
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 20 December 2022
Sec. Pediatric Infectious Diseases
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.949817
This article is part of the Research Topic Case Reports in Pediatric Infectious Diseases 2022 View all 10 articles
Human bocavirus 1 (HBoV1) belongs to the family Parvoviridae and it is acknowledged that HBoV1 is a respiratory pathogen. We report the case of a 13-month-old boy who presented with a cough, shortness of breath, and wheezing, and who eventually died of severe pneumonia and acute respiratory distress syndrome (ARDS). Metagenomics next-generation sequencing (mNGS) showed that HBoV1 was the only detected pathogen. The nasopharyngeal aspirate viral load was 2.08 × 1010 copies/ml and the serum viral load was 2.37 × 105 copies/ml. The child was still oxygen deficient under mechanical ventilation. Chest imaging suggested diffuse lesions in both lungs, an injury caused by ARDS. In this case, the clinical symptoms and signs of the child, the high viral load, viremia, and the detection of mNGS in the tracheal aspirate all supported that HBoV1 could cause severe acute respiratory tract infection in children without other pathogen infections.
Human bocavirus 1 (HBoV1) was first discovered in respiratory secretions by Allander et al. in 2005 (1); later, three other bocaviruses (HBoV2, 3, and 4) were successively found in fecal samples (2). HBoV1, which belongs to the family of Parvoviridae and the genus of Bocaparvovirus, comprises a non-enveloped capsid with a single-stranded linear DNA and is the second known human parvovirus that can replicate autonomously after parvovirus B19 (3, 4). The clinical manifestations of HBoV1 infection are often atypical and similar to other respiratory virus infections, such as rhinitis, acute otitis media, pneumonia, bronchiolitis, and asthma exacerbation (5). The symptoms of HBoV1 infection are mild and self-limited and are easy to be ignored by clinicians. HBoV1 is often combined with other respiratory viruses, and the co-infection rate is as high as 75% (6, 7). The pathogenicity of HBoV1 was earlier considered controversial because HBoV1 DNA can persist for months in airway secretions following primary infection and can also be shed in the nasopharyngeal aspirates (NPAs) of asymptomatic healthy children. However, increasing evidence supports that HBoV1 is associated with respiratory symptoms without other pathogens detected and can even cause severe lower respiratory tract infections (8). A case of severe pneumonia, acute respiratory distress syndrome (ARDS), and respiratory failure brought on by HBoV1 infection was reported in our study, along with a review of the literature.
A 13-month-old boy, the second child of consanguineous parents, was admitted to the intensive care unit (ICU) of the Children's Hospital of Chongqing Medical University in October 2018. He presented with a paroxysmal cough for 1 month, aggravated with shortness of breath and wheezing for 1 week. Regarding the patient’s family history, there was nothing unusual in the immune system. The child was delivered naturally at full term and has received his BCG vaccination. There was no history of feeding difficulties or repeated respiratory tract infections. He had not been exposed to toxic substances, gases, smog, or allergens.
A chest CT scan at a local hospital revealed a bilateral lung infection. The treatment of cefminox and interferon-α1b was not effective. The cyanosis was difficult to relieve. The patient was then admitted to our hospital with endotracheal intubation for further treatment. At admission, the boy had malnutrition with a normal body temperature and oxygen saturation of 94% under assisted ventilation (other vital signs were as follows: heart rate of 147 beats/min, the respiration rate of 30 beats/min, bodyweight of 5.5 kg, blood pressure of 77/50 mmHg). Nasal flaring, cyanosis around the lips, and an inspiratory triple concave sign were observed. Chest auscultation revealed bilateral coarse crackles.
