
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 06 July 2022
Sec. Pediatric Critical Care
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.921863
This article is part of the Research TopicGastrointestinal Function and Nutrition in Pediatric Critical CareView all 10 articles
 Frederic V. Valla1*
Frederic V. Valla1* Lyvonne N. Tume2
Lyvonne N. Tume2 Corinne Jotterand Chaparro3
Corinne Jotterand Chaparro3 Philip Arnold4
Philip Arnold4 Walid Alrayashi5
Walid Alrayashi5 Claire Morice1
Claire Morice1 Tomasz Nabialek6
Tomasz Nabialek6 Aymeric Rouchaud7
Aymeric Rouchaud7 Eloise Cercueil1
Eloise Cercueil1 Lionel Bouvet8
Lionel Bouvet8Introduction: Point-of-care ultrasound (POCUS) use is increasing in pediatric clinical settings. However, gastric POCUS is rarely used, despite its potential value in optimizing the diagnosis and management in several clinical scenarios (i.e., assessing gastric emptying and gastric volume/content, gastric foreign bodies, confirming nasogastric tube placement, and hypertrophic pyloric stenosis). This review aimed to assess how gastric POCUS may be used in acute and critically ill children.
Materials and Methods: An international expert group was established, composed of pediatricians, pediatric intensivists, anesthesiologists, radiologists, nurses, and a methodologist. A scoping review was conducted with an aim to describe the use of gastric POCUS in pediatrics in acute and critical care settings. A literature search was conducted in three databases, to identify studies published between 1998 and 2022. Abstracts and relevant full texts were screened for eligibility, and data were extracted, according to the JBI methodology (Johanna Briggs Institute).
Results: A total of 70 studies were included. Most studies (n = 47; 67%) were conducted to assess gastric emptying and gastric volume/contents. The studies assessed gastric volume, the impact of different feed types (breast milk, fortifiers, and thickeners) and feed administration modes on gastric emptying, and gastric volume/content prior to sedation or anesthesia or during surgery. Other studies described the use of gastric POCUS in foreign body ingestion (n = 6), nasogastric tube placement (n = 5), hypertrophic pyloric stenosis (n = 8), and gastric insufflation during mechanical ventilatory support (n = 4). POCUS was performed by neonatologists, anesthesiologists, emergency department physicians, and surgeons. Their learning curve was rapid, and the accuracy was high when compared to that of the ultrasound performed by radiologists (RADUS) or other gold standards (e.g., endoscopy, radiography, and MRI). No study conducted in critically ill children was found apart from that in neonatal intensive care in preterms.
Discussion: Gastric POCUS appears useful and reliable in a variety of pediatric clinical settings. It may help optimize induction in emergency sedation/anesthesia, diagnose foreign bodies and hypertrophic pyloric stenosis, and assist in confirming nasogastric tube placement, avoiding delays in obtaining confirmatory examinations (RADUS, x-rays, etc.) and reducing radiation exposure. It may be useful in pediatric intensive care but requires further investigation.
Point-of-care ultrasound (POCUS) is the routine use of ultrasound, performed by non-radiology healthcare professionals at the bedside, to guide diagnosis and patient management. POCUS can address specific clinical questions, adding to the traditional physical examination. The wide availability of portable ultrasound devices and their ability to perform repeated non-invasive examinations have rapidly increased the use of POCUS in different clinical settings.
POCUS was initially used for cardiovascular assessment (1). More recently, the use of POCUS has been expanded to other organ systems, including the gastrointestinal system, in both adults and children (2, 3), but the use of gastric POCUS is currently not common.
The pediatric literature reports some data on the use of gastric POCUS in five main domains: (i) assessing gastric emptying and gastric volume/contents, (ii) gastric foreign body identification, (iii) confirming nasogastric (or orogastric) tube (NGT) position, (iv) hypertrophic pyloric stenosis (HPS) diagnosis, and (v) other indications (e.g., assessment of ventilation on gastric insufflation) (2, 4–12).
Gastric POCUS, performed by pediatric healthcare professionals (pediatricians, anesthesiologists, surgeons, emergency department (ED) physicians, specialist nurses) may be useful in various clinical settings and for different indications, but we currently lack a robust review of the literature to understand the role of gastric POCUS in children. We have conducted a scoping review (using a robust methodology) to answer this question, identifying the key domains that gastric POCUS has been utilized in pediatrics.
An expert group was established, involving 10 members familiar with the use of gastric POCUS: two pediatricians, one clinical PICU nurse researcher, three pediatric anesthesiologists, two pediatric intensivists, one pediatric radiologist, and one methodologist. The scoping review was conducted in accordance with the JBI (Johanna Briggs Institute) methodology for scoping reviews (13). The study protocol was registered on Open Science Framework (OSF) on 28 February 2022 (doi: 10.17605/OSF.IO/P5BF9).
The review question (with five sub-questions corresponding to five aims) was discussed and defined as “What is the role of gastric POCUS in children?” (i) determining gastric emptying, (ii) assessing the placement of NGT, (iii) identifying ingested foreign bodies, (iv) diagnosing HPS, and (v) for other indications.
Studies were considered if they were conducted in preterms, term neonates, and children up to 18 years. Prenatal studies were excluded. Studies had to directly assess the use of gastric POCUS but were excluded if gastric ultrasound was performed by radiologists (RADUS), rather than any bedside healthcare professionals (POCUS). The included studies could have been conducted in any pediatric setting [ED, pediatric intensive care (PICU), operating room (OR)] and must have answered the main question or a sub-question. Tumor diagnosis was out of the scope of the review as it was considered to require RADUS, rather than POCUS.
This scoping review considered both experimental and quasi-experimental study designs (randomized or non-randomized controlled trials, before and after studies, and interrupted time-series studies), analytical observational study designs (prospective and retrospective cohort studies, case–control studies, and analytical cross-sectional studies), descriptive observational study designs (case series, individual case reports, abstracts, and descriptive cross-sectional studies), systematic reviews, gray literature (abstracts from conferences and unpublished studies), qualitative studies, and text and opinion articles.
First, an initial limited search of MEDLINE (PubMed) was undertaken to identify studies on the topic. The text contained in the titles and abstracts and the MeSH terms used to index the articles were used by an academic librarian to develop a full search strategy for MEDLINE (PubMed; Supplementary Material 1). Search equations were further adapted for other databases and/or information source (Embase and Web of Science). Search equations were run in the three aforementioned databases. Filters were applied to search for studies published in English and French between 1998 and 2022. The reference lists of all included sources of evidence were also screened for additional studies.
After duplicate removal, titles and abstracts were screened by two (or three in case of disagreement) independent reviewers (members of the expert group), following the inclusion criteria, on free online software (Rayyan QCRI) (14). Full texts of relevant studies/sources were retrieved and reviewed by one independent reviewer. They were excluded if they did not fulfill the inclusion criteria. The results were presented in a Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping review (PRISMA-ScR) flow diagram (15).
Data were extracted from included studies and consisted of study population characteristics, concept (aim of the study, gastric POCUS-related sub-question), context (setting, gastric POCUS operator), study designs, and relevant key findings.
Data are presented with a narrative summary, accompanied by tabulated and/or charted results. Where possible, quantitative summaries of extracted evidence are provided.
The literature search identified 3,666 articles, after removing duplicates and sources older than 1998, and 2,431 studies were eligible for screening (Figure 1). After abstract and full-text screening, a total of 69 articles were included, and one article was identified from another source.
Tables 1–5 summarize the study characteristics and findings for each sub-questions.
Most articles assessed gastric POCUS in one of the four main sub-questions, and the remaining four articles assessed its role in ventilatory support (see Figure 2).
Totally, 47 studies were included, in which gastric POCUS was performed by anesthesiologists and pediatricians (neonatologists) most of the time (Table 1) (2, 4, 6, 7, 16–58). Detailed results are available in Supplementary Material 2.
