
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 01 August 2022
Sec. Pediatric Nephrology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.931669
This article is part of the Research TopicThrombotic Microangiopathy In Pediatric PatientsView all 5 articles
 Yoko Takagi1
Yoko Takagi1 Yasuko Kobayashi1*
Yasuko Kobayashi1* Ayako Hirakata1
Ayako Hirakata1 Mariko Takei1
Mariko Takei1 Satoshi Ogasawara1
Satoshi Ogasawara1 Chikage Yajima1
Chikage Yajima1 Yuka Ikeuchi1
Yuka Ikeuchi1 Akira Matsumoto2
Akira Matsumoto2 Yoshiyuki Ogawa2
Yoshiyuki Ogawa2 Hiroshi Handa2
Hiroshi Handa2 Masanori Matsumoto3
Masanori Matsumoto3 Hirokazu Arakawa1
Hirokazu Arakawa1 Takumi Takizawa1
Takumi Takizawa1Background: Thrombotic microangiopathy (TMA) is a syndrome associated with hemolytic anemia, thrombocytopenia, and various organ disorders. Thrombotic thrombocytopenic purpura (TTP) is a disease that develops when a disintegrin-like and metalloproteinase with thrombospondin type l motif 13 (ADAMTS13) activity decreases to < 10% of that in normal plasma, causing platelet thrombosis in microvessels throughout the body. Currently, ADAMTS13-deficient TMA is diagnosed as TTP. Systemic lupus erythematosus (SLE)-related TMA includes both acquired TTP, in which ADAMTS13 activity is significantly reduced, and secondary TMA, in which ADAMTS13 activity is not reduced. Both diseases have different prognoses.
Case Presentation: An 11-year-old girl was admitted to our hospital on suspicion of TMA with thrombocytopenia and hemolytic anemia. Because the patient had hypocomplementemia, SLE-related TMA or complement-related TMA was considered. Therefore, we initiated plasma exchange (PE) for the patient. Subsequently, she fulfilled the pediatric SLE diagnostic criteria, and ADAMTS13 activity was shown to be decreased and the anti-ADAMTS13 antibody titer increased. She was thus diagnosed with acquired TTP caused by SLE. Treatment response was good as a platelet count and ADAMTS13 activity improved with three times of PE, followed by methylprednisolone pulse therapy and administration of mycophenolate mofetil. Renal pathology showed thrombus formation in glomerular arterioles and lupus nephritis categorized as Class III (A) of the International Society of Nephrology and the Renal Pathology Society classification. Because the patient was thought to be in the high-risk group of SLE, three courses of intravenous cyclophosphamide pulse therapy were administered as an additional induction therapy. No recurrence of TTP was observed.
Conclusion: In SLE-related TMA, measurement of ADAMTS13 activity and the anti-ADAMTS13 antibody titer are necessary for diagnosis, and for predicting prognosis and recurrence of the disease; however, in the acute phase of immune-mediated TMA, it is important to initiate proper treatments even before knowing the results to improve prognosis.
Thrombotic microangiopathy (TMA) is a syndrome associated with hemolytic anemia, thrombocytopenia, and various organ disorders (1). Collagen diseases have been reported to be the most common underlying cause of secondary TMA, among which systemic lupus erythematosus (SLE) is the most common (2).
Thrombotic thrombocytopenic purpura (TTP), a typical example of TMA, is caused by a deficiency in enzymatic activity of a disintegrin-like and metalloproteinase with thrombospondin type l motif 13 (ADAMTS13), which induces platelet aggregation and systemic microvascular thrombosis (3). Since ADAMTS13 activity became measurable in clinical practice, reduction of its activity to < 10% of that in normal plasma has become one of the diagnostic criteria for TTP. SLE-related TMA includes acquired TTP, in which ADAMTS13 activity is reduced to < 10%, and secondary TMA, in which ADAMTS13 activity is ≥ 10%. The prognoses of the two conditions are significantly different (4). In addition, anti-ADAMTS13 antibody titer measurement is important to diagnose acquired TTP in SLE; however, this process is time intensive. Because TMA is life-threatening in the acute phase, physicians are occasionally forced to decide therapeutic strategy prior to obtaining results on ADAMTS13 activity and the anti-ADAMTS13 antibody titer.