Chest radiographs showed decreased translucency in both lungs and an extensively increased density shadow in the alveoli, showing ground-glass changes. Some showed a mesh change, a slightly dilated bronchus, and localized hyperinflation of the upper lobe of the left lung. Lung injury caused by a diffuse interstitial lesion of both lungs and ARDS were considered (Figure 1). The blood routine on admission showed that the white blood cell counts were elevated (18.91 × 109/L) (reference value: 4.3–11.3 × 109/L), neutrophils mainly, and a thrombocyte count of 490 × 109/L (reference value: 100–453 × 109/L), a hemoglobin concentration of 121 g/L (reference value: 118–156 × 109 g/L), and a slightly elevated level of C-reactive protein (CRP) (9 mg/L) (reference value: <8 mg/L); other markers were within the reference ranges. After treatment with piperacillin-tazobactam for 4 days and meropenem for 6 days, the inflammatory indicators gradually decreased. However, on the 10th day of hospitalization, the white cell count and the neutrophil percentage increased again, and the level of CRP increased to 20 mg/L. The platelet count increased with the continuous decrease of red blood cells and hemoglobin (81–121 g/L), and the child had no bleeding tendencies. Amphotericin B, meropenem, and cefoperazone-sulbactam were administered as treatment, but the patient's condition did not improve. The level of CRP had no obvious change (18–20 mg/L), the white blood cell count continued to rise (12.25–18.45 × 109/L), and the level of hemoglobin decreased to 77 g/L. Arterial blood gas analysis indicated type 2 respiratory failure (PO253 mmHg, PCO262 mmHg). After admission, the assisted ventilation was continued, and the patient still had persistent oxygenation disorders. The child had hypoproteinemia, increased lactate dehydrogenase, and a decrease in cholinesterase. Immunoglobulin levels (including IgM, IgG, and IgA) were in the normal range. The C3 complement was 0.43 g/L. Tumor markers were negative. Flow cytometry was used for the detection of T cell, B cell, NK cell and other lymphocyte subsets were determined, with no obvious abnormalities (CD4/CD8 2.37, CD3 + 1610.88/μl, CD3 + CD8 + 457.59/μl, CD3 + CD4 + 1084.62/μl, NK cell 53/μl). The TREC gene was detected by fluorescence probe PCR screening for severe combined immunodeficiency diseases. The result was >1,000 copies/μl (<10 copies/μl is considered to be severe combined immune deficiency, 10–1,000 copies/μl is considered to be a primary immune syndrome or normal, and >1,000 copies/μl is considered normal). The fungal D-glucan test, T-SPOT test, purified protein derivative test, Mycoplasma pneumonia/Chlamydia PCR, sputum bacterial and fungal culture, and double blood were negative. Pneumocystis was not detected in the next-generation sequencing (NGS) of bronchoalveolar lavage (BAL) fluid, and CT findings did not support pneumocystis Jiroveci pneumonia. All tests were negative for influenza virus A and B, respiratory syncytial, adenovirus, parainfluenza 1, 2, and 3, coronaviruses (including HKU-1, OC43, 229E, NL63), rhino/enterovirus, parechovirus, Hanta pulmonary syndrome, and fungi. The results of metagenomics NGS (mNGS) in serum and respiratory secretions suggested that HBoV had a high detection sequence of 112,786 reads and 100% gene coverage. The coverage of Acinetobacter baumannii, Pseudomonas fluorescens, and Pseudomonas aeruginosa were 0.889%, 0.058%, and 0.005%, respectively, and the reads were 370, 14, and 2, which were considered colonized bacteria. The mNGS was carried out by a core facility (Kindstar Global, Wuhan, China). HBoV was the only viral pathogen detected. No other pathogens were detected. Quantitative PCR (qPCR) showed that the viral load of NPA was 2.08 × 1010 copies/ml and the viral load of serum was 2.37 × 105 copies/ml. Viral DNA and RNA were extracted from 200-µl aliquots of the NPA samples by the QIAamp MinElute Virus Spin kit (Qiagen, Hilden, Germany). The RNA was applied as the template for complementary DNA (cDNA) synthesis with the SuperScript III First-Strand Synthesis System (Invitrogen, California, USA). DNA and RNA extractions and cDNA products were used for the subsequent testing of respiratory viruses (9). HBoV1-specific primers were forward primer amplification of 5′-CCTATATAAGCTGCTGCACTTCCTG-3′ and reverse primer 5′-AAGCCATAGTAGACTCACCACAAG-3′ (10, 11). The plasmid amplified target fragment was cloned into the pMD19-T vector (TaKaRa Biotechonology, Dalian, China). The PCR process was performed exactly as described in the report (10), except for the AmpErase-UNG at 50°C. Each run included plasmid and negative controls. Standard precautions were taken throughout the PCR process to avoid cross-contamination. Negative controls and serial dilutions of the positive controls were included in every PCR assay. Therefore, HBoV was considered to be the pathogen in this case. Finally, the boy was diagnosed with severe pneumonia, ARDS, and diffuse pulmonary interstitial disease.
