- 1Pediatric Pulmonology & Respiratory Intermediate Care Unit, Sleep, and Long Term Ventilation Unit, Academic Department of Pediatrics (DPUO), Bambino Gesù Children's Hospital, IRCCS, Rome, Italy
- 2Department of Medicine, Surgery, and Health Sciences, University of Trieste, Trieste, Italy
- 3Respiratory Medicine Unit, University “Magna Graecia” of Catanzaro, Catanzaro, Italy
There is a growing need for advanced treatment in children with persistent and severe asthma symptoms. As a matter of fact, between 2 and 5% of asthmatic children experience repeated hospitalizations and poor quality of life despite optimized treatment with inhaled glucocorticoid plus a second controller. In this scenario, mepolizumab, a humanized monoclonal antibody, has proven to be effective in controlling eosinophil proliferation by targeting interleukin-5 (IL-5), a key mediator of eosinophil activation pathways. Mepolizumab is approved since 2015 for adults at a monthly dose of 100 mg subcutaneously and it has been approved for patients ≥ 6 years of age in 2019. Especially in children aged 6 to 11 years, mepolizumab showed a greater bioavailability, with comparable pharmacodynamics parameters as in the adult population. The recommended dose of 40 mg every 4 weeks for children aged 6 through 11 years, and 100 mg for patients ≥ 12 years provides appropriate concentration and proved similar therapeutic effects as in the adult study group. A marked reduction in eosinophil counts clinically reflects a significant improvement in asthma control as demonstrated by validated questionnaires, reduction of exacerbation rates, and the number of hospitalizations. Finally, mepolizumab provides a safety and tolerability profile similar to that observed in adults with adverse events mostly of mild or moderate severity. The most common adverse events were headache and injection-site reaction. In conclusion, mepolizumab can be considered a safe and targeted step-up therapy for severe asthma with an eosinophilic phenotype in children and adolescents.
Introduction
Severe asthma is a highly heterogeneous disease due to the interaction between genetic predisposition, immune response, and environmental risk factors. This complex interaction influences the onset of symptoms, the clinical evolution, and the severity of illness (1, 2). Severe asthma affects about 0.5% of the general pediatric population and 4.5% of children with asthma (3), with a wide discrepancy between countries. Although the prevalence of severe asthma in childhood is low, it is associated with high morbidity, occasionally mortality, and significant healthcare burden (4). In addition, children with severe asthma report a poor quality of life due to persistent respiratory symptoms, recurrent life-threatening attacks, anxiety and emotional distress, missed school days, and side effects of oral corticosteroids (OCS) (5, 6).
A multidisciplinary assessment is required to exclude comorbidities and modifiable factors, confirm the diagnosis, and initiate the most appropriate treatment for these patients (7). The identification of different phenotypes and endotypes, based on cellular and molecular mechanisms and related biomarkers, may guide physicians in their therapeutic choice (8).
Recently, several innovative monoclonal antibody agents have been approved to target specific inflammatory type 2 mediators and improve uncontrolled severe asthma when added to basal treatment (9). However, it is necessary that pediatric pulmonologists are aware of the benefits and risks of these medications, as well as the practical implications of providing these options for their patients (10).
In this review, especially focused on mepolizumab, we present all current evidence on the indications, use, safety, and efficacy of this fully-humanized anti-interleukin-5 (IL-5) antibody for children with severe asthma.
Search Methods
This review is based on the most significant results from an extensive PubMed search, conducted independently by three different researchers, using the search terms: “treatment in severe pediatric asthma,” “severe asthma,” “mepolizumab,” “anti-IL-5 therapy,” “anti–IL-5 antibody,” “children,” “adolescents,” and all different synonyms or word combinations. More relevance was given to clinical trials especially focused on mepolizumab treatment.
Management of Severe Asthma in Pediatrics
Definition of Severe Asthma
There is no uniformly accepted definition of severe asthma (11). Different options can be found in the scientific literature (12–15). However, international societies agree on assessing asthma severity based on the treatment level required to achieve and maintain adequate control.
According to the Global Initiative for Asthma (GINA) guidelines 2022, severe asthma isindicated by needing a high dose of ICS-LABA to maintain symptom control or uncontrolled asthma despite steps 4 and 5 of the care and management of contributory factors (12).
The European Respiratory Society and the American Thoracic Society (ERS/ATS) definitions of severe asthma require that patients have needed therapy with a high dose of ICS and a second controller, such as LABA, leukotriene modifier, or theophylline, for the previous year or have needed OCS for 50% or more of the year to prevent asthma from becoming uncontrolled or that cannot be controlled despite this therapy (13).
Step-by-Step Diagnosis
Patients that need to be moved to GINA steps 4 and 5 care have to be referred to a tertiary center for a specialist evaluation first to confirm the diagnosis and subsequently to identify the best personalized treatment (7). In the beginning, it is very important to distinguish between “difficult to treat asthma,” due to modifiable factors, and true “therapy-resistant asthma,” which is unresponsive to standard medications (16, 17). Although a concomitance between these two conditions cannot always be excluded, uncontrolled asthma is frequently caused by treatment-related issues such as poor adherence to medication, inadequate or inappropriate inhalation technique (18, 19), and persistent exposure to adverse environmental factors (smoke, irritants, allergens, etc.) (20)or emotional factors.
