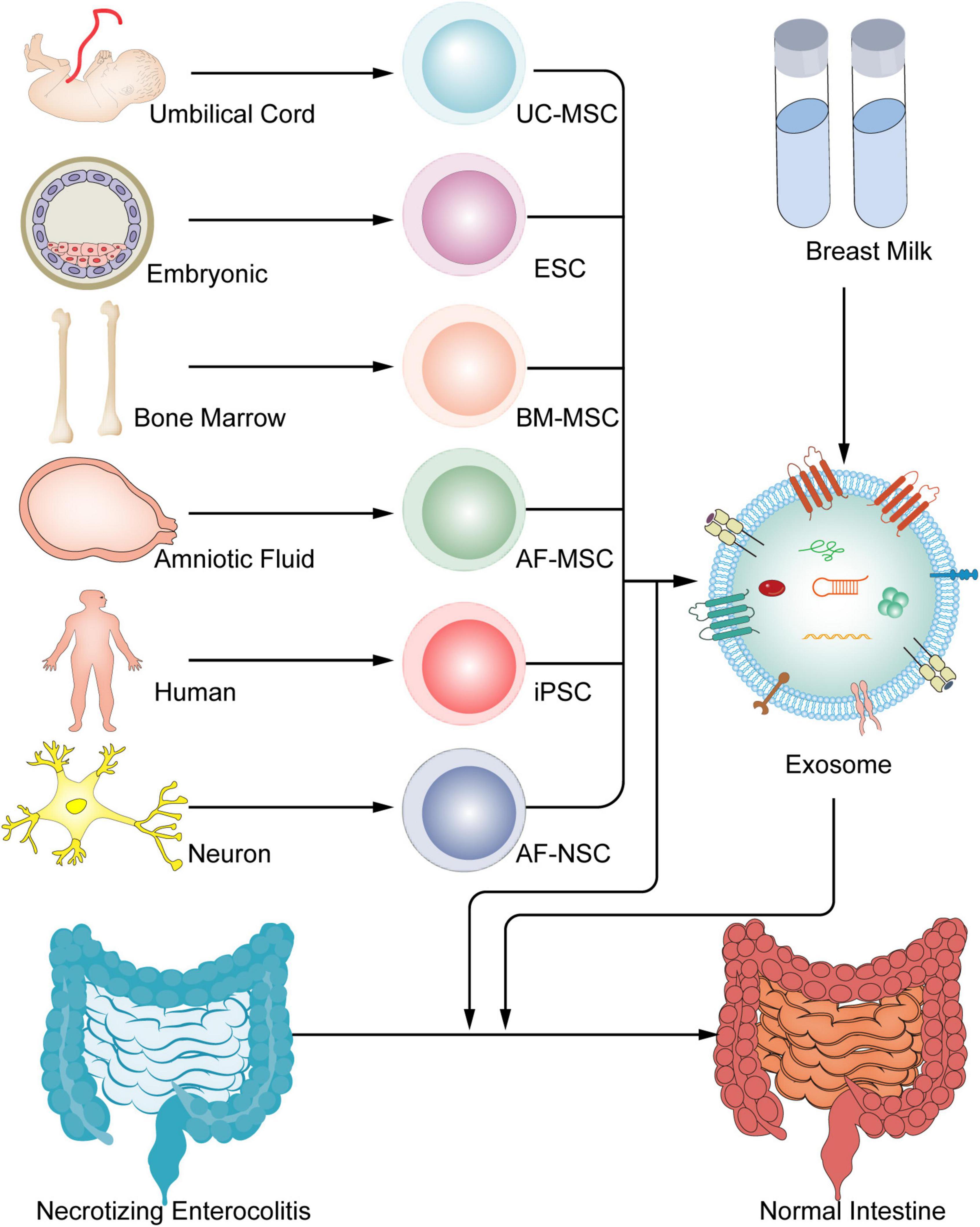
Figure 3. Stem cells and exosomes have been used in the therapy of experimental NECs. Umbilical cord, embryonic, bone marrow, amniotic fluid and so on, have the ability to create stem cells, and all stem cells have the able to generate exosomes, which both can all be treated to treat NEC.
Challenge and limitation of breast milk composition therapy
Lactoferrin, oligosaccharides and exosomes in breast milk have protective effects on NEC. However, these are difficult to implement in the clinic. For instance, lactoferrin and oligosaccharides from breast milk have outstanding anti-inflammatory properties, and relevant clinical research is now underway. Still, they are underutilized in clinical settings, making their promotion difficult. To establish the efficacy and long-term benefits of lactoferrin and oligosaccharides and the optimal dose and administration method, higher-quality, well-designed, larger, multicenter clinical trials are required. Furthermore, breast milk-derived exosomes research for the treatment of NEC is still in its early stages. For further verification and in-depth exploration of the mechanism of exosomes in the treatment of NEC, a large number of animal experiments are required.