Herein, we report a pediatric case of acquired TTP with the concurrent onset of SLE. We experienced difficulties in managing a platelet count until the ADAMTS13 activity and anti-ADAMTS13 antibody titer were confirmed. Plasma exchange (PE) and methylprednisolone pulse therapy (MPT) were quite effective, but prednisolone and plasma infusion were insufficient to improve a platelet count. In addition, typical renal pathology in TTP was observed with occlusive thrombosis in afferent glomerular arteriole in the present case (5).
An 11-year-old girl had gross hematuria from the morning 2 days before admission and a slight fever of 37°C since that night. On the next day, she visited her home doctor and was revealed to have thrombocytopenia and hemolytic anemia (platelet count, 13,000/μL; hemoglobin, 9.7 mg/dL; lactase dehydrogenase, 1,046 U/ml, with 83 schistocytes out of 1,000 erythrocytes counted), in addition to hematuria and proteinuria. The patient was transferred to our hospital with suspected TMA. Her height and weight were 142 cm [–0.6 standard deviation (SD)] and 30.8 kg (–1.0SD), respectively. Her vital signs on admission were as follows: body temperature, 37.9°C; heart rate, 105 bpm; blood pressure, 122/80 mmHg; SpO2, 98% with room air. The patient appeared pale and ill, although she was alert. Petechia was observed on her soft palate. She had a normal breath sound and no heart murmur. Her abdomen was soft and flat without tenderness. Purpura was scattered on her upper arms, lower legs, and precordium on both sides. No edema was found on her lower legs. She had no past medical history and no family history of blood diseases, autoimmune diseases, or renal diseases. Laboratory findings on admission are shown in Table 1. The data available by the end of the first hospital day revealed that her hemolytic anemia and thrombocytopenia were accompanied by hypocomplementemia. Direct Coombs test result was negative. Kidney function was normal despite abnormality in urinalysis. Although the patient did not complain of gastrointestinal symptoms or bloody stool, stool culture, verotoxin in her stool, and anti-O157 antibody in her serum were tested to exclude hemolytic uremic syndrome (HUS) caused by enterohemorrhagic Escherichia coli infection; all test results were negative. Finally, we found that ADAMTS13 activity was decreased to < 1% and the anti-ADAMTS13 antibody titer returned positive.
The clinical course is shown in Figure 1. After admission, PE was performed for 3 days for TMA, which successfully resulted in a rapid platelet count increase up to 71,000/μL, and PE was completed. Since she fulfilled the SLE diagnostic criteria, including positive antinuclear antibody and anti-ds-DNA antibody on the 3rd hospital day, prednisolone (1mg/kg) was initiated. On the 7th hospital day, her platelet count decreased to 10,000/μL. This led to the consideration of PE again; however, the patient was too distressed to undergo PE. Therefore, we first attempted to administer fresh-frozen plasma, which was found to be insufficient for a significantly improving platelet count. Meanwhile, we obtained the results of ADAMTS13 activity and anti-ADAMTS13 antibody, as described in Table 1. The patient was thus diagnosed with acquired TTP caused by SLE, and MPT was administered on the 10th hospital day. After two courses of MPT, the anti-ADAMTS13 antibody test result was negative, the ADAMTS13 activity increased, and the platelet count rapidly increased (Figure 1A). Additionally, urinary findings were normal after the first course of pulse therapy. Mycophenolate mofetil (MMF, 1,000 mg/day) and hydroxychloroquine (HCQ, 200 mg/day) were administered for SLE. Since she was considered to be in the high-risk group of SLE organ damage, and TMA is known to have frequent complications of psychiatric symptoms (4), she was additionally treated with three courses of intravenous cyclophosphamide pulse therapy [IVCY, 580 mg/dose (500 mg/m2)] as induction therapy for SLE (Figure 1B). After the three courses of IVCY, leukopenia, mainly in lymphocytes, was observed; therefore, MMF was discontinued temporarily. HCQ was discontinued because color blindness and retinopathy were suspected during a regular ophthalmologic visit. After 1 year and 4 months since the onset, belimumab (BLM) was administered instead just after BLM had become under health insurance coverage for SLE pediatric patients (Figure 1B).
