- Department of General Surgery, The Children's Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
Background: Choledochal cyst perforation is extremely rare, and early diagnosis or prediction is important for the immediate therapeutic intervention of perforations. This study aimed to define the predictor(s) of an impending or complete spontaneous perforation of choledochal cyst and establish the optimal operative timing.
Methods: All 429 consecutive choledochal cyst patients from January 2015 to December 2021, were included. A retrospective study was performed based on Kaplan-Meier analysis, and Cox univariate and multivariate analyses.
Results: A total of 429 patients were included, among which, 21 had choledochal cyst perforations (group A), and 408 did not (group B). Compared to group B, the serum alanine aminotransferase, aspartate aminotransferase, direct bilirubin, gamma-glutamyl transpeptidase, indirect bilirubin, total bilirubin, and alkaline phosphatase were significantly higher in group A (p = 0.025, 0.006, < 0.0001, 0.0001, 0.001, < 0.0001, and 0.033). High serum gamma-glutamyl transpeptidase was negatively associated with perforation-free preoperative survival, and multivariate Cox regression revealed that serum gamma-glutamyl transpeptidase was an independent predictive factor for an impending or complete perforation (p = 0.042).
Conclusions: A gamma-glutamyl transpeptidase level ≥ 346.5 U/L accompanied with significantly elevated liver enzymes and bilirubin levels was indicative of the possibility of an impending or complete choledochal cyst perforation, and a proactive surgical approach should be considered.
Introduction
Choledochal cysts (CCs) are cystic or fusiform dilations of the common bile duct. CCs occur in one in 1,000 persons in Asia, while they are less frequent, at one in 50,000–150,000 individuals, in the West. The female-to-male ratio for CCs ranges from 3:1 to 4:1 (1, 2). CC perforation is extremely rare, with a reported frequency of 1.8–2.8% (3, 4). The pathogenesis of spontaneous perforation has not been clarified, but proposed mechanisms include the reflux of pancreatic secretions (5), increased intraluminal pressure due to protein plugs (6), and viral infection (7).
CCs are treated by elective surgery, and patients usually experience a satisfiable prognosis in most cases. Whereas, as an acute abdomen, CC perforation requires emergency surgery, a delay in the diagnosis or inappropriate management can result in the development of complications (bacterial contamination of the biliary ascites, life-threatening sepsis, attacks of cholangitis, and pancreatitis) (8), which will prolong the course, increase the cost of hospitalization, and exacerbate the pain of patients.
Therefore, early diagnosis or prediction is important for the immediate therapeutic intervention of impending or complete CC perforations. As a specialized children's hospital and national clinical research center, we have performed surgeries on more than 600 CCs since January 2011. Here, we conducted a retrospective study to summarize the features of patients with CC perforations and explored the potential predictive factors that could be used to contribute to the timely diagnosis and management of CC perforations.
Materials and methods
Study design and patients
Following institutional review board approval, the electronic medical records of consecutive patients (104 males and 325 females) who underwent excision of CCs and Roux-en-Y hepaticojejunostomy in the Department of General Surgery, Children's Hospital of Zhejiang University School of Medicine between January 2015 and December 2021 were collected. Twenty-one patients (six males and 15 females) were complicated with CC perforations, as confirmed by intraoperative examinations. All operations were performed by the same group of experienced surgeons. Variables, including patient demographics, symptoms, classification of cystic or fusiform dilatation, and serum biochemical indices were recorded. A retrospective analysis was performed between patients with or without CC perforation, based on the above clinical data.
Inclusion criteria
The inclusion criteria were: (1) patients diagnosed with CC based on the intraoperative findings and postoperative pathological results; (2) the Children's Hospital of Zhejiang University School of Medicine was the first-visit hospital for patients; (3) patients did not have any other abdominal surgeries; and (4) CC perforation or cystic necrotic changes were verified by intraoperative examinations. CC patients who did not meet the above four criteria were excluded.