Stem cells therapy
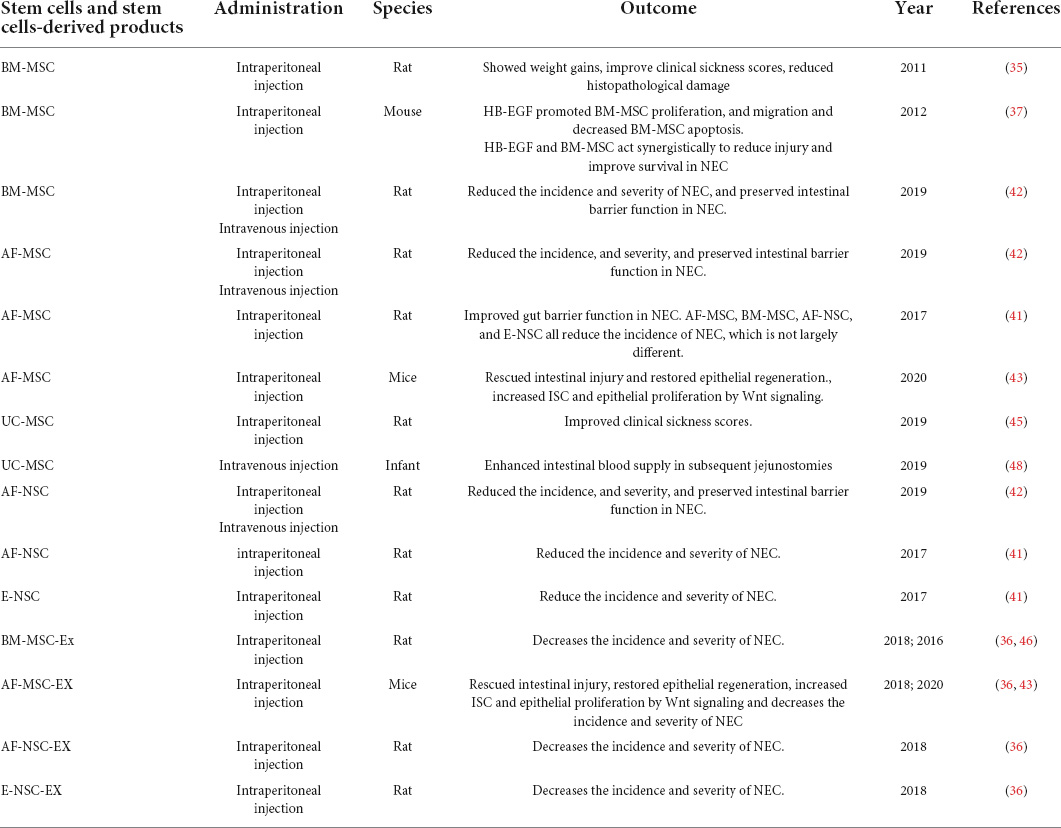
Stem cell therapy is increasingly being proposed as a novel therapeutic approach for a variety of diseases, such as spinal cord injury (SCI), stroke (33, 34). Preclinical research on the potential therapeutic role of stem cells in experimental NEC is growing. This section will cover the therapeutic effectiveness of stem cell and stem cell-derived products in the treatment of NEC and provide an overview of ongoing preclinical research (Table 2).
Bone marrow-derived mesenchymal stem cells (BM-MSCs)
In 2011, MSCs were administered intraperitoneally for the first time to treat NEC in rat models. The results illustrate that MSCs could represent a new treatment option for repairing and regenerating injured intestinal tissue in NEC due to their beneficial effects on reducing inflammation and improving tissue regeneration (35). The same study found that intraperitoneal administration of MSCs reduces injury and improves survival in experimental NEC (36). Researchers compared the therapeutic effects of intraperitoneal- and intravenous -administered MSC when treating experimental NEC. They found that intravenous -administered MSC had dramatically improved intestinal engraftment, intravenous administration may be a more effective delivery method than intraperitoneal administration (37). Even though both routes of administration may be used clinically, intravenous administration is a quick and easy way to inject MSC into the body.
Amniotic fluid-derived mesenchymal stem cells (AF-MSCs)
Amniotic fluid-derived mesenchymal stem cells are cultured using amniotic fluid collected via amniocentesis or cesarean section (38, 39). AF-MSCs therapy has three obvious benefits: AF-MSCs are abundant, are simple to collect and with ease to culture in vitro with modest amounts of medium supplement, and develop quicker than BM-MSCs (40). Due to these advantages, AF-MSCs therapy appears to be the optimum stem cell therapy for treating NEC and has piqued the interest of researchers. Other studies also demonstrated that intraperitoneal injection of AF-MSCs decreased the incidence of NEC and enhanced the intestinal barrier function in rats (41, 42). Similarly, Li et al. (43) discovered that Wnt-β signaling increased cell proliferation while decreased inflammatory factor release, restoring intestinal epithelial regeneration after intraperitoneal injection of AF-MSCs.