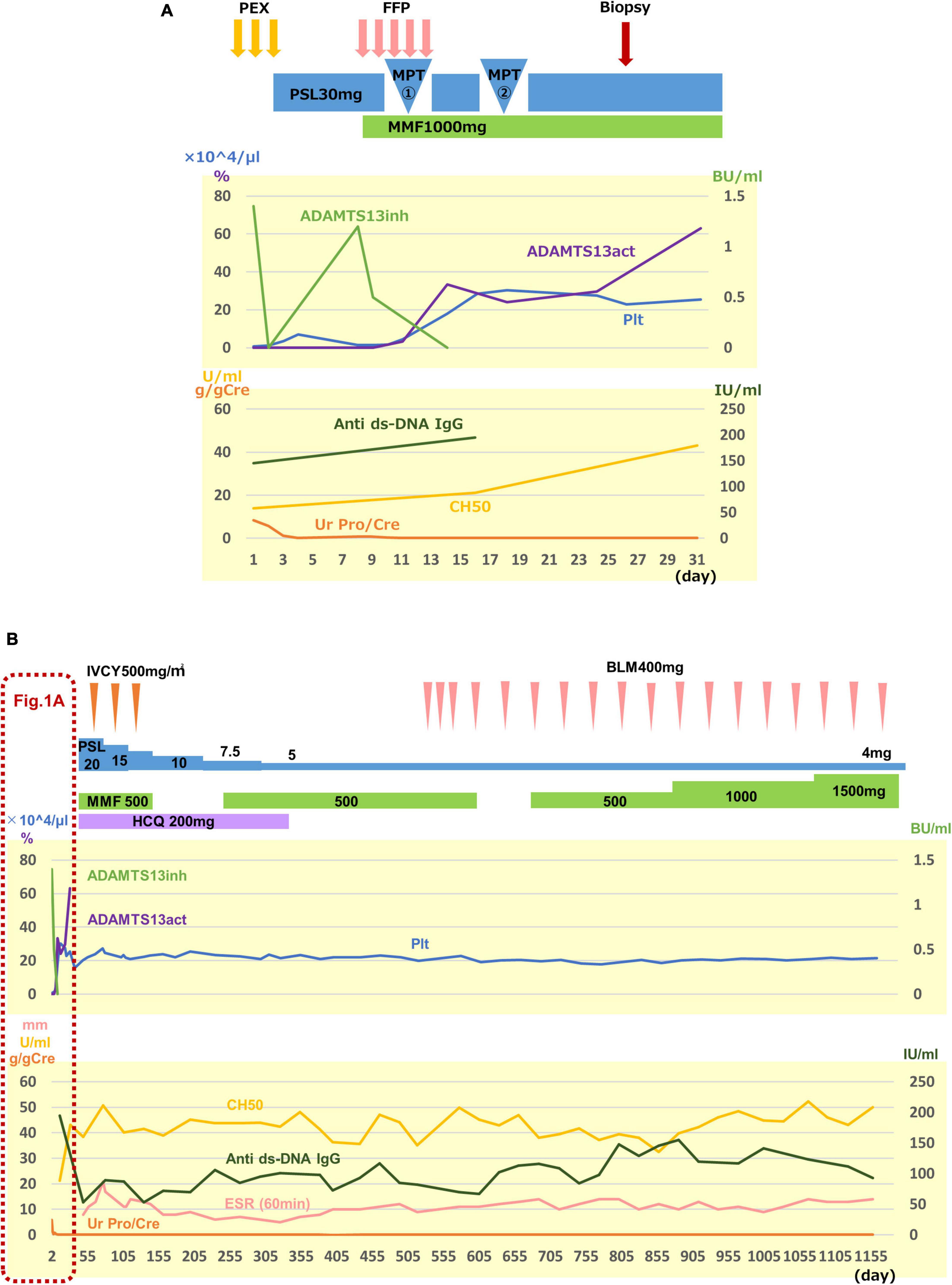
Figure 1. Clinical course. The upper graph shows the activity of TTP, and the lower graph shows the disease activity of SLE and lupus nephritis, both in (A,B). (A) Clinical course for the first 31 days after admission is shown. Three courses of plasma exchange (PE) were effective to remove anti-ADMTS13 antibody (ADMTS13inh) and to increase a platelet count (Plt) rapidly. However, 30 mg of prednisolone (PSL) injection and fresh-frozen plasma (FFP) infusion were not sufficient to suppress antibody production to recover ADAMTS13 activity (ADAMTS13act) and to maintain the platelet count. After two courses of methylprednisolone pulse therapy (MPT), the recovery of the ADAMTS13 activity and the platelet count was observed. Proteinuria disappeared after the first course of pulse therapy. Renal biopsy was performed on the 25th hospital day. (B) Overall clinical course is shown. Treatment details and clinical data are shown following (A). Since the patient was considered to be in the high-risk group of SLE organ damage, and TMA is known to have frequent complications of psychiatric symptoms, she was additionally treated with three courses of intravenous cyclophosphamide pulse therapy (IVCY) as induction therapy for SLE, in addition to mycophenolate mofetil (MMF) and hydroxychloroquine (HCQ). HCQ was discontinued because color blindness and retinopathy were suspected at a regular ophthalmologic visit, and belimumab (BLM) was started instead just after BLM had become under health insurance coverage for SLE pediatric patients (B). Ur Pro/Cre, urinary protein creatinin ratio; anti ds-DNA IgG, anti-double-stranded DNA immunogloburin G; ESR, erythrocyte sedimentation rate.