These studies aimed (i) to assess gastric POCUS use to determine the gastric content volume; (ii) to assess gastric emptying from different amounts or types of breast milk (fortifier or not) or breakfast in infants or children; (iii) to determine the gastric contents/volume according to fasting duration, or in the setting of elective or emergency surgery in children; (iv) to assess whether ear nose and throat (ENT) surgery was associated with the change in the gastric content volume.
Figure 3 presents an example of gastric antrum measurements and details the gastric POCUS technique.
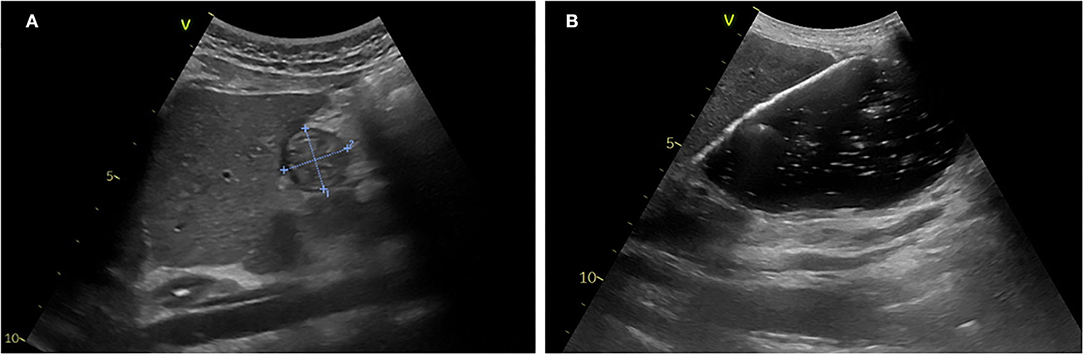
Figure 3. Gastric POCUS to assess gastric volume and content. (A) Empty stomach. (B) Full stomach (liquid content). Curvilinear low-frequency (2–5 MHz) or high-frequency linear transducers were used for examination (the former providing better scanning in older children). The gastric antrum was scanned in the epigastric sagittal plane, in the supine and/or in the right lateral decubitus position, for qualitative assessment and/or for the measurement of the antral cross-sectional area. In some studies, a longitudinal scan of the stomach was performed, allowing measurement of 3 diameters (anteroposterior, transverse, and longitudinal axes) for the calculation of the spheroid stomach volume (43, 44, 46). Repeated measurements minimize intra-rater variability. Gastric content volume was calculated in four studies using the mathematical model by (52) (R2 = 0.60), and in one study, it was calculated using the mathematical model by Schmitz et al. (7) (R2 = 0.582).
(i) Gastric ultrasound as a tool for estimating gastric content volume.
In most studies, the gastric antrum cross-sectional area (CSA) was measured, and gastric volume was calculated as per Spencer or Schmitz formulas (7, 47, 48, 52). In one research group, a longitudinal scan of the stomach was performed, allowing measurement of three diameters (anteroposterior, transverse, and longitudinal axes) for the calculation of the spheroid stomach volume (43, 44, 46).
In children older than 1 year, several studies reported a significant correlation between the antral CSA and gastric volume that was improved when ultrasound examination was performed in the right lateral decubitus position (7, 30, 35, 39, 47, 48, 52). Cut-off values for an antral CSA of 219 mm2 in the supine position and 307 mm2 in the right lateral decubitus position allowed discrimination between an empty or full stomach, with a sensitivity of 75% and a specificity of 36% in the supine position, and a sensitivity of 76% and a specificity of 67% in the right lateral decubitus position (39). Overall, three studies described mathematical models for predicting gastric contents volume compared to gastric endoscopy (52) or MRI (7) or gastric aspiration through the NGT (35), and one study developed a model for hypertrophic pyloric stenosis infants (31).
Spencer et al. (52) validated adult gastric contents. Perlas et al. made qualitative classification of gastric contents (77) to children aged 12 months−17 years; they reported that grade 0 (empty antrum in supine and right lateral decubitus (RLD) positions), grade 1 (fluid content seen in RLD only), and grade 2 (fluid content seen in both supine and RLD positions) were associated with gastric fluid volumes of <0.3, 0.3–1.5, and >1.5 ml/kg, respectively.
(ii) Feed type and amount impact on gastric emptying.
Repeated measurements of the antral cross-sectional area or spheroid stomach volume have been used to assess gastric emptying patterns in preterm neonates, term neonates, and children, not only to adapt to preoperative fasting rules but also as a surrogate of feed tolerance in term and preterm neonates and to identify factors associated with gastric emptying.
In term and preterm neonates, several studies investigated the impact of different feeding formula compositions on gastric emptying. These different feeds included formula thickening, feed formulas with various energy concentrations, fortified or non-fortified expressed breast milk, hydrolyzed protein feeds, and the child's position and feed volume, with inconsistent results among the studies (18, 28, 33, 38, 44–46, 57). In preterms, Beck et al. found the mean gastric emptying time of breast milk was <4 h, and Lee et al. reported a mean gastric emptying time after formula feeding of 93 min, ranging from 45 to 150 min (20, 36).
In children, several studies assessed gastric emptying times of different meals, formulas, and clear fluids. The gastric emptying time was <4 h after low-fat milk, breast milk, and a light breakfast (16, 50, 54, 78); <1 h after clear fluids (21); and <90 min after a carbohydrate-rich drink (58). Song et al. reported gastric fluid volume 2 h after a carbohydrate drink were lower than that after prolonged fasting (51), while a pilot study reported that gastric emptying of apple juice, 2% milk, or a high-protein drink was >4 h in children aged 8–14 years (25). Furthermore, fluid volume (3 vs. 5 ml/kg) did not affect gastric emptying in 44 children older than 6 years (55), and gastric emptying of clear fluids was enhanced when children were positioned in a semi-seated position (26).
(iii) Preoperative gastric volume assessment.
Several studies assessed the percentage of full stomach in elective and emergency anesthesia/surgery. In fasting children planned for elective surgery, gastric content volume was low (79) and the percentage of “stomachs at risk of aspiration” was 1% (2), but in the emergency setting, Gagey et al. and Evain et al. reported the incidence of full stomach to be between 51 and 37%, respectively (27, 31, 32). In children, fasting for more than 6 h admitted to the ED and requiring procedural sedation, 18–69% of these had a full stomach (6, 37, 41). Gagey et al. reported that an ultrasound-guided anesthetic strategy led to an 85% appropriate induction sequence technique compared to 49% after clinical assessment alone in non-elective children (31). In addition, ultrasound-monitored gastric aspiration allowed the performance of a non-rapid induction sequence in 88% of 34 infants scheduled for pyloromyotomy (31, 32).
(iv) Monitoring of gastric content during surgery.
Desgranges et al. reported no significant changes assessed by gastric POCUS in gastric volume occurred during ear nose and throat (ENT) surgery. This surgery bleeding did not increase the risk of postoperative aspiration (24).
Totally, six studies (10, 59–63) were identified, and all were conducted in the pediatric ED (Table 2). They reported the use of gastric POCUS to diagnose foreign body ingestion and detect the presence of foreign bodies in the stomach and follow up in relation to gastric clearance. They noted only short training of ED physicians was required to perform gastric POCUS. Figure 4 presents an example of an intragastric trichobezoar (hair or wool solid mass) and details the gastric POCUS technique. These studies showed that gastric POCUS could be used to confirm foreign body ingestion, compared to x-ray. These findings may reduce the need for routine x-rays, which are not valuable in the case of both radiolucent and non-radiolucent masses. Gastric water filling may further help foreign body visualization.