Classification of perforation type and perforation locations
Type I: complete cystic perforation; type II: sealed perforation with a small amount of bile contained in a pseudocyst; and type III: cystic necrotic changes with an intact serosal layer. Perforation locations of choledochal cyst were shown in Table 1.
Statistical analysis
Data are shown as the medians ± interquartile ranges (IQRs). Statistical analyses were performed using SPSS22.0 and GraphPad Prism 6. Statistical differences were analyzed by two-tailed Student's t-tests. Survival curves were drawn using Kaplan–Meier univariate estimates according to the Youden index. Multivariate analysis was performed by Cox regression. A p-value < 0.05 was considered statistically significant.
Result
Clinical data and analyses of serum biochemical indices
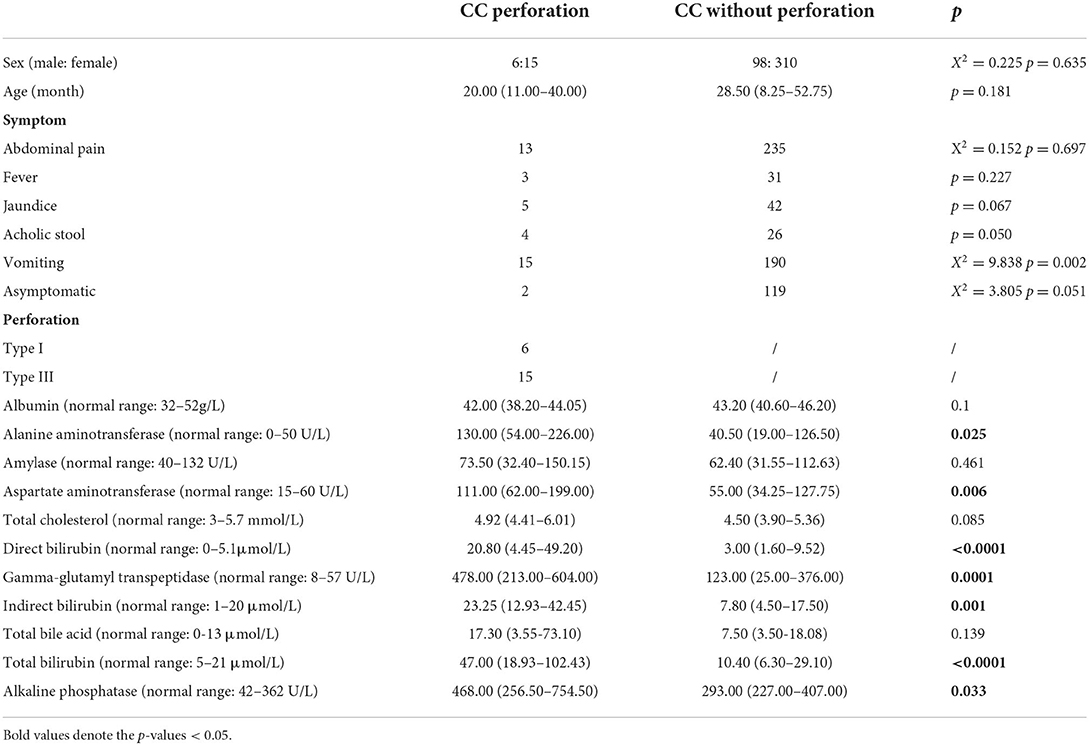
A total of 429 CC patients (104 males and 325 females) were included in this study, among whom 21 (six males and 15 females) were complicated with perforation (group A), and 409 (98 males and 310 females) did not experience perforation (group B). The two groups of patients were similar in sex, and age. The predominant symptoms were abdominal pain (61.9% in group A and 57.6% in group B) and vomiting (71.4% in group A and 46.5% in group B), followed by jaundice, fever and acholic stool. Within group A, six patients (28.6%) had type I perforations, and 15 patients (71.4%) had type III perforations. Group A exhibited higher serum ALT levels [130.00 (54.00–226.00) vs. 40.50 (19.00–126.50), p = 0.025], and serum AST levels [111.00 (62.00–199.00) vs. 55.00 (34.25–127.75), p = 0.006] compared to group B. The serum levels of direct bilirubin [20.80 (4.45–49.20) vs. 3.00 (1.60–9.52), p < 0.0001], indirect bilirubin [23.25 (12.93–42.45) vs. 7.80 (4.50–17.50), p = 0.001], total bilirubin [47.00 (18.93–102.43) vs. 10.40 (6.30–29.10), p < 0.0001], and alkaline phosphatase [468.00 (256.50–754.50) vs. 10.40 (6.30–29.10), p = 0.033] in group A were significantly higher than those in group B. Significantly elevated serum GGT levels [478.00 (213.00–604.00) vs. 123.00 (25.00–376.00), p = 0.0001] were also observed in group A compared to group B (data are shown in Table 2).