Renal biopsy was performed on the 25th hospital day. Light microscopic findings in the renal pathology showed focal and segmental mild subendothelial deposition, basement membrane duplication, and endothelial cell proliferation. The findings led to a pathological diagnosis of lupus nephritis International Society of Nephrology and the Renal Pathology Society (ISN/RPS) Classification III (A) (Figure 2A). Notably, granular periodic acid-Schiff stain-positive thrombi were found in afferent arterioles, and glomeruli with luminal stenosis in afferent arterioles were observed in 6 of 26 glomeruli, which was considered to be consistent with TTP (Figure 2B). Immunofluorescent staining (IF) showed full house positive at the capillary wall and, mainly, in the paramesangial region (Figure 2C). Electron-dense deposits were observed in the paramesangial region with electron microscopy, which were comparable to IF findings (Figure 2D).
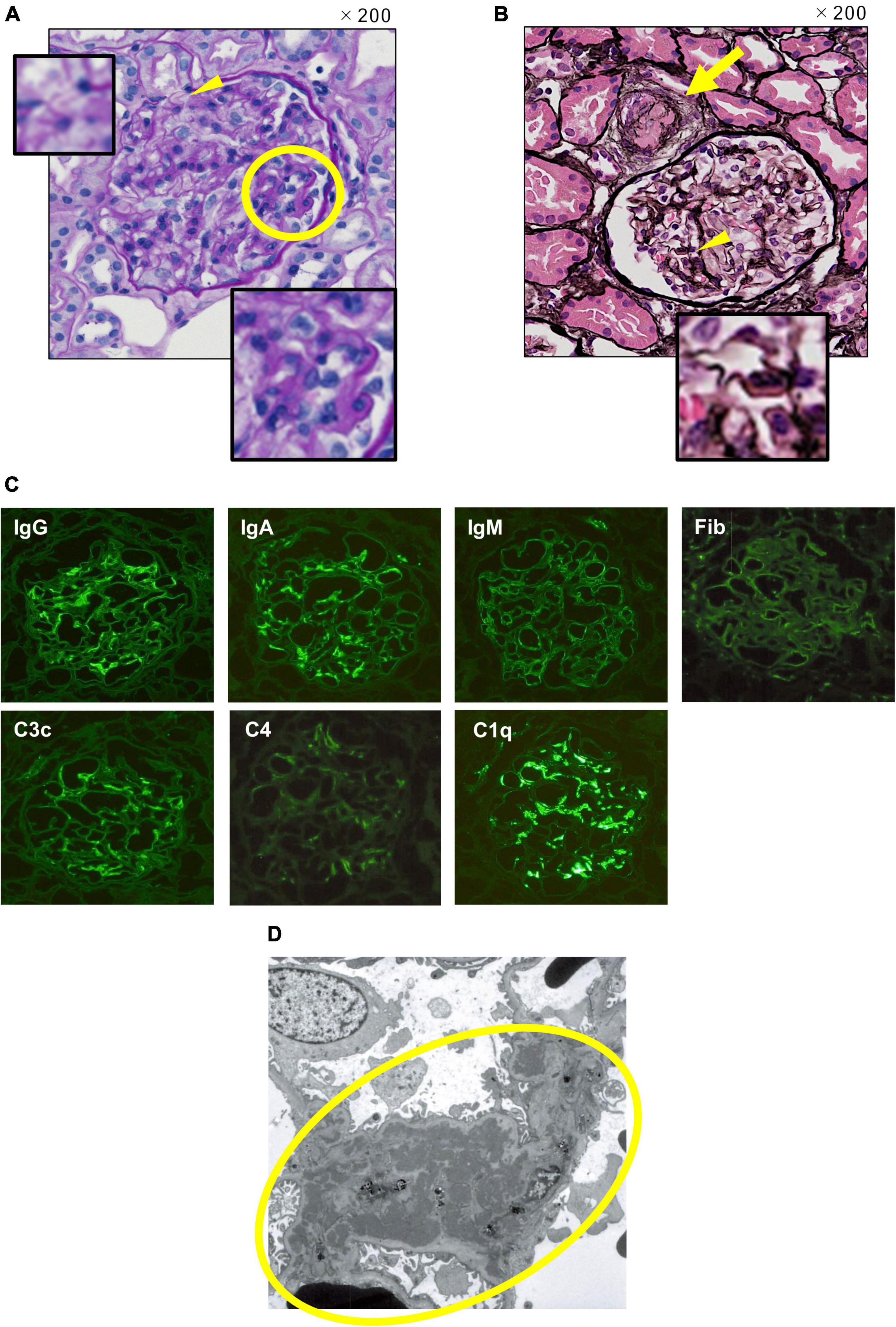
Figure 2. Renal pathology. (A) Light microscopy. Periodic acid Schiff stain, original magnification 200 ×. A wire loop lesion and endocapillary hypercellularity (the yellow circle), and duplication in glomerular basement membrane (the yellow arrowhead) are observed. (B) Light microscopy. Periodic acid silver-methenamin + hematoxylin-eosin staining, original magnification 200 ×. Thrombus formation with luminal stenosis in afferent arteriole (the yellow arrow) and wire loop lesion (the yellow arrowhead) are observed. (C) Immunofluorescent staining. Full-house positive staining at the capillary wall and, mainly, in the paramesangial region is shown. (D) Electronic microscopy. Electron-dense deposits in the paramesangial region (the yellow circle) are observed comparable to IF findings.
Herein, a pediatric case of acquired TTP with the concurrent onset of SLE is described. In TTP, platelet thrombosis in microvasculature causes severe damage to kidneys, brain, and other end organs due to low ADAMTS13 metalloprotease activity (3). ADAMTS13 specifically cleaves von Willebrand factor (VWF) into various size multimers, which are mainly produced in vascular endothelial cells. Uncleaved large VWF multimers, due to the low ADAMTS13 activity, bind to platelets with high affinity and form platelet thrombi under high fluid shear stress in microvessels, resulting in subsequent thrombosis in patients with TTP (6, 7).