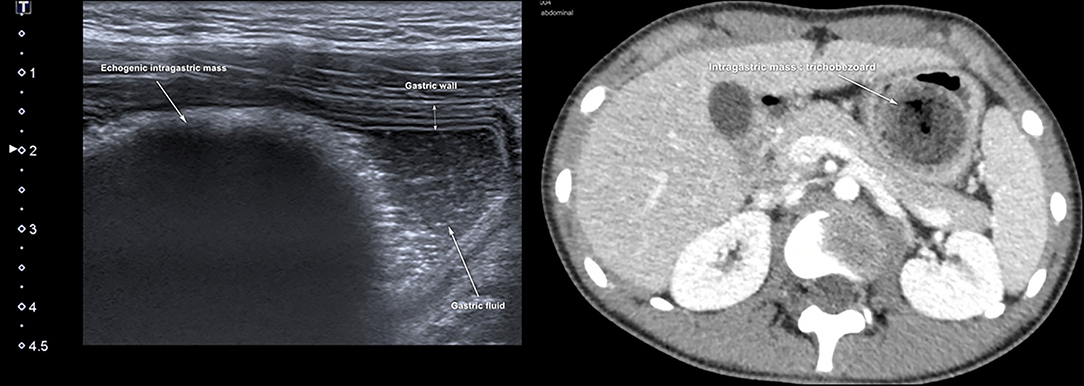
Figure 4. Intragastric foreign body. Large hyperechoic mass within the stomach may suggest the diagnosis of an intragastric foreign body (e.g., voluminous trichobezoar). In an uncertain diagnosis, and before surgery, an abdomino-pelvic CT scan should be performed to examine the extension of the foreign body. Gastric POCUS techniques found in the literature: various ultrasound probes have been used (high- or low-frequency linear or curvilinear transducers) and the child was positioned in a supine and/or a right lateral decubitus to enhance the quality scanning of the thoracic and epigastric areas. Liquid filling may help in visualizing the foreign body.
In all, five articles were included (8, 9, 64–66) (Table 3). They all focused on the accuracy of confirmation of the NGT position by bedside ultrasound in children and preterm neonates; two studies involved only newborns and preterms (8, 64), the others involved children older than 16 years (9, 65, 66); two studies compared ultrasound placements to abdominal x-ray (as gold standard), and the other ultrasound placements to “standard confirmation techniques.” POCUS was performed by a pediatrician, an ED physician, or a neonatologist, with only two of the studies being blinded. Figure 5 presents an example of NGT in the stomach and details the gastric POCUS technique.
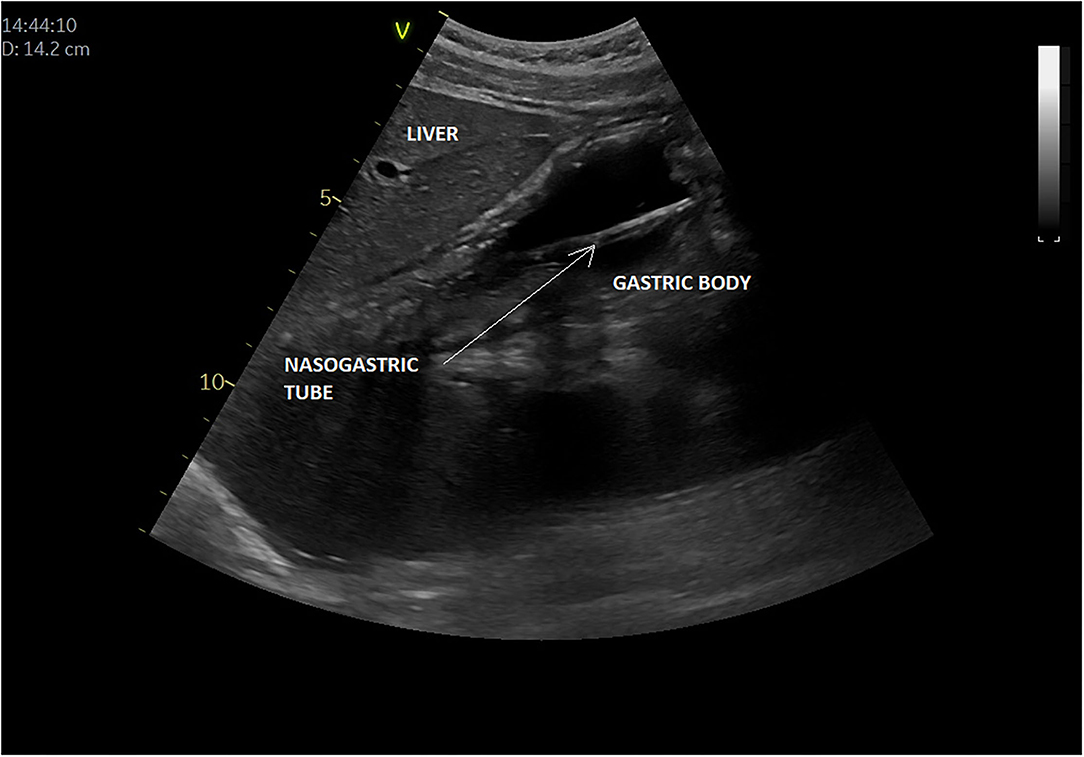
Figure 5. Intragastric nasogastric tube (or orogastric tube). The gastric POCUS technique found in the literature: The NGT was visualized using the curvilinear transducer or the phased transducer (with the iScan feature to optimize the view). Probe frequency was adapted to the size of the patient. The child was positioned in a dorsal decubitus position. The transducer was positioned in the middle of the epigastric region, allowing for visualization of the tube passing through the cardia and entering the gastric area. Then the transducer was positioned in the upper right quadrant toward the duodenum, to verify whether the tube was entering the pylorus. The correct position of the NGT corresponded to a hyperechogenic line passing through the cardia with its length continuing within the gastric area but not entering the pylorus. Otherwise, the transducer was placed transversely over the xiphisternum and was fanned downward and aimed toward the left upper quadrant to visualize the gastric body through the left lobe of the liver. Then, sagittal and transverse sweeps were performed over the epigastric area. If the NGT was not identified, the transducer was placed over the left flank in the sagittal position using the spleen as a window. The study was considered positive when the NGT could be visualized in the stomach as two parallel hyperechoic lines.
Of the three studies reporting sensitivity of POCUS to confirm the GT position, this ranged from 88% (95% confidence interval, 70.0–97.6%) to 98.1% (8, 9, 64). However, there was considerable variability between the studies regarding POCUS being unable to determine the position of the NGT: 7.8% (Atalay et al.), 48% (Choi et al.), but in <2% (Dias et al.). In the Choi study (65), broken down by age of the child, at the gastric antrum level, POCUS showing successful NGT placement was limited to 15 of 29 patients [52% (95% CI: 33–71%)]. A subgroup analysis showed that tube placement in the stomach was visualized in four of nine patients [44% (95% CI: 14–79%)] between 0 and 2 years old, 7/10 [70% (95% CI: 35–93%)] aged 3–6 years and in four of 10 patients [40% (95% CI: 12–74%)] > 6 years old. The use of an air bolus (1 ml/kg) improved NGT visibility in some children.
Totally, eight studies were identified (Table 4) (5, 12, 67, 69–72, 80), and 652 infants younger than 6 months presenting with signs of HPS were recruited in the ED; three studies examined gastric POCUS performed by surgeons (5, 67, 69) and five by ED physicians (12, 70–72, 80). In these studies, POCUS was compared to RADUS; in four other studies, the learning curve and training were assessed (surgeons training surgeons, surgeons training ED physicians, or ED physicians training ED physicians) (69, 71, 72). Figure 6 presents an example of HPS and details the gastric POCUS technique. Regarding the accuracy of POCUS compared to RADUS, sensitivity and specificity ranged from 96.6 to 100% and from 94 to 100%, respectively (12, 70). The measurements of the pylorus muscle obtained by radiologists or surgeons/ED physicians did not significantly differ. One study assessed the impact of POCUS on the length of ED stays and found that POCUS significantly shortened ED stay in children with HPS and time to disposition in children with no HPS (12). The ability of healthcare professionals trained in POCUS to train their ED colleagues was good, with no false positives or false negatives in two studies (69, 72), and as shown the third study, pre-/post-training test scores improved from a mean of 61–83% correct, respectively (71).