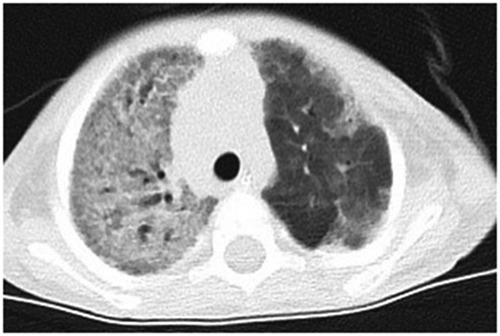
Figure 1. The chest-CT on the 14th day after hospitalization showed both lungs decreased translucency, extensive parenchymal opacities, and ground glass change. Some showed grid change and slightly dilated bronchus throughout the course.
Due to the high suspicion of the possibility of respiratory system-related genetic diseases, complete exon sequencing (completed by Beijing Mygeno) was completed during hospitalization. It indicated that the patient had a heterozygous variant in the STAT1 gene: C. 1127 + 10G > A, causing amino acid change splicing. After 16 days of treatment in the ICU, the patient's pulmonary symptoms were not alleviated, and the chest CT scan showed that the diffuse lesions in both lungs were still severe. The patient's family was required to give up treatment, and the patient died.
Since 2005, there has been increasing evidence supporting HBoV1 as an actual human pathogen that causes mild to severe respiratory infections. HBoV1 DNA is detectable in respiratory secretions in 2%–20% of children with acute respiratory tract infections (12). However, HBoV is not used to routinely detect respiratory viruses in clinical practice, which can easily lead to the neglect of clinicians and a missed diagnosis. However, increasing cases of severe and even fatal respiratory HBoV1 infections have been reported in recent years. In this study, the clinical characteristics, risk factors, methods of detection, and treatment of severe HBoV1 infection are discussed. Here, we present a case of severe HBoV1 infection and review the literature to provide a reference for its clinical diagnosis and treatment.
The major manifestations of severe HBoV1 infection were a respiratory failure or respiratory distress, fever, and wheezing. A total of 17 cases of HBoV1-related pediatric severe respiratory infection admitted to the ICU were collected from 10 pieces of literature (8, 13–21) (Table 1). Among these 17 children, fever and wheezing were the first symptoms in most cases. Specifically, the symptoms included respiratory failure or respiratory distress (n = 12), cough (n = 2), wheezing (n = 5), fever (n = 4), and diarrhea (n = 1). The diagnosis was respiratory failure (n = 5), ARDS (n = 4), bronchiolitis (n = 3), atelectasis (n = 2), and status asthma (n = 4), suggesting that severe HBoV infection often leads to respiratory failure and ARDS. Except for a 4-year-old child and two newborn patients, the children were aged between 6 months and 2 years. Chest imaging suggested interstitial infiltration and atelectasis. Specifically, it included atelectasis (n = 4), infiltration (n = 7), and hyperinflation (n = 1), which were consistent with our case. Our patient was 13 months old, and his first symptoms were a cough and fever, aggravated by shortness of breath, cyanosis, and hypoxemia. The chest CT scan suggested ground-glass changes, with diffuse lesions in both lungs. In the laboratory tests, with the increase in disease severity, the proportion of neutropenia and lymphocytopenia significantly increased. Procalcitonin and CRP obviously increased. In severe cases reported in the past, the levels of CRP and leukocytes were also slightly elevated. Among them, HBoV1 was the only pathogen detected. Jula et al. (12) reported that a tiny amount of HRV RNA was detected. However, the copy number of HBoV1 DNA was significant, so it was still highly correlated with HBoV1 infection.