Stem cells of other sources
Other sources of stem cells, such as embryonic stem cells (ESCs), umbilical cord-derived mesenchymal stem cells (UC-MSCs), enteral neural stem cells (E-NSCs), amniotic fluid-derived neural stem cells (AF-NSCs) and induced pluripotent stem cells (iPSCs) also have been shown to reduce the incidence of NEC (44, 45). Overall, these findings suggests that stem cell therapy represent a promising treatment for NEC.
Stem cell-derived products
Exosome may reduce the incidence and severity of experimental NEC as effectively as the stem cells from which they derive (Figure 3) (36). According to the study, they showed that the effect on intestinal injury repair was similar with that of BM-MSCs, AF-MSCs, AF-NSCs, and E-NSCs therapy in rat model of NEC (36). Exosomes produced by AF-MSCs largely activated the Wnt/catenin signaling pathway to increase enterocyte proliferation, reduce inflammatory response, and promote normal intestinal epithelium regeneration (43). Researchers reveal that intraperitoneal -administered BM-MSCs-derived exosomes can independently maintain the integrity of the intestinal barrier from experimental NEC (46). Further, the results of the first comprehensive review and meta-analysis of preclinical models examining the role of stem cells- derived exosomes in experimental NEC demonstrated that exosomes derived from stem cells improved survival and reduced the incidence and severity of cases were diagnosed NEC in rat model (47). The results of these studies suggest exosomes are an effective approach in prevention of NEC development.
Challenge and limitation of stem cell therapy
Despite these positive outcomes in animal models, there is currently no ongoing stem cell therapy clinical trial for human NEC. Although an instance of supraventricular tachycardia led to a case of NEC. UC-MSCs were administered intravenously to show enhanced intestinal blood supply in subsequent jejunostomies, without any signs of small bowel syndrome (48). A single instance, though, is insufficient to show that stem cell therapy is available in clinics, and there may be other unidentified aspects that merit research as well. Besides, stem cell therapy is limited in the clinical treatment of NEC due to ethical concerns, immunological rejection and a significant risk of tumorigenesis (49–51). Stem cell therapy is a hard task to convert for preclinical and clinical application since it must also address issues including an augmented immune response, cancer, gene mutation, and ethical concerns. It is crucial to find an efficient therapeutic method that does not directly use stem cells in these conditions. Exosome may reduce the incidence and severity of experimental NEC as effectively as the stem cells from which they derive. The use of stem cell-derived exosomes, may be the best way to overcome some of the limitations of stem cell therapy (36).
Exosome therapy is easier to be administered than stem cell therapy because there is no chance of teratoma formation or ethical concerns. However, researchers continue to face considerable challenges in expanding the use of exosome treatment in clinics. Limitations and Challenge might be from three aspects: (1) long-term exosome extraction, low purity, and partial disintegration of obtained exosome (52–56); (2) poor targeting capability and probable “dilution effect” that could reduce treatment efficacy (57); and (3) absence of research on the precise mechanism of action of exosomes in NEC treatment. Numerous attempts have been made to overcome these limitations, such as enhancing the extraction process for exosomes and extending targeting capability by modification. Chen et al. (58) proposed an anion exchange method for efficiently extracting and detecting exosomes. Furthermore, aptamer-mediated surface modification may boost the specificity of exosomes’ ability to reach injured tissues and organs, displaying enhanced targeting capability (59–62). Exosomes’ unique properties and biological impacts must be comprehended and studied, as well as the underlying mechanism in NEC treatment and their scale-up utilizing existing technology. With sustained research, it is envisaged that exosome therapy will become one of the most promising therapies for NEC.
Therapy with fecal microbiota transplantation
A dysbiosis of the gut microbiome is a risk factor of NEC (63). FMT, a strategy in which healthy feces are transferred to patients with dysbiosis to balance their intestinal flora, has been used to treat clostridium difficile infected diseases (64). Experimental models of NEC have shown positive results when dysbiosis is corrected with FMT. A recent study by Liu et al. concluded that FMT has a unique effect on treating NEC by decreasing inflammation in the intestines, decreasing intestinal permeability, and strengthening the intestinal barrier (65). Brunse et al. examined gut colonization patterns and host reactions to FMT according to different administration routes (66). Rectal administration is the most preferable method of administering FMT, since oral FMT administration increases lethal sepsis incidence and overall mortality by exposing the proximal gut to potentially pathogenic organisms (66). However, according to another study, intragastric administration of FMT appears safe in postsurgical newborn piglets with SBS, with no sepsis and no mortality (67). Hence, there is a need to further explore the security of administration of FMT by different routes.