ROC curve and Kaplan-Meier analysis
Univariate ROC curve analyses of the effect of CC perforation on each serum biochemical index was performed. The AUCs and p-values of ALT, AST, GGT, ALB, TB, DB, IDB, ALP, TBA, TC, and AMY are shown in Figure 1. The optimum cutoffs of serum GGT (cutoff = 346.5 U/L, sensitivity = 71.4%, specificity = 73.2%) and indirect bilirubin (cutoff = 12.8 μmol/L, sensitivity = 80.0%, specificity = 79.4%) levels were set according to the Youden index. Next, we evaluated the prognostic values of serum GGT (cutoff = 346.5 U/L) and indirect bilirubin (cutoff = 12.8 μmol/L) levels, and divided each into high and low groups according to the cut-offs. Using Kaplan–Meier curves, we identified that high levels of serum GGT and indirect bilirubin were negatively associated with preoperative perforation-free survival (p-values were both <0.0001, Figure 2).
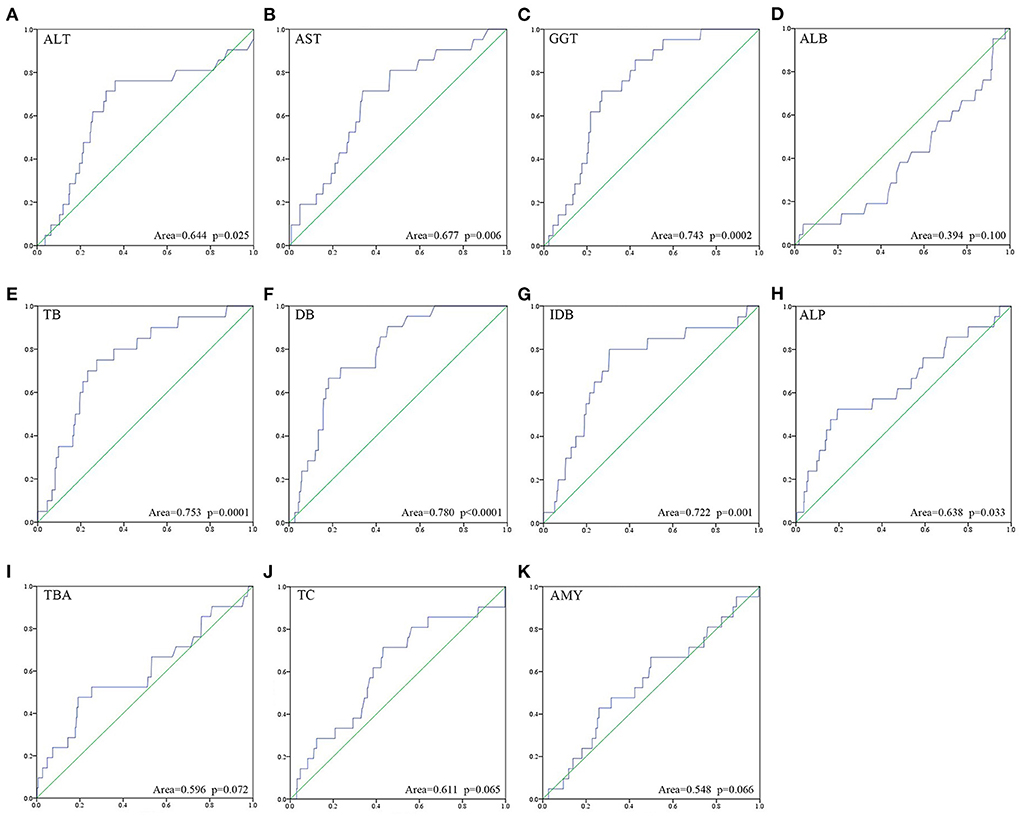
Figure 1. Univariate ROC curve analysis about risk of perforation onto each of the eleven serum biochemical indices (A–K) was performed.