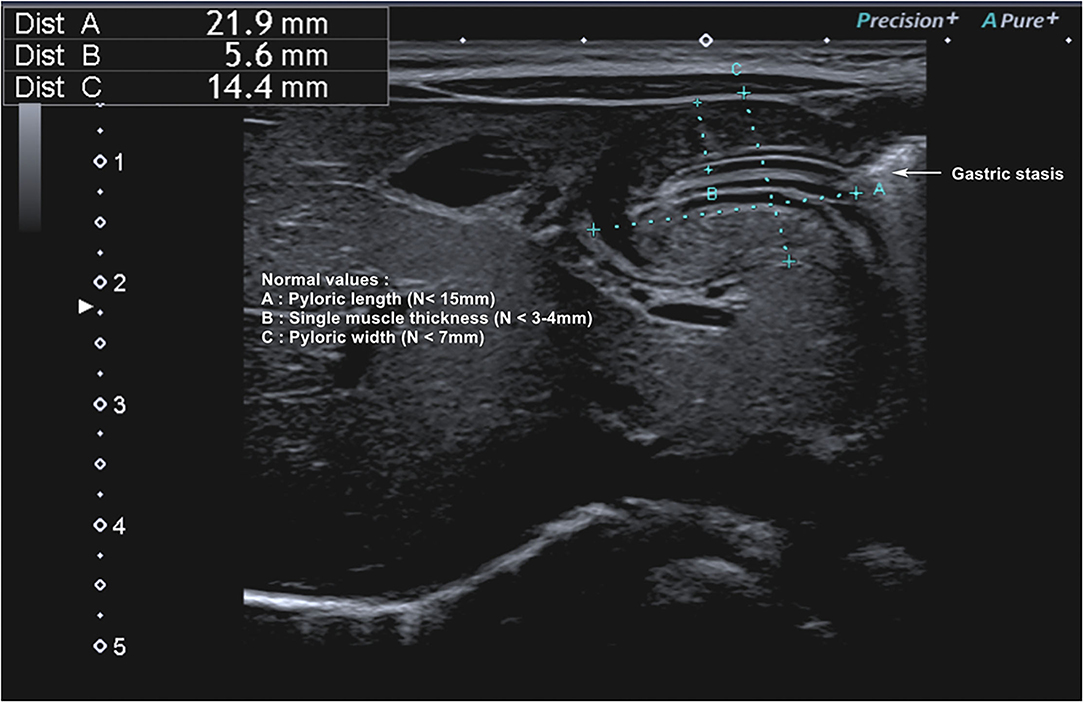
Figure 6. Hypertrophic pyloric stenosis. Gastric POCUS consisted of the measurements of pylorus muscle thickness and length, and HPS diagnosis was confirmed if they were >3 and 15 mm, respectively. A 6–10 MHz linear probe in a transverse position allows identifying the gallbladder in the supine position. The pylorus is usually located slightly medial and posterior in relation to the gallbladder.
Totally, four randomized controlled trials (73–76) were conducted in a total of 337 children undergoing general anesthesia for elective surgery (Table 5). Different ventilation modes and methods were assessed, and POCUS was used as a primary or secondary outcome to assess gastric insufflation induced by ventilatory support (face mask vs. ventilator, different positive inspiratory pressure (PIP) levels, manual vs. pressure-controlled face mask). The technique was similar to the one described in Figure 3. POCUS was performed by trained anesthesiologists and aimed to measure the gastric antrum CSA as a surrogate of gastric insufflation. One study (74) compared these measurements with gastric auscultation and showed that all cases of gastric insufflation detected by auscultation were also detected by US, although not vice versa. Gastric POCUS helped determine the optimal level of positive inspiratory pressure (PIP) and suggested that pressure-controlled face masks limited gastric insufflation.
This scoping review found that gastric POCUS was assessed or used in a significant number of clinical scenarios in pediatrics. However, most commonly, it was used to assess gastric contents/volume in the peri-operative setting. We did not find any studies conducted in the PICU, but a few in neonatal intensive care. Most studies were recent (published in the last 10 years) as the availability of portable ultrasound machines was not common before the 2010's, when radiologists used to be responsible for ultrasounding patients. POCUS has been shown to decrease the time to diagnosis and treatment (12). Overall, the gastric POCUS learning curve is short, and several studies showed good reliability of measurements and interpretations (7–9, 12, 52, 64, 69, 70, 72). Gastric POCUS is likely to increase in different pediatric clinical settings in future. However, POCUS may not always be cost- or time-efficient, especially in elective sedation/anesthesia considering the low prevalence of a full stomach in this setting (40, 42, 49, 81).
Different techniques (and mathematical extrapolation of gastric volume from the surrogate antrum or cardia length measurements) have been proposed. Even if the spheroid calculation of the stomach appears the most accurate (43–46), the use of the antrum CSA has been used for decades in adults and is easier to perform (7, 52), especially when monitoring is required. Mathematical models to calculate gastric volume compared to NGT aspiration showed study design limitations and produced rather inaccurate models, preventing their use in clinical practice (35). So, gastric ultrasound may be useful to estimate the gastric content and volume status, mainly based on qualitative assessment, possibly completed by gastric fluid volume calculation (82). The use of gastric POCUS to assess gastric emptiness prior to sedation/anesthesia may limit the risk of aspiration during induction (with increased morbidity and mortality rates). But considering the low prevalence of a full stomach after recommended fasting times, this might be better to be applied to emergency sedation/anesthesia, rather than all children systematically (2, 6, 7, 82). However, it would allow for a fortuitous diagnosis of full stomach (22, 40, 42).
Inconsistent results were found among these studies (18, 28, 33, 38, 44–46, 57), thus questioning the use of gastric emptying time as a clinically relevant surrogate for assessing feeding tolerance. However, these studies allowed for reducing fasting times recommended in recent guidelines: breast milk feeds, fortified or not, are encouraged until 3 h before anesthesia induction (82, 83).
Gastric POCUS was useful and limited the use of conventional radiography and irradiation in children. However, all studies were low-quality case series, and larger studies are required to better define the role of gastric POCUS in this setting.
Blind insertions of the NGT can be sub-optimal in adult and pediatric patients (84). The risk of NGT malposition is increased in certain populations including critically ill patients, which may lead to complications such as gastric perforation, placement within the tracheobronchial tree, aspiration, or pneumothorax (85). Gastric POCUS can help identify the correct NGT placement and may reduce the need for ionizing radiation (x-ray) to confirm position (8, 9, 86, 87), which currently remains the gold standard method. Of the four studies (excluding the case report), two included only preterms and newborns. One study conducted in children lacked the gold standard abdominal x ray comparison (65). However, of the studies that report sensitivity, this was high to very high (88–98%) with good positive predictive values. No study was able to calculate specificity. The injection of an air bolus in the NGT (creates “bubbles” in the gastric fluid) may help confirm the correct placement of the tube as these bubbles are more easily detected during POCUS assessment. As NGT misplacement in the lower airways may have catastrophic consequences, the gold standard assessment for correct NGT placement is essential when gastric POCUS fails to confirm correct the intragastric position. In addition, as NGTs are often placed in the gastric fundus, gastric POCUS will neither consist of a sole antrum assessment nor fundus exploration, as described in Figure 5. Further robustly undertaken blinded studies are required in children beyond newborns and would be of value in the acutely and critically ill pediatric population.
Gastric POCUS appeared reliable compared to RADUS, and this technique could be implemented more broadly in the ED to reduce time diagnosing HSP and allowing patient care or discharge.