Challenge and limitation of fecal microbiota transplantation
Even though FMT has shown promising properties in preventing NEC, FMT is associated with safety concerns because no screening method will be able to exclude transfer of an infectious agent from the donor. Yan et al. suggest that the guts of recipients had higher levels of pathogenic signatures from Escherichia coli and Salmonella enterica, which may be a risk factor (68). Oral FMT administration increases lethal sepsis incidence and overall mortality by exposing the proximal gut to potentially pathogenic organisms (66). To improve the safety of FMT, Fecal filtrate transplantation (FFT) and FMT sterilization by ultraviolet radiation are techniques that remove the bacterial component from donor feces by sterile filtration (69, 70). Most studies found that fecal donors are mainly 10-day-old healthy piglets (66, 68, 69). However, there are no standard procedures for selecting donors in NEC animal models. To sum up, there are few published studies on FMT’s effects on NEC, and a greater number are still experimental. Therefore, it is essential to conduct a comprehensive screening procedure in order to determine the characteristics of FMT donors, screen conditions, the preferred route of administration and improve the quality of FMT in the future.
Immunotherapy
TLR4-targeting agents
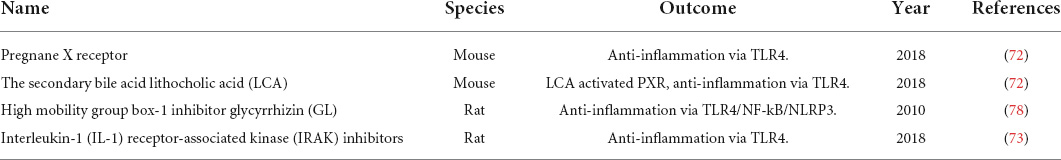
Toll-like receptors (TLRs) are pattern recognition receptors (PRR) of the innate immune system, and each TLR may identify particular pathogen-associated molecular patterns (PAMP). It is generally established that TLRs have a role in NEC pathogenesis, particularly TLR4 which identifies lipopolysaccharides in Gram-negative bacteria. TLR4 was reported to be highly activated in both neonatal rats and human infants in the event of NEC (71). Researchers have shown that TLR4-deficient mice don’t display significant inflammatory responses (72, 73). Studies have demonstrated the importance of TLR4 signal activation in the development of NEC, as it can provoke excessive intestinal inflammation and increase the apoptosis and necrosis of enterocytes (74–77). TLR4-targeted agents have the potential to be useful in the treatment of NEC (Table 3).
Pregnane X receptor (PXR) can function as an external biosensor and signal intermediate in producing various host-bacterial metabolites. It has been proven with an ability to inhibit TLR4 signal expression. According to an animal study, mice with PXR knockout exhibited more severe disease symptoms following experimental NEC induction (72). Lithocholic acid (LCA), a liver-distributed PXR agonist, could activate intestinal PXR, reducing NEC-related intestinal inflammation (72). The high mobility group box 1 (HMGB1) is essentially required for the incidence and progression of NEC. In animal investigations, it was revealed that when NEC developed, HMGB1 expression increased, and inflammatory cell migration was facilitated (78). Yu et al. (78) examined the effect of glycyrrhizin (GL), and HMGB1 inhibitor, in NEC and reported that it might inhibit TLR4 and the downstream NF-κB/NLRP3 signaling pathway, resulting in decreased intestinal inflammation. Hou et al. (73) revealed that an interleukin-1 receptor-associated kinase (IRAK) inhibitor lowered inflammatory factor production by downregulating TLR4 receptor expression, thereby reducing the severity of NEC-induced intestinal inflammation. The possibility that TLR4-targeted drugs particular to the pathophysiology of NEC suggest that they may represent an innovative treatment strategy.
Challenge and limitation of immunotherapy
There is evidence that targeting TRL4 and employing biological agents to treat NEC has therapeutic effects, but related research is still in the phase of animal testing. Furthermore, the exact mechanism of action remains a mystery that must be clarified. In this context, greater emphasis should be made on the specific mode of action of TLR4-targeted drugs and appropriate biological agents to repair small intestinal injuries. As a result, it is anticipated that more effective, specialized novel drugs will be developed at the molecular level and subsequently used in NEC treatment.
Conclusion
This review outlines lactoferrin, oligosaccharides, exosomes in breast milk, stem cells and stem cells derived-exosomes, TLR4-targeted agents, and FMT, have demonstrated promising therapeutic effects and clinical application potential for the NEC therapy. Further elucidation of mechanisms, advancements in preparation, bioengineering, and application, as well as strict clinical trials, will support the use of Lactoferrin, oligosaccharides, exosomes in breast milk, stem cells and stem cells derived-exosomes, TLR4-targeted agents, and FMT, as new therapeutics for pediatric diseases.
Author contributions
HW, KG, and ZZ: drafting and revising manuscript. RZ, YL, QY, JL, RJ, and ZH: participating in revising the manuscript. WS and HC: reviewing the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82171698, 82170561, 81741067, 81300279, and 82000355), the National Science Fund for Distinguished Young Scholars of Guangdong Province (2021B1515020003), the Climbing Program of Introduced Talents and High-level Hospital Construction Project of Guangdong Provincial People’s Hospital (DFJH201803, KJ012019099, KY012021183, and KJ012021143), the Guangdong Provincial Natural Science Foundation (2018A030310016), and the Guangzhou Science and Technology Program (202102080037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. (2016) 13:590–600.