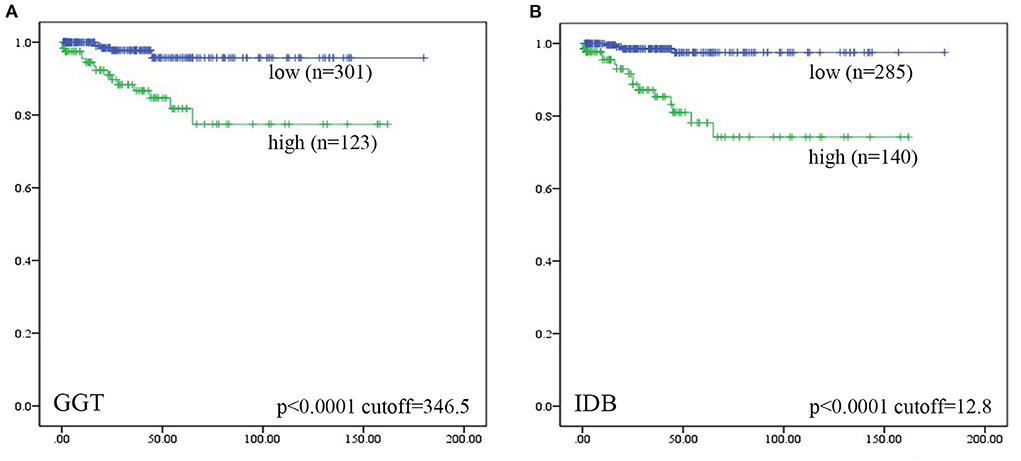
Figure 2. Patients were divided into “high” and “low” groups according to the optimal cut-offs which were determined by Youden index. Kaplan–Meier analyses of preoperative perforation-free survival for (A) serum GGT level, and (B) serum IDB level were analyzed. Among them, five patients lost data of GGT levels and four lost data of IDB levels. Log-rank test was used.