Totally, four high-quality studies (RCTs) used, rather than assessed, gastric POCUS in children receiving ventilation support, to evaluate gastric insufflation. However, the validation of this technique has not been clearly established in this setting.
Recent guidelines on the use of POCUS in pediatrics rarely mention gastric POCUS. In critically ill children (European guidelines published in 2020 on POCUS for critically ill children and neonates), cardiac, lung, cerebral, and vascular line placement and abdominal POCUS use are well-detailed (88). For abdominal POCUS, guidelines focused on intra-abdominal fluid detection and drainage or aspiration, parenchymal changes of solid organs and urinary obstruction, and peristalsis assessment and necrotizing enterocolitis detection only. Gastric POCUS was mentioned only for HPS detection. Recent neonatal guidelines (89) mention the use of abdominal POCUS to detect necrotizing enterocolitis, gut dysmotility, or anuria, but again gastric POCUS is not mentioned. The most recent European anesthesia guidelines (82) do discuss the benefits of using gastric POCUS in children when compliance with fasting instructions is unsure or in case of emergency anesthesia. Some recent pediatric emergency POCUS reviews (90, 91) have also mentioned gastric POCUS, and O'Brien et al. rated it as probably useful to identify foreign bodies and HSP, respectively. Other published reviews have not mentioned gastric POCUS (92, 93).
An expert group of adult and pediatric physicians has recently created a website dedicated to gastric POCUS, which describes the technique and presents a few examples of findings in various clinical settings (94). It provides useful information to strengthen clinicians' knowledge about gastric POCUS.
Most studies reported gastric POCUS performed by physicians (ED, intensivists, pediatricians, surgeons, anesthesiologists), but its use could be extended to other healthcare professionals like specialist nurses and advanced nurse practitioners who spend more time at the bedside (especially in the PICU and NICU), and manage NGT placement, and also potentially assess stomach volume and reduce fasting times for common planned procedures. Indeed, nurse-led urinary output algorithms have already been published using POCUS bladder scanning (95).
We did not find any study published in the PICU setting. However, two studies using gastric POCUS in the PICU are currently registered on www.clinicaltrial.gov: the GastriPed study (NCT04119089) aims to compare gastric residual measurements performed by NGT aspiration and gastric POCUS; the GastrExtub study (NCT05181904) aims to monitor gastric content/volume with gastric POCUS in the peri-operative setting. Furthermore, findings presented in this scoping review could be used to implement and evaluate its use in the PICU and to utilize it more for foreign body ingestion and HSP diagnosis. Ensuring correct placement of the NGT is also crucial in critically ill children for whom recent guidelines strongly recommend early enteral nutrition (96, 97). Gastric POCUS could also help confirm gastric emptiness, which is often expected prior to procedures like extubation and procedures requiring recurrent sedation/anesthesia (burn patients). This could reduce the often prolonged fasting times and related nutrition debt, and incidence of gastric aspiration. Finally, gastric insufflation may also be problematic in children in the PICU, especially if uncuffed endotracheal tubes are used, in situations requiring ventilatory pressures or high-frequency oscillatory ventilation or in children on non-invasive ventilation in whom gastric air distension sometimes may compromise ventilation and/or enteral nutrition efficiency. Thus, gastric POCUS may also help in optimizing ventilatory support. Ideally, the implementation of gastric POCUS in the PICU should undergo further robust evaluation studies (98).
POCUS use is currently increasing in a variety of pediatric settings, including the ED, NICU, and PICU. Gastric POCUS has been validated in other clinical situations and is used prior to sedation or anesthesia and in the ED. The implementation of gastric POCUS in the PICU setting appears beneficial but requires further robust studies and closer scrutiny.
FV, LT, and CJ designed the study. FV, LT, CJ, PA, WA, CM, TN, AR, EC, and LB reviewed the study abstracts and full texts. FV, LT, PA, CM, TN, EC, and LB extracted and analyzed the data. FV, LT, CM, TN, and LB wrote a synthesis of the findings per sub-questions. EC and AR provided illustrations of gastric POCUS. FV wrote the draft manuscript which was reviewed and approved by all authors. LT English-edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Florence Bouriot, the academic librarian for her precious help building the search equations, running the equations in the databases, and removing the duplicates.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.921863/full#supplementary-material
Supplementary Material 1. Search equations.
Supplementary Material 2. Long rational of gastric emptying/content.
1. Canty DJ, Royse CF, Kilpatrick D, Bowman L, Royse AG. The impact of focused transthoracic echocardiography in the pre-operative clinic. Anaesthesia. (2012) 67:618–25. doi: 10.1111/j.1365-2044.2012.07074.x
2. Bouvet L, Bellier N, Gagey-Riegel A-C, Desgranges F-P, Chassard D, De Queiroz Siqueira M. Ultrasound assessment of the prevalence of increased gastric contents and volume in elective pediatric patients: a prospective cohort study. Paediatr Anaesth. (2018) 28:906–13. doi: 10.1111/pan.13472
3. Kruisselbrink R, Gharapetian A, Chaparro LE, Ami N, Richler D, Chan VWS, et al. Diagnostic accuracy of point-of-care gastric ultrasound. Anesth Analg. (2019) 128:89–95. doi: 10.1213/ANE.0000000000003372
4. Adler A, Matisoff A, DiNardo J, Miller-Hance W. Point-of-care ultrasound in pediatric anesthesia: perioperative considerations. Curr Opin Anesthesiol. (2020) 33:343–53. doi: 10.1097/ACO.0000000000000852
5. Bonasso PC, Dassinger MS, Wyrick DL, Gurien LA, Burford JM, Smith SD. Review of bedside surgeon-performed ultrasound in pediatric patients. J Pediatr Surg. (2018) 53:2279–89. doi: 10.1016/j.jpedsurg.2018.04.040
6. Leviter J, Steele DW, Constantine E, Linakis JG, Amanullah S. “Full stomach” despite the wait: point-of-care gastric ultrasound at the time of procedural sedation in the pediatric emergency department. Acad Emerg Med Off J Soc Acad Emerg Med. (2019) 26:752–60. doi: 10.1111/acem.13651
7. Schmitz A, Schmidt AR, Buehler PK, Schraner T, Frühauf M, Weiss M, et al. Gastric ultrasound as a preoperative bedside test for residual gastric contents volume in children. Paediatr Anaesth. (2016) 26:1157–64. doi: 10.1111/pan.12993
8. Dias F de SB, Alvares BR, Jales RM, Franco APV, Silva JEF, da Fabene SMS, et al. The use of ultrasonography for verifying gastric tube placement in newborns. Adv Neonatal Care. (2019) 19:219–25. doi: 10.1097/ANC.0000000000000553
9. Claiborne M, Gross T, McGreevy J, Riemann M, Temkit M, Augenstein J. Point-of-care ultrasound for confirmation of nasogastric and orogastric tube placement in pediatric patients. Pediatr Emerg CARE. (2021) 37:E1611–5. doi: 10.1097/PEC.0000000000002134
10. Buonsenso D, Chiaretti A, Curatola A, Morello R, Giacalone M, Parri N. Pediatrician performed point-of-care ultrasound for the detection of ingested foreign bodies: case series and review of the literature. J Ultrasound. (2021) 24:107–14. doi: 10.1007/s40477-020-00452-z
11. Jeckovic M, Anupindi SA, Balj S, Lovrenski J. Ultrasound in detecting and following gastric foreign bodies in children. Pediatr Radiol. (2013) 43:S559. doi: 10.1007/s00247-013-2675-4
12. Park JS, Byun Y-H, Choi SJ, Lee JS Ryu J-M, Lee J-Y. Feasibility of point-of-care ultrasound for diagnosing hypertrophic pyloric stenosis in the emergency department. Pediatr Emerg Care. (2021) 37:550–4. doi: 10.1097/PEC.0000000000002532
13. Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. (2020) 18:2119–26. doi: 10.11124/JBIES-20-00167
14. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
15. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
16. Andersson H, Frykholm P. Gastric content assessed with gastric ultrasound in paediatric patients prescribed a light breakfast prior to general anaesthesia: a prospective observational study. Paediatr Anaesth. (2019) 29:1173–8. doi: 10.1111/pan.13755
17. Azad AM, Al Madi HA, Abdull Wahab SF, Shokoohi H, Kang YJ, Liteplo AS. Gastric ultrasonography in evaluating NPO status of pediatric patients in the emergency department. Am J Emerg Med. (2019) 37:355–6. doi: 10.1016/j.ajem.2018.06.013
18. Baldassarre M, Di Mauro A, Montagna O, Fanelli M, Capozza M, Wampler J, et al. Faster gastric emptying is unrelated to feeding success in preterm infants: randomized controlled trial. Nutrients. (2019) 11:71670. doi: 10.3390/nu11071670
19. Bansal P, Saini S. A study to compare gastric volume after six hours of fasting and two hours after carbohydrate drink by ultrasonography in children undergoing elective surgery. Anesth Analg. (2021) 133:1189–90.