2. Thyoka M, de Coppi P, Eaton S, Khoo K, Hall NJ, Curry J, et al. Advanced necrotizing enterocolitis part 1: mortality. Eur J Pediatr Surg. (2012) 22:8–12.
3. Spencer AU, Kovacevich D, McKinney-Barnett M, Hair D, Canham J, Maksym C, et al. Pediatric short-bowel syndrome: the cost of comprehensive care. Am J Clin Nutr. (2008) 88:1552–9.
4. Bazacliu C, Neu J. Necrotizing enterocolitis: long term complications. Curr Pediatr Rev. (2019) 15:115–24.
5. Weintraub AS, Ferrara L, Deluca L, Moshier E, Green RS, Oakman E, et al. Antenatal antibiotic exposure in preterm infants with necrotizing enterocolitis. J Perinatol. (2012) 32:705–9.
6. Wong D, Abdel-Latif M, Kent A, Network N. Antenatal steroid exposure and outcomes of very premature infants: a regional cohort study. Arch Dis Child Fetal Neonatal Ed. (2014) 99:F12–20.
7. Downard CD, Grant SN, Maki AC, Krupski MC, Matheson PJ, Bendon RW, et al. Maternal cigarette smoking and the development of necrotizing enterocolitis. Pediatrics. (2012) 130:78–82.
8. Garcia-Munoz Rodrigo F, Galan Henriquez G, Figueras Aloy J, Garcia-Alix Perez A. Outcomes of very-low-birth-weight infants exposed to maternal clinical chorioamnionitis: a multicentre study. Neonatology. (2014) 106:229–34. doi: 10.1159/000363127
9. Grandi C, Tapia JL, Cardoso VC. Impact of maternal diabetes mellitus on mortality and morbidity of very low birth weight infants: a multicenter Latin America study. J Pediatr (Rio J). (2015) 91:234–41. doi: 10.1016/j.jped.2014.08.007
10. Cetinkaya M, Ozkan H, Koksal N. Maternal preeclampsia is associated with increased risk of necrotizing enterocolitis in preterm infants. Early Hum Dev. (2012) 88:893–8.
12. Denning TL, Bhatia AM, Kane AF, Patel RM, Denning PW. Pathogenesis of NEC: role of the innate and adaptive immune response. Semin Perinatol. (2017) 41:15–28.
13. Wang C, Zhang M, Guo H, Yan J, Chen L, Teng W, et al. Human milk oligosaccharides activate epidermal growth factor receptor and protect against hypoxia-induced injuries in the mouse intestinal epithelium and Caco2 cells. J Nutr. (2020) 150:756–62. doi: 10.1093/jn/nxz297
14. Liu J, Zhu H, Li B, Robinson SC, Lee C, O’Connell JS, et al. Lactoferrin reduces necrotizing enterocolitis severity by upregulating intestinal epithelial proliferation. Eur J Pediatr Surg. (2020) 30:90–5.
15. Queiroz VA, Assis AM, Júnior Hda R. Protective effect of human lactoferrin in the gastrointestinal tract. Rev Paul Pediatr. (2013) 31:90–5.
16. Nguyen DN, Jiang P, Stensballe A, Bendixen E, Sangild PT, Chatterton DE. Bovine lactoferrin regulates cell survival, apoptosis and inflammation in intestinal epithelial cells and preterm pig intestine. J Proteomics. (2016) 139:95–102. doi: 10.1016/j.jprot.2016.03.020
17. Serce Pehlevan O, Benzer D, Gursoy T, Aktas Cetin E, Karatekin G, Ovali MF. Cytokine responses to symbiotic and lactoferrin combination in very low birth weight neonates: a randomized control trial. Arch Argent Pediatr. (2020) 118:e8–15. doi: 10.5546/aap.2020.eng.e8
18. Manzoni P, Meyer M, Stolfi I, Rinaldi M, Cattani S, Pugni L, et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: a randomized clinical trial. Early Hum Dev. (2014) 90:S60–5.
19. Gao Y, Hou L, Lu C, Wang Q, Pan B, Wang Q, et al. Enteral lactoferrin supplementation for preventing sepsis and necrotizing enterocolitis in preterm infants: a metaanalysis with trial sequential analysis of randomized controlled trials. Front Pharmacol. (2020) 11:1186. doi: 10.3389/fphar.2020.01186
20. Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. (2012) 61:1417–25.