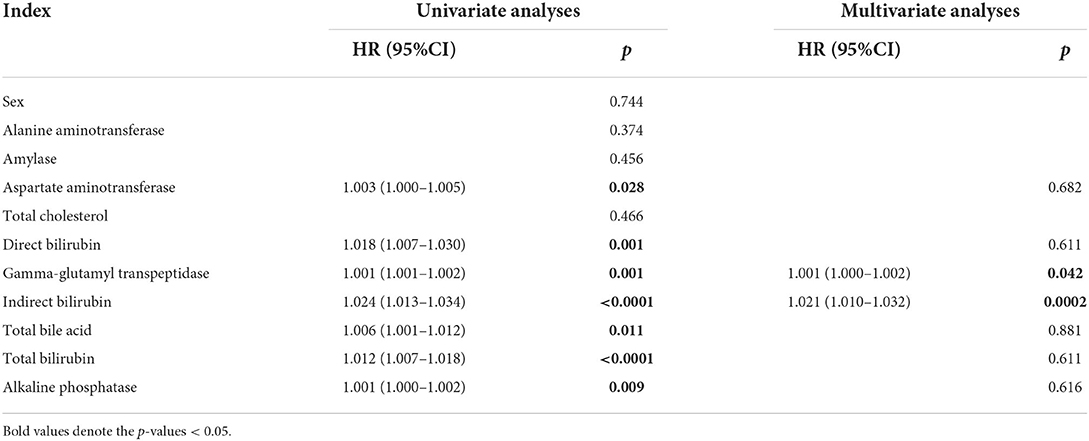
Cox univariate and multivariate analyses
To illustrate whether independent preoperative predictive factors existed, each serum biochemical index that was significant based on univariate analyses were adopted as covariates for multivariate Cox regression analyses. Patients with high serum GGT levels harbored a 1.001—fold higher risk of perforation than patients with low serum GGT levels (HR, 1.001; 95% CI, 1.000–1.002; p = 0.001). High serum levels of indirect bilirubin also imparted a 1.021—fold higher risk of perforation (HR, 1.021; 95% CI, 1.010–1.032; p = 0.021, Table 3). However, the cutoff level (12.8 μmol/L) of indirect bilirubin was within the normal range clinical testing (1–20 μmol/L in our hospital), and thus, we excluded indirect bilirubin and the clinical application of this index in predicting CC perforation remained further discussion.
Discussion
Choledochal cyst perforation is an uncommon complication, while bile peritonitis can be fatal (9). Because of their atypical clinical manifestations, preoperative misdiagnoses of CC perforation are high, and early pre-operative diagnoses are notoriously difficult to perform. Here, we aimed to summarize the clinical characteristics of patients with CC perforations and explore potential non-invasive serum biomarkers for the detection of perforations at early stages.
The exact pathogenesis of CC perforations remains controversial. Previous studies concluded three main potential causes of spontaneous perforation. First, the distal obstruction of the bile duct and consequent high intraluminal pressure may result in the perforation of the fragile cystic wall (10, 11). Thus, some researchers suggested that CC perforation is not rare in infancy because of the immature ducal wall with fewer elastic fibers (10, 12). Second, pancreaticobiliary malunion may cause epithelial irritation of the biliary tract due to refluxed pancreatic juice (10, 13, 14). Third, the poor blood supply of the common bile ducts' anterior wall, which is only from the marginal artery, is more susceptible to an ischemic event (15, 16). In addition to their undefined etiologies, CC perforations may also manifest clinically with atypical symptoms, such as acute abdomen with peritonitis and vomiting (10, 17). This was also observed in our series with abdominal pain (61.9%) and vomiting (71.4%) as the primary complaints. One patient with complete cystic perforation was even asymptomatic in our study. If the site of perforation is small, patients may present without any symptoms since the bile may escape slowly enough to permit a pseudocyst to develop (12). Thus, the early diagnosis of CC perforation is difficult and the frequencies of misdiagnoses are high. Not to mention cystic necrotic changes with an intact serosal layer, patients may not have any clinical symptoms with an impending perforation.
One previous study pointed out that the interruption of the continuity of the bile duct in ultrasound was a diagnostic feature for CC perforations, and exhibited a specificity of 100%. However, the detection sensitivity of the interruption on ultrasound was low (18.6%) (8). The loss of local gallbladder tension, a thickened gallbladder wall, abnormal morphology of the gallbladder, and peritoneal effusion could also be used as imaging features for CC perforation (18). Ultrasound allows for real-time dynamic monitoring and has good reproducibility, but it requires subjective assessments by the operator, which requires sufficient experience to become proficient. Thus, a non-invasive and objective serum biomarker is clinically superior. Serum GGT levels were used to predict biliary atresia, however, its accuracy varied (19, 20). Some studies also included GGT levels to develop a grading system (Grade I–III) for bile leakage severity in CC, with the serum GGT level for a Grade I diagnosis being 343.75 ± 215.6 U/L (6). In the current study, we found that preoperative serum GGT levels were positively correlated cystic necrotic change and complete CC perforation. Thus, we supported the early detection of elevated GGT level as an important reference for the possibility of CC perforation.