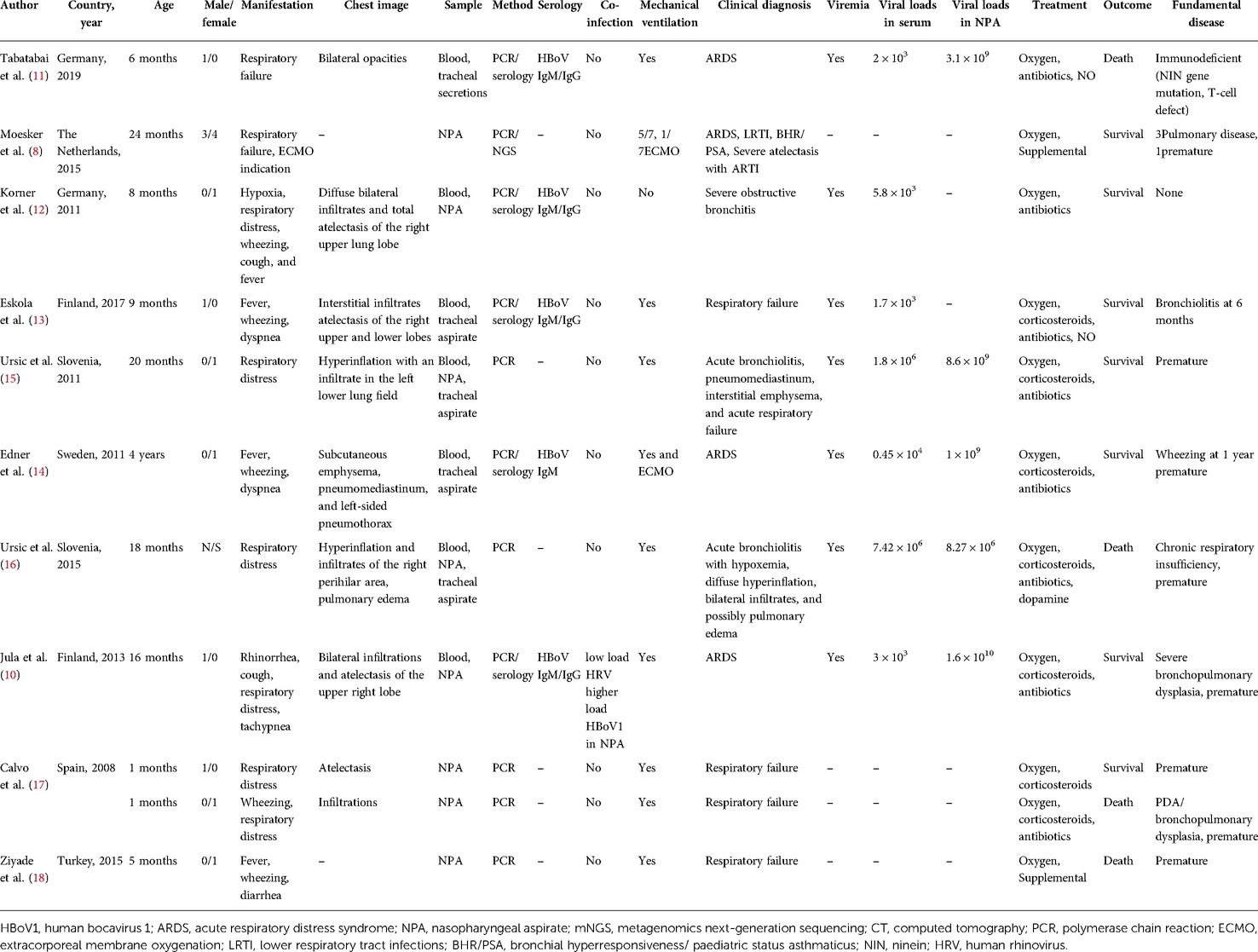
Table 1. The reported severe respiratory tract infection cases admitted to ICU were caused by HBoV1.
The risk factors for severe HBoV1 infections include underlying chronic conditions, such as congenital heart disease, chronic lung disease, premature birth, cancer, and immune deficiency (5). Of the 17 patients, 8 were premature infants, 6 had underlying pulmonary disease, 1 had patent ductus arteriosus (PDA), 2 had a history of wheezing, and 1 was an immunodeficient child. In terms of treatment, all the children were given oxygen: 8 cases were given antibiotic therapy, corticosteroids were given in 7 cases, mechanical ventilation was given in 14 cases, and ECMO in 2 cases. Four children died, and the remaining 13 survived. In this case, we performed qPCR detection of HBoV1 in the serum and NPA. The NGS and other PCR results showed that HBoV1 was the only detected pathogen. The viral load in the NPA was high, accompanied by viremia, suggesting that HBoV1 was the most likely cause of severe respiratory tract infection in this case. It is reported that the single detection of HBoV1 was more prevalent among children with a high viral load than those with a low viral load in severe acute cases (11). Christensen et al. (6) showed that mono-detection, high viral load, and viremia are associated with respiratory tract infection. The samples include blood, NPA, or tracheal intubation aspirates of the seven cases reviewed here for qPCR detection, and viremia was indicated in all seven critically ill patients. Unlike other markers (14, 22), the detection of HBoV1 DNA in the blood is more closely associated with the symptoms of the present infection. Therefore, blood testing is one of the important diagnostic methods for the study of HBoV1 (3, 5). It includes both PCR and serodiagnoses.