21. Autran CA, Kellman BP, Kim JH, Asztalos E, Blood AB, Spence ECH, et al. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut. (2018) 67:1064–70. doi: 10.1136/gutjnl-2016-312819
22. Zhang W, He-Yang J, Tu W, Zhou X. Sialylated human milk oligosaccharides prevent intestinal inflammation by inhibiting toll like receptor 4/NLRP3 inflammasome pathway in necrotizing enterocolitis rats. Nutr Metab (Lond). (2021) 18:5. doi: 10.1186/s12986-020-00534-z
23. Wang C, Zhang M, Guo H, Yan J, Liu F, Chen J, et al. Human milk oligosaccharides protect against necrotizing enterocolitis by inhibiting intestinal damage via increasing the proliferation of crypt cells. Mol Nutr Food Res. (2019) 63:e1900262. doi: 10.1002/mnfr.201900262
24. Rasmussen SO, Martin L, Ostergaard MV, Rudloff S, Roggenbuck M, Nguyen DN, et al. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J Nutr Biochem. (2017) 40:141–54. doi: 10.1016/j.jnutbio.2016.10.011
25. Whiteside TL. The role of tumor-derived exosomes (TEX) in shaping anti-tumor immune competence. Cells. (2021) 10:3054. doi: 10.3390/cells10113054
26. Martin C, Patel M, Williams S, Arora H, Brawner K, Sims B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. (2018) 24:278–84. doi: 10.1177/1753425918785715
27. Wang X, Yan X, Zhang L, Cai J, Zhou Y, Liu H, et al. Identification and peptidomic profiling of exosomes in preterm human milk: insights into necrotizing enterocolitis prevention. Mol Nutr Food Res. (2019):e1801247. doi: 10.1002/mnfr.201801247
28. Dong P, Zhang Y, Yan DY, Wang Y, Xu X, Zhao YC, et al. Protective effects of human milk-derived exosomes on intestinal stem cells damaged by oxidative stress. Cell Transplant. (2020) 29:963689720912690. doi: 10.1177/0963689720912690
29. Gao R, Zhang R, Qian T, Peng X, He W, Zheng S, et al. A comparison of exosomes derived from different periods breast milk on protecting against intestinal organoid injury. Pediatr Surg Int. (2019) 35:1363–8. doi: 10.1007/s00383-019-04562-6
30. Hock A, Miyake H, Li B, Lee C, Ermini L, Koike Y, et al. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg. (2017) 52:755–9.
31. Xie MY, Hou LJ, Sun JJ, Zeng B, Xi QY, Luo JY, et al. Porcine milk exosome MiRNAs attenuate LPS-induced apoptosis through inhibiting TLR4/NF-kappaB and p53 pathways in intestinal epithelial cells. J Agric Food Chem. (2019) 67:9477–91. doi: 10.1021/acs.jafc.9b02925
32. Li B, Hock A, Wu RY, Minich A, Botts SR, Lee C, et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS One. (2019) 14:e0211431. doi: 10.1371/journal.pone.0211431
33. Shao A, Tu S, Lu J, Zhang J. Crosstalk between stem cell and spinal cord injury: pathophysiology and treatment strategies. Stem Cell Res Ther. (2019) 10:238. doi: 10.1186/s13287-019-1357-z
34. Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. (2017) 158:94–131. doi: 10.1016/j.pneurobio.2017.07.004
35. Tayman CN, Uckan D, Ulus AT, Tonbul A, Hirfanoglu IM, Helvacioglu F, et al. mesenchymal stem cell therapy in necrotizing enterocolitis a rat study. Pediatr Res. (2011) 70:489–94.
36. McCulloh CJ, Olson JK, Wang Y, Zhou Y, Tengberg NH, Deshpande S, et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg. (2018) 53:1215–20.
37. Yang J, Watkins D, Chen C, Bhushan B, Zhou Y, Besner G. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg. (2012) 215:534–45. doi: 10.1016/j.jamcollsurg.2012.05.037
38. Murphy SV, Atala A. Amniotic fluid and placental membranes: unexpected sources of highly multipotent cells. Semin Reprod Med. (2013) 31:62–8. doi: 10.1055/s-0032-1331799
39. Bottai D, Cigognini D, Nicora E, Moro M, Grimoldi MG, Adami R, et al. Third trimester amniotic fluid cells with the capacity to develop neural phenotypes and with heterogeneity among sub-populations. Restor Neurol Neurosci. (2012) 30:55–68. doi: 10.3233/RNN-2011-0620
40. McCulloh CJ, Olson JK, Zhou Y, Wang Y, Besner GE. Stem cells and necrotizing enterocolitis: a direct comparison of the efficacy of multiple types of stem cells. J Pediatr Surg. (2017) 52:999–1005. doi: 10.1016/j.jpedsurg.2017.03.028
41. McCulloh CJ, Olson JK, Wang Y, Vu J, Gartner S, Besner GE. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J Surg Res. (2017) 214:278–85. doi: 10.1016/j.jss.2017.03.026
42. Pisano C, Besner GE. Potential role of stem cells in disease prevention based on a murine model of experimental necrotizing enterocolitis. J Pediatr Surg. (2019) 54:413–6. doi: 10.1016/j.jpedsurg.2018.07.025
43. Li B, Lee C, O’Connell JS, Antounians L, Ganji N, Alganabi M, et al. Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis. (2020) 11:750. doi: 10.1038/s41419-020-02964-2
44. Kagia A, Tzetis M, Kanavakis E, Perrea D, Sfougataki I, Mertzanian A, et al. Therapeutic effects of mesenchymal stem cells derived from bone marrow, umbilical cord blood, and pluripotent stem cells in a mouse model of chemically induced inflammatory bowel disease. Inflammation. (2019) 42:1730–40. doi: 10.1007/s10753-019-01033-x
45. Drucker NA, Te Winkel JP, Shelley WC, Olson KR, Markel TA. Inhibiting hydrogen sulfide production in umbilical stem cells reduces their protective effects during experimental necrotizing enterocolitis. J Pediatr Surg. (2019) 54:1168–73. doi: 10.1016/j.jpedsurg.2019.02.037
46. Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. (2016) 51:942–7. doi: 10.1016/j.jpedsurg.2016.02.061
47. Villamor-Martinez E, Hundscheid T, Kramer BW, Hooijmans CR, Villamor E. Stem cells as therapy for necrotizing enterocolitis: a systematic review and meta-analysis of preclinical studies. Front Pediatr. (2020) 8:578984. doi: 10.3389/fped.2020.578984
48. Akduman H, Dilli D, Ergun E, Cakmakci E, Celebi SK, Citli R, et al. Successful mesenchymal stem cell application in supraventricular tachycardia-related necrotizing enterocolitis: a case report. Fetal Pediatr Pathol. (2019) 40:250–55. doi: 10.1080/15513815.2019.1693672