To diagnose a patient with hemolytic anemia and thrombocytopenia of unknown etiology, ADAMTS13 activity is a key test in the diagnostic process of TTP, after excluding HUS caused by Shiga toxin-producing E. coli infection (3). Patients in whom ADAMTS13 activity is reduced to < 10% are diagnosed with TTP. Among patients with TTP, those negative for anti-ADAMTS13 antibody are diagnosed with congenital TTP, such as Upshaw–Schulman syndrome (USS), and those positive for the antibody are diagnosed with acquired TTP. On the other hand, if ADAMTS13 activity is ≥ 10%, atypical HUS (aHUS) with complement abnormality or secondary TMA with underlying conditions is considered in the differential diagnosis. In the present case, the patient was found to fulfill the SLE diagnostic criteria. Therefore, acquired TTP or secondary TMA was considered in relation to SLE. In addition, aHUS and even USS had been considered in the differential diagnosis, until the ADAMTS13 activity was revealed to have decreased to < 10%. Since anti-ADAMTS13 antibody was reported positive, the patient was diagnosed with acquired TTP and USS was excluded. Generally, SLE is complicated with both acquired TTP with decreased ADAMTS13 activity and secondary TMA without decreased ADAMTS13 activity. Thus, the ADAMTS13 activity test is important for the differential diagnosis of SLE-related TMA.
Yue et al. reported that the incidence of TMA in patients with SLE was 3% (62 of 2,062 patients with SLE), and the incidence of acquired TTP was 0.29% (6 of 2,062 patients with SLE) (4). Sato et al. reported that 5 (2.2%) of 222 patients with SLE were diagnosed with TTP with four or five of the classic pentad of TTP, and no patient fulfilled the recent criteria for acquired TTP with < 10% of ADAMTS13 activity among the SLE cohort (8). Reese et al. identified 79 acquired TTP with a severe reduction in ADAMTS13 activity in children from a systematic literature review. Of the 79 patients, 10 (12.6%) were described to have SLE (9). Fujimura and Matsumoto reported that collagen disease-related TMA accounted for 24% (221 out of 919) of patients with TMA and was the most common underlying disease in acquired secondary TMA, according to the Japanese TMA registry from 1998 to 2008. SLE was the most common disease (41%, 92 out of 221 collagen disease-related TMA) among them (2).