20. Beck CE, Witt L, Albrecht L, Winstroth A-M, Lange M, Dennhardt N, et al. Ultrasound assessment of gastric emptying time in preterm infants: a prospective observational study. Eur J Anaesthesiol. (2019) 36:406–10. doi: 10.1097/EJA.0000000000001007
21. Beck C, Chandrakumar T, Sumpelmann R, Nickel K, Keil O, Heiderich S, et al. Ultrasound assessment of gastric emptying time after intake of clear fluids in children scheduled for general anesthesia-a prospective observational study. Pediatr Anesth. (2020) 30:1384–9. doi: 10.1111/pan.14029
22. Boretsky KR, Perlas A. Gastric ultrasound imaging to direct perioperative care in pediatric patients: a report of 2 cases. AA Pract. (2019) 13:443–5. doi: 10.1213/XAA.0000000000001100
23. Charlesworth M, Wiles MD. Pre-operative gastric ultrasound - should we look inside Schrödinger's gut? Anaesthesia. (2019) 74:109–12. doi: 10.1111/anae.14516
24. Desgranges F-P, Gagey Riegel A-C, Aubergy C, de Queiroz Siqueira M, Chassard D, Bouvet L. Ultrasound assessment of gastric contents in children undergoing elective ear, nose and throat surgery: a prospective cohort study. Anaesthesia. (2017) 72:1351–6. doi: 10.1111/anae.14010
25. Du T, Hill L, Ding L, Towbin AJ, DeJonckheere M, Bennett P, et al. Gastric emptying for liquids of different compositions in children. Br J Anaesth. (2017) 119:948–55. doi: 10.1093/bja/aex340
26. Elmetwally S, Hasanin A, Sobh L, Gohary M, Sarhan K, Ghazy D. Semi sitting position enhances gastric emptying of clear fluids in children: a randomized controlled trial. Egypt J Anaesth. (2020) 36:170–5. doi: 10.1080/11101849.2020.1814185
27. Evain J-N, Durand Z, Dilworth K, Sintzel S, Courvoisier A, Mortamet G, et al. Assessing gastric contents in children before general anesthesia for acute extremity fracture: an ultrasound observational cohort study. J Clin Anesth. (2022) 77:110598. doi: 10.1016/j.jclinane.2021.110598
28. Fabiani E, Bolli V, Pieroni G, Corrado G, Carlucci A, De Giacomo C, et al. Effect of a water-soluble fiber (galactomannans)-enriched formula on gastric emptying time of regurgitating infants evaluated using an ultrasound technique. J Pediatr Gastroenterol Nutr. (2000) 31:248–50. doi: 10.1097/00005176-200009000-00009
29. Frykholm P, Schindler E, Sumpelmann R, Walker R, Weiss M. Preoperative fasting in children: review of existing guidelines and recent developments. Br J Anaesth. (2018) 120:469–74. doi: 10.1016/j.bja.2017.11.080
30. Fukunaga C, Sugita M, Yamamoto T. Validity of ultrasonographic measurement of gastric volume in fasted pediatric patients without sedation. J Anesth. (2016) 30:900–3. doi: 10.1007/s00540-016-2204-3
31. Gagey A-C, de Queiroz Siqueira M, Desgranges F-P, Combet S, Naulin C, Chassard D, et al. Ultrasound assessment of the gastric contents for the guidance of the anaesthetic strategy in infants with hypertrophic pyloric stenosis: a prospective cohort study. Br J Anaesth. (2016) 116:649–54. doi: 10.1093/bja/aew070
32. Gagey A-C, de Queiroz Siqueira M, Monard C, Combet S, Cogniat B, Desgranges F-P, et al. The effect of pre-operative gastric ultrasound examination on the choice of general anaesthetic induction technique for non-elective paediatric surgery. A prospective cohort study. Anaesthesia. (2018) 73:304–12. doi: 10.1111/anae.14179
33. Gathwala G, Shaw C, Shaw P, Yadav S, Sen J. Human milk fortification and gastric emptying in the preterm neonate. Int J Clin Pract. (2008) 62:1039–43. doi: 10.1111/j.1742-1241.2006.01201.x
34. Geddes D, Gridneva Z, Cannon A, Hepworth A, Tie W, Lai C, et al. Effect of breast-milk leptin and macronutrient content on gastric emptying in term breastfed infants. Pediatr Res. (2017) 82:72–8. doi: 10.1038/pr.2017.79
35. Kim E-H, Yoon H-C, Lee J-H, Kim H-S, Jang Y-E, Ji S-H, et al. Prediction of gastric fluid volume by ultrasonography in infants undergoing general anaesthesia. Br J Anaesth. (2021) 127:275–80. doi: 10.1016/j.bja.2021.03.039
36. Lee JJ, Price JC, Duren A, Shertzer A, Hannum R, Akita FA, et al. Ultrasound evaluation of gastric emptying time in healthy term neonates after formula feeding. Anesthesiology. (2021) 2021:845–51. doi: 10.1097/ALN.0000000000003773
37. Miller A, Levy J, Krauss B, Gravel C, Vieira R, Neuman M, et al. Does point-of-care gastric ultrasound correlate with reported fasting time? Pediatr Emerg CARE. (2021) 37:E1265–9. doi: 10.1097/PEC.0000000000001997
38. Miyazawa R, Tomomasa T, Kaneko H, Morikawa A. Effect of formula thickened with locust bean gum on gastric emptying in infants. J Paediatr Child Health. (2006) 42:808–12. doi: 10.1111/j.1440-1754.2006.00982.x
39. Moser JJ, Walker AM, Spencer AO. Point-of-care paediatric gastric sonography: can antral cut-off values be used to diagnose an empty stomach? Br J Anaesth. (2017) 119:943–7. doi: 10.1093/bja/aex249
40. Munlemvo D, Moharir A, Yamaguchi Y, Khan S, Tobias J. Utility of gastric ultrasound in evaluating nil per os status in a child. Saudi J Anaesth. (2021) 15:46–9. doi: 10.4103/sja.SJA_702_20
41. Na H, Do HH, Lee SC, Lee JH, Seo JS, Kim YW, et al. Gastric point-of-care ultrasound evaluation in pediatric emergency department procedural sedation patients; is the stomach empty at the point of scheduled revisit? Signa Vitae. (2021) 17:59–65.