49. Schwartz SD, Hubschman J-P, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. (2012) 379:713–20.
50. Moradi S, Mahdizadeh H, Saric T, Kim J, Harati J, Shahsavarani H, et al. Research and therapy with induced pluripotent stem cells (iPSCs): social, legal, and ethical considerations. Stem Cell Res Ther. (2019) 10:341. doi: 10.1186/s13287-019-1455-y
51. Deng J, Zhang Y, Xie Y, Zhang L, Tang P. Cell transplantation for spinal cord injury: tumorigenicity of induced pluripotent stem cell-derived neural stem/progenitor cells. Stem Cells Int. (2018) 2018:5653787.
52. Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. (2013) 13:3354–64. doi: 10.1002/pmic.201300282
53. Liga A, Vliegenthart AD, Oosthuyzen W, Dear JW, Kersaudy-Kerhoas M. Exosome isolation: a microfluidic road-map. Lab Chip. (2015) 15:2388–94. doi: 10.1039/c5lc00240k
54. Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. (2012) 56:293–304. doi: 10.1016/j.ymeth.2012.01.002
55. Wan Y, Cheng G, Liu X, Hao SJ, Nisic M, Zhu CD, et al. Rapid magnetic isolation of extracellular vesicles via lipid-based nanoprobes. Nat Biomed Eng. (2017) 1:0058. doi: 10.1038/s41551-017-0058
56. Woo HK, Sunkara V, Park J, Kim TH, Han JR, Kim CJ, et al. Exodisc for rapid, size-selective, and efficient isolation and analysis of nanoscale extracellular vesicles from biological samples. ACS Nano. (2017) 11:1360–70. doi: 10.1021/acsnano.6b06131
57. Fuhrmann G, Chandrawati R, Parmar PA, Keane TJ, Maynard SA, Bertazzo S, et al. Engineering extracellular vesicles with the tools of enzyme prodrug therapy. Adv Mater. (2018) 30:e1706616. doi: 10.1002/adma.201706616
58. Chen J, Xu Y, Lu Y, Xing W. Isolation and visible detection of tumor-derived exosomes from plasma. Anal Chem. (2018) 90:14207–15. doi: 10.1021/acs.analchem.8b03031
59. Liu C, Zhao J, Tian F, Chang J, Zhang W, Sun J. lambda-DNA- and aptamer-mediated sorting and analysis of extracellular vesicles. J Am Chem Soc. (2019) 141:3817–21. doi: 10.1021/jacs.9b00007
60. Luo ZW, Li FX, Liu YW, Rao SS, Yin H, Huang J, et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. (2019) 11:20884–92. doi: 10.1039/c9nr02791b
61. Tran PH, Xiang D, Nguyen TN, Tran TT, Chen Q, Yin W, et al. Aptamer-guided extracellular vesicle theranostics in oncology. Theranostics. (2020) 10:3849–66. doi: 10.7150/thno.39706
62. Zou J, Shi M, Liu X, Jin C, Xing X, Qiu L, et al. Aptamer-functionalized exosomes: elucidating the cellular uptake mechanism and the potential for cancer-targeted chemotherapy. Anal Chem. (2019) 91:2425–30. doi: 10.1021/acs.analchem.8b05204
63. Brehin C, Dubois D, Dicky O, Breinig S, Oswald E, Serino M. Evolution of gut microbiome and metabolome in suspected necrotizing enterocolitis: a case-control study. J Clin Med. (2020) 9:2278. doi: 10.3390/jcm9072278
64. Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection: a randomized trial. Ann Intern Med. (2016) 165:609–16. doi: 10.7326/M16-0271
65. Liu J, Miyake H, Zhu H, Li B, Alganabi M, Lee C, et al. Fecal microbiota transplantation by enema reduces intestinal injury in experimental necrotizing enterocolitis. J Pediatr Surg. (2020) 55:1094–8. doi: 10.1016/j.jpedsurg.2020.02.035
66. Brunse A, Martin L, Rasmussen TS, Christensen L, Skovsted Cilieborg M, Wiese M, et al. Effect of fecal microbiota transplantation route of administration on gut colonization and host response in preterm pigs. ISME J. (2019) 13:720–33. doi: 10.1038/s41396-018-0301-z