The results of whole exon sequencing showed a heterozygous variant in the STAT1 gene: c.1127 + 10G > A, causing amino acid change splicing. According to the public database ClinVar, the clinical significance of this variant was considered to be uncertain significance. The family history was negative. STAT proteins are key transcription factors that regulate cellular responses to interferon (IFNs), cytokines, growth factors, and hormones and are associated with diseases related to regulating immune responses. The protein plays a vital role in immune responses to viral, fungal, and mycobacterium pathogens. The invasion of macrophages is the most common mechanism for infectious agents and STAT1 is probably fundamental for the activation of the corresponding intracellular killing programs (23). Genetic variants in STAT1 can lead to four different phenotypes. There are three outcomes of reduced or absent STAT1 function (loss of function [LOF]) and one outcome of gained STAT1 function (GOF). STAT1 plays a crucial role in the cellular response to IFNA/IFNB (type I interferon) and IFNG (type III interferon). Autosomal dominant (AD) LOF STAT1 selectively affects the IFNG pathway but does not affect the IFNA/IFNB pathway and mainly leads to susceptibility to mycobacterial infections and no susceptibility to viral infections. AD LOF STAT1 has low penetrance, a mild clinical phenotype, and a good prognosis (24). However, AD GOF STAT1 with susceptibility to candida has a highly variable prognosis (25). Two patients with a heterozygous variant of STAT1 have been reported to have an increased susceptibility to adult-onset herpes simplex encephalitis (HSE) without a history of other significant infections (24, 26). Autosomal recessive (AR) complete LOF STAT1 affects the IFNA/IFNB and IFNG pathways, leading to susceptibility to mycobacteria, Salmonella, and viruses, often leading to a severe course of the disease and fatal results (24). Patients with AR partial LOF STAT1 present with clinical insufficiency, and the severity of illness is variable (23). However, we have not collected samples from the parents of the child for genetic testing, so we cannot determine the source of the genetic variant. The clinical pathogenicity of this gene variant is uncertain.
In this case, we noted a continuous decline in hemoglobin and red blood cells, presenting as small cell hypochromic anemia. Still, the child had no signs of bleeding, considered to be iron-deficiency anemia or related to iron death. Jayaweera et al. (27) found a significant correlation between the risk of acute respiratory tract infections and iron deficiency anemia in children. Iron supplementation in blood played a critical protective role in recurrent acute respiratory tract infections and gastroenteritis in children. The child, in this case, had severe malnutrition, low oxygen-carrying capacity in pulmonary vessels and lung parenchyma, and low protective immunity against invading pathogens as well as a significant decrease in hemoglobin from day 10 after admission, suggesting a severe infection (28).
In this study, we used qPCR and mNGS for etiological detection, which further confirmed that HBoV1 was the only pathogen that could cause severe respiratory tract infection. Because the PCR method is often targeted at suspected pathogens, unknown or rare pathogenic microorganisms cannot be quickly identified, and certain limitations exist. The mNGS high-throughput sequencing of nucleic acids in clinical samples is then compared with the database analysis. The detection range is more extensive and suitable for diagnosing severe infections (29). The routine clinical use of mNGS is still under development. Assume that NGS is added to clinical and routine laboratory data. In that case, it may be combined with the PCR detection method for clinical diagnosis in the future, which has high clinical specificity and broad application prospects (30). Our study, using mNGS, provides further evidence that HBoV1 can cause severe acute respiratory tract infections in children without other viral and bacterial infections.
It is reported that at least two of the following five factors should be present for the diagnosis of an acute primary HBoV1 infection: high DNA load by qPCR (>106 HBoV1 DNA copies/ml of NPA); HBoV1 mRNA in NPA; positive IgM; low IgG avidity; or a fourfold increase or more of IgG titre in paired serum samples (5). One of the limitations of this study is that we did not include the serological analysis of HBoV1. Second, as this study was a retrospective study, blood samples of the patient's parents were not collected; therefore, gene sequencing and variant analysis could not be performed for verification.