Matsuyama et al. analyzed clinical characteristics and plasma ADAMTS13 levels in 127 patients with collagen disease-related TMAs, including 64 patients with SLE. Half of the patients with SLE-related TMA have a subnormal to normal range (> 25%) of ADAMTS13 activity, and 23.4% of the patients with SLE-related TMA have a severe deficiency (< 5%) of ADAMTS13 activity. Furthermore, 32 of the patients with SLE-related TMA were ADAMTS13 inhibitor-positive. The group with severe ADAMTS13 deficiency responded well to treatments, and the prognosis was rather favorable (10). Yue et al. compared two groups of patients with SLE-related TMA: one group included 6 patients with acquired TTP secondary to SLE, and the other included 13 patients with SLE-related TMA negative for the ADAMTS13 inhibitor and whose ADAMTS13 activities were within the normal range. They concluded that the TTP group is associated with more severe thrombocytopenia, central nervous system involvement, mild renal involvement, rapid resolution, and relatively good treatment response (4).
Plasmapheresis is the only treatment that has been scientifically shown to be effective in the treatment of acquired TTP (11). Since it has been reported that delayed PE worsens prognosis (12), PE should be initiated as soon as possible when acquired TTP is suspected. Currently, as ADAMTS13 activity measurement result takes several days in daily clinical practice, treatment should be initiated even without results. Steroid therapy is also recommended with the expectation of suppressing autoantibody production (13). Both steroid pulse therapy and high-dose steroids are administered, although it is unclear which therapy is more efficacious (13). In our case, as shown in Figure 1, the platelet count rapidly increased after three courses of PE and two courses of MPT after the anti-ADAMTS13 antibody became negative and the ADAMTS13 activity increased in response to the treatments. Thrombus formation in afferent arterioles in glomeruli with luminal stenosis was still observed in renal pathology (Figure 2) obtained on the 25th hospital day, indicating the severity of organ damage in the acute phase of the disease.
In conclusion, measurement of ADAMTS13 activity and the anti-ADAMTS13 antibody titer is necessary for the diagnosis and proper treatment of SLE-related TMA; however, in the acute phase of immune-mediated TMA, it is important to initiate PE even before knowing the results to improve prognosis. Pediatric SLE-related TTP cases need to be accumulated to elucidate appropriate treatment and to improve long-term prognoses as this is a rare condition, and clinical features of pediatric SLE are partially different from adult SLE.
It’s already been three years since I was discharged from the hospital. At first I wasn’t sure what happened to me, and I really don’t remember anything from when I was in the ICU. When I moved from the ICU to the pediatric ward, I was worried when I heard from my family that my condition was pretty bad, but with the support of doctors, nurses, other patients in the same room, and my family, now, I am here. All the children in the same room were nice, and each was having a hard time with their own disease. I realized that for a child fighting illness, every single day in the hospital is a battle against time and their body. I am very grateful to my family who accompanied me in the hospital and to the doctors and pharmacists who helped me with the treatment and medicine.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin.
YT and YK wrote the manuscript and performed the practical work. AH, MT, SO, CY, and YI collected clinical data. AM, YO, HH, and MM contributed to measuring ADAMTS13 activity and anti-ADAMTS13 antibody sequentially and to analyzing the data. HA and TT revised the manuscript. All authors contributed to the article and approved the submitted version.
Publication costs were funded by Doctors’ Association of Department of Pediatrics, Gunma University Graduate School of Medicine. This work was supported by the JSPS KAKENHI, Grant-in-Aid for Scientific Research (C), Grant Numbers 19K08696, 21K08247, and 22K08324.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the patient and her family for their contribution to this study. We would like to thank Editage (www.editage.cn) for English language editing.
aHUS, atypical HUS; ADAMTS13, a disintegrin-like and metalloproteinase with thrombospondin type l motifs 13; BLM, belimumab; HCQ, hydroxychloroquine; HUS, hemolytic uremic syndrome; IF, immunofluorescent staining; ISN/RPS, International Society of Nephrology and the Renal Pathology Society; IVCY, intravenous cyclophosphamide pulse therapy; MMF, mycophenolate mofetil; MPT, methylprednisolone pulse therapy; PE, plasma exchange; SD, standard deviation; SLE, systemic lupus erythematosus; TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura; VWF, von Willebrand factor; USS, Upshaw–Schulman syndrome.