67. Hinchliffe T, Pauline ML, Wizzard PR, Jovel J, Nation PN, Wales PW, et al. The effect of fecal microbial transplant on intestinal microbial composition in short-bowel neonatal piglets. JPEN J Parenter Enteral Nutr. (2022) doi: 10.1002/jpen.2333
68. Hui Y, Vestergaard G, Deng L, Kot WP, Thymann T, Brunse A, et al. Donor-dependent fecal microbiota transplantation efficacy against necrotizing enterocolitis in preterm pigs. NPJ Biofilms Microbiomes. (2022) 8:48. doi: 10.1038/s41522-022-00310-2
69. Brunse A, Deng L, Pan X, Hui Y, Castro-Mejia JL, Kot W, et al. Fecal filtrate transplantation protects against necrotizing enterocolitis. ISME J. (2022) 16:686–94. doi: 10.1038/s41396-021-01107-5
70. Prado C, Abatti MR, Michels M, Corneo E, Cucker L, Borges H, et al. Comparative effects of fresh and sterile fecal microbiota transplantation in an experimental animal model of necrotizing enterocolitis. J Pediatr Surg. (2022) doi: 10.1016/j.jpedsurg.2021.12.013
71. Liu T, Zong H, Chen X, Li S, Liu Z, Cui X, et al. Toll-like receptor 4-mediated necroptosis in the development of necrotizing enterocolitis. Pediatr Res. (2021) 91:73–82. doi: 10.1038/s41390-021-01457-y
72. Huang K, Mukherjee S, DesMarais V, Albanese JM, Rafti E, Draghi Ii A, et al. Targeting the PXR-TLR4 signaling pathway to reduce intestinal inflammation in an experimental model of necrotizing enterocolitis. Pediatr Res. (2018) 83:1031–40. doi: 10.1038/pr.2018.14
73. Hou Y, Lu X, Zhang Y. IRAK Inhibitor protects the intestinal tract of necrotizing enterocolitis by inhibiting the toll-like receptor (TLR) inflammatory signaling pathway in rats. Med Sci Monit. (2018) 24:3366–73. doi: 10.12659/MSM.910327
74. Jia H, Sodhi CP, Yamaguchi Y, Lu P, Ladd MR, Werts A, et al. Toll like receptor 4 mediated lymphocyte imbalance induces nec-induced lung injury. Shock. (2019) 52:215–23. doi: 10.1097/SHK.0000000000001255
75. Du M, Yuan L, Tan X, Huang D, Wang X, Zheng Z, et al. The LPS-inducible lncRNA Mirt2 is a negative regulator of inflammation. Nat Commun. (2017) 8:2049. doi: 10.1038/s41467-017-02229-1
76. Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. (2016) 126:495–508.
77. Zhou Y, Li Y, Zhou B, Chen K, Lyv Z, Huang D, et al. Inflammation and apoptosis: dual mediator role for toll-like receptor 4 in the development of necrotizing enterocolitis. Inflamm Bowel Dis. (2017) 23:44–56. doi: 10.1097/MIB.0000000000000961
Keywords: necrotizing enterocolitis, breast milk composition, stem cell, fecal microbiota transplantation, immunotherapy
Citation: Wu H, Guo K, Zhuo Z, Zeng R, Luo Y, Yang Q, Li J, Jiang R, Huang Z, Sha W and Chen H (2022) Current therapy option for necrotizing enterocolitis: Practicalities and challenge. Front. Pediatr. 10:954735. doi: 10.3389/fped.2022.954735
Received: 27 May 2022; Accepted: 07 July 2022;
Published: 28 July 2022.
Edited by:
Bo Li, University of Toronto, CanadaReviewed by:
Xiaoyan Feng, University Hospital Leipzig, GermanyGiuseppe Lauriti, G. d’Annunzio University of Chieti and Pescara, Italy
Copyright © 2022 Wu, Guo, Zhuo, Zeng, Luo, Yang, Li, Jiang, Huang, Sha and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihong Sha, c2hhd2VpaG9uZ0BnZHBoLm9yZy5jbg==; Hao Chen, Y2hlbmhhb0BnZHBoLm9yZy5jbg==
†These authors have contributed equally to this work
 Huihuan Wu
Huihuan Wu Kehang Guo1,2†
Kehang Guo1,2† Zewei Zhuo
Zewei Zhuo Ruijie Zeng
Ruijie Zeng Zena Huang
Zena Huang Weihong Sha
Weihong Sha Hao Chen
Hao Chen