Serum GGT level was an independent predictive factor of CC perforation, and patients with low GGT levels (cutoff = 346.5U/L) experienced a long perforation-free preoperative survival duration. Collectively, we identified a potential non-invasive and objective biomarker to predict CC perforation. More importantly, we applied serum GGT level to predict potential cystic necrotic changes, which are indicative of an impending perforation, to help with timely clinical decision making. We also found that CC patients with cystic necrotic changes or complete perforation had significantly elevated levels of serum ALT, AST and bilirubin. Thus, those markers could be used as important auxiliary indicators to enhance the accuracy of clinical assessments. Thus, when encountering CC patients with GGT levels >346.5 U/L combined with significantly elevated liver enzymes and bilirubin, dynamic monitoring of ultrasound is recommended. We should especially focus on gallbladder fossa or right upper abdomen effusion indicated by ultrasound and finish MRCP examination as soon as possible. Then, a proactive surgical approach should be considered to avoid bile leakage, preferably before perforation.
Conclusions
In summary, we identified the applicability of serum GGT levels in predicting CC perforation, particularly in patients with potential cystic necrotic changes, which were indicative of an impending perforation. Serum GGT level relied on routine clinical data, and thus, it is practical and easily accessible for clinical decision-making purposes. However, validation of its relationship with CC perforation is required in the future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Children's Hospital, Zhejiang University School of Medicine (Approval No. 2022-IRB-047). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SZ and DC contributed to study conception and design. SZ, DH, ZH, and QC contributed to data acquisition. SZ, YZ, KC, YJ, and WL contributed to analysis and data interpretation. SZ and ZG contributed to drafting of the manuscript, while ZG contributed to critical revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Clinical Medical Research of Minimally Invasive Diagnosis and Treatment of Abdominal Organs in Zhejiang Province (Grant No. 01492-02).
Acknowledgments
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arora M, Kaur J, Aggarwal SK. Biliary Pseudocyst: A Rare Complication of Antenatal Perforation of Forme Fruste Choledochal Cyst. J Indian Assoc Pediatr Surg. (2019) 24:291. doi: 10.4103/jiaps.JIAPS_212_18
2. Tsai CC, Huang PK, Liu HK, Su YT, Yang MC, Yeh ML. Pediatric types I and VI choledochal cysts complicated with acute pancreatitis and spontaneous perforation: A case report and literature review. Medicine. (2017) 96:e8306. doi: 10.1097/MD.0000000000008306
3. Ohba G, Yamamoto H, Nakayama M, Honda S, Taketomi A. Single-stage operation for perforated choledochal cyst. J Pediatr Surg. (2017) 53:653–5. doi: 10.1016/j.jpedsurg.2017.07.014
4. Masao Y. Congenital choledochal cyst. Analysis of 1,433 patients in the Japanese literature. Am J Surg. (1980) 140:653–7. doi: 10.1016/0002-9610(80)90051-3
5. Zhu L, Xiong J, Lv Z, Liu J, Xu W. Type C Pancreaticobiliary maljunction is associated with perforated choledochal cyst in children. Front Pediatr. (2020) 8:1–6. doi: 10.3389/fped.2020.00168
6. Li P, Zhang D, Zheng C, Guo C. Development and validation the bile leakage grading criterion in patients following Roux-en-Y hepaticojejunostomy. Asian J Surg. (2020) 1:358–62. doi: 10.1016/j.asjsur.2020.09.001
7. Moore TC. Massive bile peritonitis in infancy due to spontaneous bile duct perforation with portal vein occlusion. J Pediatr Surg. (1975) 10:537–8. doi: 10.1016/0022-3468(75)90200-6
8. Xin Y, Wang XM, Wang Y, Hu YX, Jia LQ. Value of ultrasound in diagnosing perforation of congenital choledochal cysts in children. J Ultras Med. (2021) 40:2157–63. doi: 10.1002/jum.15604
9. Lilly JR, Weintraub WH, Altman RP. Spontaneous perforation of the extrahepatic bile duct and bile peritonitis in infancy. Surgery. (1974) 75:664–73.