1. George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. (2014) 371:1847–8. doi: 10.1056/NEJMc1410951
2. Fujimura Y, Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: database of Nara Medical University during 1998-2008. Intern Med. (2010) 49:7–15. doi: 10.2169/internalmedicine.49.2706
3. Matsumoto M, Fujimura Y, Wada H, Kokame K, Miyakawa Y, Ueda Y, et al. Diagnostic and treatment guidelines for thrombotic thrombocytopenic purpura (TTP) 2017 in Japan. Int J Hematol. (2017) 106:3–15. doi: 10.1007/s12185-017-2264-7
4. Yue C, Su J, Gao R, Wen Y, Li C, Chen G, et al. Characteristics and outcomes of patients with systemic lupus erythematosus-associated thrombotic microangiopathy, and their acquired ADAMTS13 inhibitor profiles. J Rheumatol. (2018) 45:1549–56. doi: 10.3899/jrheum.170811
5. Laszik ZG, Kambham N, Silva FG. Thrombotic microangiopathies. In: JC Jennette, JL Olson, FG Silva, VD D’Agati editors. Heptinstall’s Pathology of the Kidney. Philadelphia: Wolters Kluwer (2015). p. 739.
6. Furlan M. Von Willebrand factor: molecular size and functional activity. Ann Hematol. (1996) 72:341–8. doi: 10.1007/s002770050184
7. Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. (1996) 84:289–97. doi: 10.1016/S0092-8674(00)80983-
8. Sato T, Hanaoka R, Ohshima M, Miwa Y, Okazaki Y, Yajima N, et al. Analyses of ADAMTS13 activity and its inhibitor in patients with thrombotic thrombocytopenic purpura secondary to connective tissue diseases: observations in a single hospital. Clin Exp Rheumatol. (2006) 24:454–5.
9. Reese JA, Muthurajah DS, Kremer Hovinga JA, Vesely SK, Terrell DR, George JN. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. (2013) 60:1676–82. doi: 10.1002/pbc.24612
10. Matsuyama T, Kuwana M, Matsumoto M, Isonishi A, Inokuma S, Fujimura Y. Heterogeneous pathogenic processes of thrombotic microangiopathies in patients with connective tissue diseases. Thromb Haemost. (2009) 102:371–8. doi: 10.1160/TH08-12-0825
11. Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian apheresis study group. N Engl J Med. (1991) 325:393–7. doi: 10.1056/NEJM199108083250604
12. Pereira A, Mazzara R, Monteagudo J, Sanz C, Puig L, Martínez A, et al. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome: a multivariate analysis of factors predicting the response to plasma exchange. Ann Hematol. (1995) 70:319–23. doi: 10.1007/BF01696619
Keywords: systemic lupus erythematosus, thrombotic microangiopathy, thrombotic thrombocytopenic purpura, ADAMTS13 activity, anti-ADAMTS13 antibody, platelet thrombosis, von Willebrand factor, lupus nephritis
Citation: Takagi Y, Kobayashi Y, Hirakata A, Takei M, Ogasawara S, Yajima C, Ikeuchi Y, Matsumoto A, Ogawa Y, Handa H, Matsumoto M, Arakawa H and Takizawa T (2022) Systemic Lupus Erythematosus Presenting With Thrombotic Thrombocytopenic Purpura at Onset: A Case Report. Front. Pediatr. 10:931669. doi: 10.3389/fped.2022.931669
Received: 29 April 2022; Accepted: 09 June 2022;
Published: 01 August 2022.
Edited by:
Toshihiro Sawai, Shiga University of Medical Science, JapanReviewed by:
Hesham Safouh, Cairo University, EgyptCopyright © 2022 Takagi, Kobayashi, Hirakata, Takei, Ogasawara, Yajima, Ikeuchi, Matsumoto, Ogawa, Handa, Matsumoto, Arakawa and Takizawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasuko Kobayashi, a29iYXlhc3VAZ3VubWEtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.