42. Parekh UR, Rajan N, Iglehart RC, McQuillan PM. Bedside ultrasound assessment of gastric content in children noncompliant with preoperative fasting guidelines: is it time to include this in our practice? Saudi J Anaesth. (2018) 12:318–20. doi: 10.4103/sja.SJA_452_17
43. Perrella SL, Hepworth AR, Simmer KN, Geddes DT. Validation of ultrasound methods to monitor gastric volume changes in preterm infants. J Pediatr Gastroenterol Nutr. (2013) 57:741–9. doi: 10.1097/MPG.0b013e3182a938d7
44. Perrella SL, Hepworth AR, Simmer KN, Hartmann PE, Geddes DT. Repeatability of gastric volume measurements and intragastric content using ultrasound in preterm infants. J Pediatr Gastroenterol Nutr. (2014) 59:254–63. doi: 10.1097/MPG.0000000000000397
45. Perrella SL, Hepworth AR, Simmer KN, Geddes DT. Influences of breast milk composition on gastric emptying in preterm infants. J Pediatr Gastroenterol Nutr. (2015) 60:264–71. doi: 10.1097/MPG.0000000000000596
46. Perrella SL, Hepworth AR, Gridneva Z, Simmer KN, Hartmann PE, Geddes DT. Gastric emptying of different meal volumes of identical composition in preterm infants: a time series analysis. Pediatr Res. (2018) 83:778–83. doi: 10.1038/pr.2017.292
47. Schmitz A, Kellenberger C, Weiss M, Schraner T. Ultrasonographic gastric antral area to assess gastric contents in children: comparison with total gastric fluid volume determined by magnetic resonance imaging. Swiss Med Wkly. (2010) 140:15S. Available online at: https://smw.ch/fileadmin/content/supplements/SMW_Suppl_184.pdf
48. Schmitz A, Thomas S, Melanie F, Rabia L, Klaghofer R, Weiss M, et al. Ultrasonographic gastric antral area and gastric contents volume in children. Paediatr Anaesth. (2012) 22:144–9. doi: 10.1111/j.1460-9592.2011.03718.x
49. Schmitz A, Schmidt A. Can we use ultrasound examination of gastric content as a diagnostic test in clinical anaesthesia? Pediatr Anesth. (2019) 29:112–3. doi: 10.1111/pan.13555
50. Sethi AK, Chatterji C, Bhargava SK, Narang P, Tyagi A. Safe pre-operative fasting times after milk or clear fluid in children. A preliminary study using real-time ultrasound. Anaesthesia. (1999) 54:51–9. doi: 10.1046/j.1365-2044.1999.00660.x
51. Song I-K, Kim H-J, Lee J-H, Kim E-H, Kim J-T, Kim H-S. Ultrasound assessment of gastric volume in children after drinking carbohydrate-containing fluids. Br J Anaesth. (2016) 116:513–7. doi: 10.1093/bja/aew031
52. Spencer AO, Walker AM, Yeung AK, Lardner DR, Yee K, Mulvey JM, et al. Ultrasound assessment of gastric volume in the fasted pediatric patient undergoing upper gastrointestinal endoscopy: development of a predictive model using endoscopically suctioned volumes. Paediatr Anaesth. (2015) 25:301–8. doi: 10.1111/pan.12581
53. Spencer AO, Walker AM. Antral sonography in the paediatric patient: can transducer choice affect the view? Br J Anaesth. (2015) 114:1002–3. doi: 10.1093/bja/aev122
54. Sümpelmann AE, Sümpelmann R, Lorenz M, Eberwien I, Dennhardt N, Boethig D, et al. Ultrasound assessment of gastric emptying after breakfast in healthy preschool children. Paediatr Anaesth. (2017) 27:816–20. doi: 10.1111/pan.13172
55. Taye S, Mohammed S, Bhatia P, Kumar M, Chhabra S, Kumar R, et al. Gastric emptying time of two different quantities of clear fluids in children: a double-blinded randomized controlled study. Paediatr Anaesth. (2021) 31:1187–93. doi: 10.1111/pan.14261
56. Yamaguchi Y, Zadora S, Flahive C, Russo J, Maves G, Moharir A, et al. Point-of-care gastric ultrasound in a pediatric patient after bowel preparation: a case report. Clin Exp Gastroenterol. (2020) 13:245–8. doi: 10.2147/CEG.S254793
57. Yigit S, Akgoz A, Memisoglu A, Akata D, Ziegler E. Breast milk fortification: effect on gastric emptying. J Matern Fetal Neonatal Med. (2008) 21:843–6. doi: 10.1080/14767050802287176
58. Zhang Y-L, Li H, Zeng H, Li Q, Qiu L-P, Dai R-P. Ultrasonographic evaluation of gastric emptying after ingesting carbohydrate-rich drink in young children: a randomized crossover study. Paediatr Anaesth. (2020) 30:599–606. doi: 10.1111/pan.13853
59. Horowitz R, Cico SJ, Bailitz J. Point-of-care ultrasound: a new tool for the identification of gastric foreign bodies in children? J Emerg Med. (2016) 50:99–103. doi: 10.1016/j.jemermed.2015.07.022
60. Jecković M, Anupindi SA, Barbir SB, Lovrenski J. Is ultrasound useful in detection and follow-up of gastric foreign bodies in children? Clin Imaging. (2013) 37:1043–7. doi: 10.1016/j.clinimag.2013.08.004
61. Salmon M, Doniger SJ. Ingested foreign bodies: a case series demonstrating a novel application of point-of-care ultrasonography in children. Pediatr Emerg Care. (2013) 29:870–3. doi: 10.1097/PEC.0b013e3182999ba3
62. Spina P, Minniti S, Bragheri R. Usefulness of ultrasonography in gastric foreign body retention. Pediatr Radiol. (2000) 30:840–1. doi: 10.1007/s002470000353
63. Yamamoto M, Koyama T, Agata M, Ouchi K, Kotoku T, Mizuno Y. Diagnosis of ingested foreign body in the stomach by point-of-care ultrasound in the upright and slightly forward tilting position (bowing position). Pediatr Emerg Care. (2017) 33:365–6. doi: 10.1097/PEC.0000000000001125
64. Atalay Y, Polat A, Ozkan E, Tomak L, Aygun C, Tobias J. Bedside ultrasonography for the confirmation of gastric tube placement in the neonate. Saudi J Anaesth. (2019) 13:23–7. doi: 10.4103/sja.SJA_413_18
65. Choi E, Korostensky M, Walker A, Spencer A. Validation of sonographic assistance for placement of a nasogastric tube in pediatric patients. J Clin Ultrasound. (2021) 49:101–5. doi: 10.1002/jcu.22963
66. Mori T, Takei H, Ihara T, Hagiwara Y, Nomura O. Ultrasound-guided nasogastric tube placement in a pediatric emergency department. J Clin Ultrasound. (2021) 49:106–9. doi: 10.1002/jcu.22958
67. Boneti C, McVay MR, Kokoska ER, Jackson RJ, Smith SD. Ultrasound as a diagnostic tool used by surgeons in pyloric stenosis. J Pediatr Surg. (2008) 43:87–91. doi: 10.1016/j.jpedsurg.2007.09.027
68. Malcolm WF, Smith PB, Mears S, Goldberg RN, Cotten CM. Transpyloric tube feeding in very low birthweight infants with suspected gastroesophageal reflux: impact on apnea and bradycardia. J Perinatol. (2009) 29:372–5. doi: 10.1038/jp.2008.234
69. McVay MR, Copeland DR, McMahon LE, Cosper GH, McCallie TG, Kokoska ER, et al. Surgeon-performed ultrasound for diagnosis of pyloric stenosis is accurate, reproducible, and clinically valuable. J Pediatr Surg. (2009) 44:169–72. doi: 10.1016/j.jpedsurg.2008.10.028
70. Sivitz AB, Tejani C, Cohen SG. Evaluation of hypertrophic pyloric stenosis by pediatric emergency physician sonography. Acad Emerg Med. (2013) 20:646–51. doi: 10.1111/acem.12163