In this case report, the clinical symptoms and signs of the child and the high viral load, viremia, and mNGS detection in the tracheal aspirate all supported that HBoV1 could cause a severe acute respiratory tract infection in children without other viral and bacterial infections. This case suggested that bocavirus can cause severe infection, especially in immunodeficiency conditions, and more vigilance is needed.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The study procedure was approved by the ethics committee of the Children’s Hospital of Chongqing Medical University, Chongqing, China. Written informed consent was obtained from the parent or guardian of all participants.
JL analyzed and interpreted the data, and wrote the manuscript. ZY, YH, and W collected the clinical information and assisted in the analysis. NZ contributed to the design of the study and assisted in the analysis and interpretation of data, and revised the manuscript. LR assisted with the revision of the manuscript. EL contributed to the conception, collection of clinical information, and revision of the manuscript. All authors contributed to the article and approved the submitted version.
We would like to acknowledge the assistance of the patient involved in the study, the staff in the Department of Respiratory Medicine, and the Intensive Care Unit of Children's Hospital of Chongqing Medical University for their help with the nasopharyngeal aspirates and serum collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. (2005) 102(36):12891–6. doi: 10.1073/pnas.0504666102
2. Kapoor A, Slikas E, Simmonds P, Chieochansin T, Naeem A, Shaukat S, et al. A newly identified bocavirus species in human stool. J Infect Dis. (2009) 199(2):196–200. doi: 10.1086/595831
3. Qiu J, Söderlund-Venermo M, Young NS. Human parvoviruses. Clin Microbiol Rev. (2017) 30(1):43–113. doi: 10.1128/CMR.00040-16
4. Cotmore SF, Agbandje-McKenna M, Chiorini JA, Mukha DV, Pintel DJ, Qiu J, et al. The family Parvoviridae. Arch Virol. (2014) 159(5):1239–47. doi: 10.1007/s00705-013-1914-1
5. Christensen A, Kesti O, Elenius V, Eskola AL, Døllner H, Altunbulakli C, et al. Human bocaviruses and paediatric infections. Lancet Child Adolesc Health. (2019) 3(6):418–26. doi: 10.1016/S2352-4642(19)30057-4
6. Christensen A, Nordbø SA, Krokstad S, Rognlien AG, Døllner H. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J Clin Virol. (2010) 49(3):158–62. doi: 10.1016/j.jcv.2010.07.016
7. Martin ET, Fairchok MP, Kuypers J, Magaret A, Zerr DM, Wald A, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis. (2010) 201(11):1625–32. doi: 10.1086/652405
8. Moesker FM, van Kampen JJ, van der Eijk AA, van Rossum AM, de Hoog M, Schutten M, et al. Human bocavirus infection as a cause of severe acute respiratory tract infection in children. Clin Microbiol Infect. (2015) 21(10):964.e1–8. doi: 10.1016/j.cmi.2015.06.014
9. Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis. (2007) 44:904–10. doi: 10.1086/512196
10. Kantola K, Sadeghi M, Antikainen J, Kirveskari J, Delwart E, Hedman K, et al. Real-time quantitative PCR detection of four human bocaviruses. J Clin Microbiol. (2010) 48:4044–50. doi: 10.1128/JCM.00686-10
11. Zhou L, Zheng S, Xiao Q, Ren L, Xie X, Luo J, et al. Single detection of human bocavirus 1 with a high viral load in severe respiratory tract infections in previously healthy children. BMC Infect Dis. (2014) 14:424. doi: 10.1186/1471-2334-14-424
12. Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Söderlund-Venermo M. Human bocavirus – the first 5 years. Rev Med Virol. (2012) 22(1):46–64. doi: 10.1002/rmv.720
13. Jula A, Waris M, Kantola K, Peltola V, Söderlund-Venermo M, Hedman K, et al. Primary and secondary human bocavirus 1 infections in a family, Finland. Emerg Infect Dis. (2013) 19(8):1328–31. doi: 10.3201/eid1908.130074
14. Christensen A, Kesti O, Elenius V, Eskola AL, Døllner H, Altunbulakli C, et al. Severe human bocavirus 1 respiratory tract infection in an immunodeficient child with fatal outcome. Pediatr Infect Dis J. (2019) 38(9):e219–22. doi: 10.1097/INF.0000000000002354