10. Müller U, Frickmann H, Jungblut S, Bargon J. Spontaneous perforation of choledochal cyst: a study of 13 cases*. Eur J Pediatr Surg. (1998) 8:23–5. doi: 10.1055/s-2008-1071113
11. Yamoto M, Urushihara N, Fukumoto K, Miyano G, Nouso H, Morita K, et al. Usefulness of laparoscopic cholecystostomy in children with complicated choledochal cyst. Asian J Endosc Surg. (2015) 8:153–7. doi: 10.1111/ases.12170
12. Yasufuku M, Hisamatsu C, Nozaki N, Nishijima E. A very low-birth-weight infant with spontaneous perforation of a choledochal cyst and adjacent pseudocyst formation. J Pediatr Surg. (2012) 47:E17–9. doi: 10.1016/j.jpedsurg.2012.03.055
13. Ohkawa H, Takahashi H, Maie MA. malformation of the pancreatico-biliary system as a cause of perforation of the biliary tract in childhood. J Pediatr Surg. (1977) 12:541–6. doi: 10.1016/0022-3468(77)90194-4
14. Ng W, Cheung C, Chan S. Is spontaneous perforation of the bile duct in children due solely to pancreatico-biliary maljunction? Pediatr Surg Int. (2002) 18:565–6. doi: 10.1007/s00383-002-0745-z
15. Northover JMA, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Brit J Surg. (1979) 66:379–84. doi: 10.1002/bjs.1800660603
16. Lloyd JR. The etiology of gastrointestinal perforations in the newborn. J Pediatr Surg. (1969) 4:77–84. doi: 10.1016/0022-3468(69)90186-9
17. Chiang L, Chui CH, Low Y, Jacobsen AS. Perforation: a rare complication of choledochal cysts in children. Pediatr Surg Int. (2011) 27:823–7. doi: 10.1007/s00383-011-2882-8
18. Chen J, Tang Y, Wang Z, Wang Q, Wang D. Clinical value of ultrasound in diagnosing pediatric choledochal cyst perforation. Am J Roentgenol. (1976). 2015 2015-01-01;204:630. doi: 10.2214/AJR.14.12935
19. Shankar S, Bolia R, Foo HW, D'Arcy CE, Hardikar W. Normal gamma glutamyl transferase levels at presentation predict poor outcome in biliary atresia. J Pediatr Gastr Nutr. (2019) 70:1. doi: 10.1097/MPG.0000000000002563
Keywords: choledochal cyst, perforation, cystic necrotic changes, gamma-glutamyl transpeptidase, prediction
Citation: Zhang S, Cai D, Chen Q, Zhang Y, Chen K, Jin Y, Luo W, Huang Z, Hu D and Gao Z (2022) Value of serum GGT level in the timing of diagnosis of choledochal cyst perforation. Front. Pediatr. 10:921853. doi: 10.3389/fped.2022.921853
Received: 16 April 2022; Accepted: 28 July 2022;
Published: 15 August 2022.
Edited by:
Pedro Gutierrez-Castrellon, Hospital General Dr. Manuel Gea Gonzalez, MexicoCopyright © 2022 Zhang, Cai, Chen, Zhang, Chen, Jin, Luo, Huang, Hu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Gao, ZWJ3ayYjeDAwMDQwO3pqdS5lZHUuY24=
 Shuhao Zhang
Shuhao Zhang Zhigang Gao
Zhigang Gao