71. Tejwani NA, Phatak T, Rutkin M, Sivitz AB. Pylor US imaging: Learning curve for pediatric emergency medicine fellows. Acad Emerg Med. (2014) 21:S37–8. doi: 10.1111/acem.12365
72. Wyrick DL, Smith SD, Dassinger MS. Surgeon as educator: bedside ultrasound in hypertrophic pyloric stenosis. J Surg Educ. (2014) 71:896–8. doi: 10.1016/j.jsurg.2014.05.001
73. Qian X, Hu Q, Zhao H, Meng B, Nan Y, Cao H, et al. Determination of the optimal inspiratory pressure providing adequate ventilation while minimizing gastric insufflation using real-time ultrasonography in Chinese children: a prospective, randomized, double-blind study. BMC Anesthesiol. (2017) 17:126. doi: 10.1186/s12871-017-0417-0
74. Park JH, Kim JY, Lee JM, Kim YH, Jeong HW, Kil HK. Manual vs. pressure-controlled facemask ventilation for anaesthetic induction in paralysed children: a randomised controlled trial. Acta Anaesthesiol Scand. (2016) 60:1075–83. doi: 10.1111/aas.12737
75. Li J, Hu Q. Clinical value of ultrasonography in monitoring gastric insufflation related to facemask ventilation in children during induction of general anesthesia. Anesth Analg. (2016) 123:324. doi: 10.1213/01.ane.0000492644.57194.e7
76. Lee J-H, Jung H, Jang Y-E, Kim E-H, Song I-K, Kim H-S, et al. Manual vs. pressure-controlled facemask ventilation during the induction of general anesthesia in children: a prospective randomized controlled study. Paediatr Anaesth. (2019) 29:331–7. doi: 10.1111/pan.13594
77. Perlas A, Davis L, Khan M, Mitsakakis N, Chan VWS. Gastric sonography in the fasted surgical patient: a prospective descriptive study. Anesth Analg. (2011) 113:93–7. doi: 10.1213/ANE.0b013e31821b98c0
78. Beck CE, Witt L, Albrecht L, Dennhardt N, Böthig D, Sümpelmann R. Ultrasound assessment of gastric emptying time after a standardised light breakfast in healthy children: a prospective observational study. Eur J Anaesthesiol. (2018) 35:937–41. doi: 10.1097/EJA.0000000000000874
79. Degeeter T, Demey B, Van Caelenberg E, De Baerdemaeker L, Coppens M. Prospective audit on fasting status of elective ambulatory surgery patients, correlated to gastric ultrasound. Acta Chir Belg. (2021) 23:1–6. doi: 10.1080/00015458.2021.1940438
80. Malcom GE 3rd, Raio CC, Del Rios M, Blaivas M, Tsung JW. Feasibility of emergency physician diagnosis of hypertrophic pyloric stenosis using point-of-care ultrasound: a multi-center case series. J Emerg Med. (2009) 37:283–6. doi: 10.1016/j.jemermed.2007.11.053
81. Boretsky K. Perioperative point-of-care ultrasound in children. Child-Basel. (2020) 7:213. doi: 10.3390/children7110213
82. Frykholm P, Disma N, Andersson H, Beck C, Bouvet L, Cercueil E, et al. Pre-operative fasting in children: a guideline from the European Society of Anaesthesiology and Intensive Care. Eur J Anaesthesiol. (2022) 39:4–25. doi: 10.1097/EJA.0000000000001599
83. Thomas M, Morrison C, Newton R, Schindler E. Consensus statement on clear fluids fasting for elective pediatric general anesthesia. Pediatr Anesth. (2018) 28:411–4. doi: 10.1111/pan.13370
84. Sorokin R, Gottlieb JE. Enhancing patient safety during feeding-tube insertion: a review of more than 2000 insertions. J Parenter Enter Nutr. (2006) 30:440–5. doi: 10.1177/0148607106030005440
85. Creel AM, Winkler MK. Oral and nasal enteral tube placement errors and complications in a pediatric intensive care unit. Pediatr Crit Care Med. (2007) 8:161–4. doi: 10.1097/01.PCC.0000257035.54831.26
86. Lyman B, Kemper C, Northington L, Yaworski JA, Wilder K, Moore C, et al. Use of temporary enteral access devices in hospitalized neonatal and pediatric patients in the United States. J Parenter Enter Nutr. (2016) 40:574–80. doi: 10.1177/0148607114567712
87. McMullen CD, Anstey C, Garrett P, Moore J. Nasogastric tube placement under sonographic observation: a comparison study of ultrasound and chest radiography in mechanically ventilated patients. Aust Crit Care. (2022) 35:181–5. doi: 10.1016/j.aucc.2021.03.006
88. Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care. (2020) 24:65. doi: 10.1186/s13054-020-2787-9
89. Miller LE, Stoller JZ, Fraga MV. Point-of-care ultrasound in the neonatal ICU. Curr Opin Pediatr. (2020) 32:216–27. doi: 10.1097/MOP.0000000000000863
90. Le Coz J, Orlandini S, Titomanlio L, Rinaldi VE. Point of care ultrasonography in the pediatric emergency department. Ital J Pediatr. (2018) 44:87. doi: 10.1186/s13052-018-0520-y
91. O'Brien AJ, Brady RM. Point-of-care ultrasound in paediatric emergency medicine. J Paediatr Child Health. (2016) 52:174–80. doi: 10.1111/jpc.13098
92. Bortcosh W, Shaahinfar A, Sojar S, Klig JE. New directions in point-of-care ultrasound at the crossroads of paediatric emergency and critical care. Curr Opin Pediatr. (2018) 30:350–8. doi: 10.1097/MOP.0000000000000621
93. Shaahinfar A, Ghazi-Askar ZM. Procedural applications of point-of-care ultrasound in pediatric emergency medicine. Emerg Med Clin North Am. (2021) 39:529–54. doi: 10.1016/j.emc.2021.04.006
94. Gastric, UltraSound. A Point-of-Care Tool. Available online at: https://www.gastricultrasound.org/en/home (accessed April 4, 2022).
95. Pierson M, Cretella B, Roussel M, Byrne P, Parkosewich J. A nurse-led voiding algorithm for managing urinary retention after general thoracic surgery. Crit Care Nurse. (2022) 42:23–31. doi: 10.4037/ccn2022727
96. Mehta NM, Skillman HE, Irving SY, Coss-Bu JA, Vermilyea S, Farrington EA, et al. Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: society of critical care medicine and American Society for Parenteral and Enteral Nutrition. J Parenter Enteral Nutr. (2017) 41:706–42. doi: 10.1177/0148607117711387
97. Tume LN, Valla FV, Joosten K, Chaparro CJ, Latten L, Marino LV, et al. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Med. (2020) 46:411–25. doi: 10.1007/s00134-019-05922-5
Keywords: pediatric intensive care, pediatric emergency, pediatric anesthesia, POCUS, nasogastric tube, foreign body, gastric insufflation, hypertrophic pyloric stenosis
Citation: Valla FV, Tume LN, Jotterand Chaparro C, Arnold P, Alrayashi W, Morice C, Nabialek T, Rouchaud A, Cercueil E and Bouvet L (2022) Gastric Point-of-Care Ultrasound in Acutely and Critically Ill Children (POCUS-ped): A Scoping Review. Front. Pediatr. 10:921863. doi: 10.3389/fped.2022.921863
Received: 16 April 2022; Accepted: 31 May 2022;
Published: 06 July 2022.
Edited by:
Enid Martinez, Boston Children's Hospital, United StatesReviewed by:
Daniela De Souza, University of São Paulo, BrazilCopyright © 2022 Valla, Tume, Jotterand Chaparro, Arnold, Alrayashi, Morice, Nabialek, Rouchaud, Cercueil and Bouvet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frederic V. Valla, ZnJlZGVyaWMudmFsbGFAY2h1LWx5b24uZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.