15. Körner RW, Söderlund-Venermo M, van Koningsbruggen-Rietschel S, Kaiser R, Malecki M, Schildgen O. Severe human bocavirus infection, Germany. Emerg Infect Dis. (2011) 17(12):2303–5. doi: 10.3201/eid1712.110574
16. Eskola V, Xu M, Söderlund-Venermo M. Severe lower respiratory tract infection caused by human bocavirus 1 in an infant. Pediatr Infect Dis J. (2017) 36:1107–8. doi: 10.1097/INF.0000000000001681
17. Edner N, Castillo-Rodas P, Falk L, Hedman K, Söderlund-Venermo M, Allander T. Life-threatening respiratory tract disease with human bocavirus-1 infection in a 4-year-old child. J Clin Microbiol. (2012) 50(2):531–2. doi: 10.1128/JCM.05706-11
18. Ursic T, Steyer A, Kopriva S, Kalan G, Krivec U, Petrovec M. Human bocavirus as the cause of a life-threatening infection. J Clin Microbiol. (2011) 49(3):1179–81. doi: 10.1128/JCM.02362-10
19. Uršič T, Krivec U, Kalan G, Petrovec M. Fatal human bocavirus infection in an 18-month-old child with chronic lung disease of prematurity. Pediatr Infect Dis J. (2015) 34(1):111–2. doi: 10.1097/INF.0000000000000509
20. Calvo C, García-García ML, Blanco C, Santos MJ, Pozo F, Pérez-Breña P, et al. Human bocavirus infection in a neonatal intensive care unit. J Infect. (2008) 57(3):269–71. doi: 10.1016/j.jinf.2008.06.004
21. Ziyade N, Şirin G, Elgörmüş N, Daş T. Detection of human bocavirus DNA by multiplex PCR analysis: postmortem case report. Balkan Med J. (2015) 32(2):226–9. doi: 10.5152/balkanmedj.2015.15254
22. Foulongne V, Olejnik Y, Perez V, Elaerts S, Rodière M, Segondy M. Human bocavirus in French children. Emerg Infect Dis. (2006) 12(8):1251–3. doi: 10.3201/eid1708.060213.
23. Olbrich P, Freeman AF. STAT1 and STAT3 mutations: important lessons for clinical immunologists. Expert Rev Clin Immunol. (2018) 14(12):1029–41. doi: 10.1080/1744666X.2018.1531704
24. Al-Muhsen S, Casanova JL. The genetic heterogeneity of Mendelian susceptibility to mycobacterial diseases. J Allergy Clin Immunol. (2008) 122(6):1043–51; quiz 1052–3. doi: 10.1016/j.jaci.2008.10.037
25. Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. (2003) 33(3):388–91. doi: 10.1038/ng1097
26. Mørk N, Kofod-Olsen E, Sørensen KB, Bach E, Ørntoft TF, Østergaard L, et al. Mutations in the TLR3 signaling pathway and beyond in adult patients with herpes simplex encephalitis. Genes Immun. (2015) 16(8):552–66. doi: 10.1038/gene.2015.46
27. Jayaweera J, Reyes M, Joseph A. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci Rep. (2019) 9(1):12637. doi: 10.1038/s41598-019-49122-z
28. Viana MB. Anemia and infection: a complex relationship. Rev Bras Hematol Hemoter. (2011) 33(2):90–2. doi: 10.5581/1516-8484.20110024
29. Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the leap from research laboratory to clinic: challenges and opportunities for next-generation sequencing in infectious disease diagnostics. mBio. (2015) 6(6):e01888–15. doi: 10.1128/mBio.01888-15
Keywords: human bocavirus 1, severe infection, children, respiratory, STAT1 variant
Citation: Liao J, Yang Z, He Y, Wei J, Ren L, Liu E and Zang N (2022) Respiratory tract infection of fatal severe human bocavirus 1 in a 13-month-old child: A case report and literature review. Front. Pediatr. 10:949817. doi: 10.3389/fped.2022.949817
Received: 21 May 2022; Accepted: 29 November 2022;
Published: 20 December 2022.
Edited by:
Kazumichi Fujioka, Kobe University, JapanReviewed by:
Oliver Schildgen, Kliniken der Stadt Köln, Germany© 2022 Liao, Yang, He, Wei, Ren, Liu and Zang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Zang emFuZ25hMTIxNEAxMjYuY29t
Specialty Section: This article was submitted to Pediatric Infectious Diseases, a section of the journal Frontiers in Pediatrics
Abbreviations HBoV1: human bocavirus 1; ARDS, acute respiratory distress syndrome; NPA, nasopharyngeal aspirate; mNGS, metagenomics next-generation sequencing; CT, computed tomography; PCR, polymerase chain reaction.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.