Moreover, the presence of comorbidities, including allergic conditions (rhinitis, eczema, atopic dermatitis, food or drug allergy, etc), sinus disease, gastroesophageal reflux, obesity, obstructive sleep apnea, anxiety, and depression, may reduce the response to therapy (21–23).
Finally, the exclusion of asthma-mimicking conditions (24), such as tracheobronchomalacia, bronchopulmonary dysplasia, cystic fibrosis, primary ciliary dyskinesia, immunodeficiencies, obliterative bronchiolitis, pertussis, tuberculosis, vascular rings, and foreign body in toddlers (25) and vocal cord dysfunction, exercise-induced hyperventilation, and habitual cough in children and adolescents (26), is necessary.
Once all the following steps are carried out, that is, revising treatment issues, excluduing or treating comorbidities or other differential diagnoses, and confirming that it is severe asthma, it is possible to consider add-on medications, including biologics.
Phenotype and Endotype-Guided Therapy
The introduction of biologics has revolutionized the care of severe asthma in adult and pediatric populations (27–29). Recent studies have suggested that physicians should characterize patients with severe asthma not only by their phenotype but also by their endotype before starting a biological treatment (30). This approach is in line with personalized medicine, which aims to achieve a better characterization of patients with the purpose to prescribe the most suitable treatment at an individual level (31).
The phenotype is the summation of clinical features while the endotype is determined by biomolecular mechanisms leading to the pathogenesis of the disease (32). To date, two endotypes of severe asthma have been described, based on the pathogenetic processes linked to airway inflammation: T2-low endotype and T2-high endotype (33).
The T2-low endotype is more frequent in adults and is characterized by the following: neutrophilic or paucigranulocytic inflammation, T-helper lymphocytes type 17, innate lymphoid type 3 cells, and IL-1, IL-8, IL-17, and IL-23 are the respective implied molecules (34, 35).
The T2-high endotype typically affects children and it is characterized by two specific characteristics: allergic sensitization and eosinophilia (1). Allergic asthma is the most common in childhood; it often presents an early onset, and it is associated with a family or personal history of allergic disease, a positive skin prick test, an elevated serum total IgE level, and increased fractional exhaled nitric oxide (FeNO) (36). Patients with eosinophilia and diffuse airway inflammation often are well responsive to corticosteroids (37, 38). However, a subgroup of T2-high asthma has poor control of symptoms despite corticosteroids, probably due to very high levels of type 2 inflammation. T2-high asthma constitutes 50% of mild to moderate asthma and probably a larger proportion of patients with more severe asthma (37).
In the T2-high endotype, T-helper lymphocytes type 2 signal the production of IL-4, IL-5, and IL-13, while innate lymphoid cells type 3 are activated by the epithelial alarmins TSLP, IL-33, and IL-25. Specifically, IL-4 and IL-13 promote B lymphocyte activation, inducing plasma cell formation, isotype switching to IgE, and their production. On the other hand, IL-5 induces chemoattraction, proliferation, and activation of eosinophils and also decreases their apoptosis (32).
Current research has suggested that these molecular pathways of severe asthma should guide the choice of the most adequate biologic treatment (39). The identification of biomarkers such as total IgE, peripheral eosinophil count, and FeNO, as a surrogate of mechanism generating and maintaining type 2 inflammation, helps design a personalized therapy for patients (30). In this scenario which is constantly and progressively oriented toward tailored treatments, mepolizumab might be the more indicated add-on target therapy for severe eosinophilic asthma.
Mepolizumab as Target Therapy for Severe Eosinophilic Asthma
License and Mechanism of Action
Mepolizumab (Nucala, GlaxoSmithKline) has been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in patients aged 12 years and older since November 2015 and was licensed in September 2019 in patients aged 6 years and older as an add-on maintenance therapy for severe eosinophilic asthma (40, 41). Mepolizumab is a murine humanized monoclonal antibody belonging to the IgG1 kappa subclass. It binds circulating IL-5 and prevents its interaction with IL-5 receptor alfa. Mepolizumab target (i.e., IL-5) is a 134-amino acid dimeric glycoprotein with a four-helix bundle motif, which consists of a 52-kDa homodimer. Mepolizumab specifically binds to the α-chain of IL-5 with an IC50 of <1 nM, a dissociation constant of 4.2 pM, and stoichiometry of 2.2, so that two IL-5 dimers are cross-linked by two molecules of mepolizumab. Therefore, through this mechanism of action, mepolizumab effectively inhibits IL-5 ligation to IL-5Rα (42). As mentioned above, IL-5 is fundamental for the recruitment, activation, and survival of eosinophils.
Eligibility Criteria and Dosage
The eligibility criteria of treatment with mepolizumab for severe eosinophilic asthma in children and adolescents include blood eosinophilia > 0.15 × 109/l in the absence of OCS treatment and > 0.3 × 109/l in the previous year plus two or more asthma exacerbations requiring hospitalization or OCS treatment. In contrast to adults, among children with severe asthma, a smaller proportion of patients appear to have clear features of type 2 mucosal inflammation, including greater eosinophilia (43).
The recommended dosage of mepolizumab differs according to age: it is administered subcutaneously at a dose of 40 mg every 4 weeks in children aged 6–11 years and at a dose of 100 mg in children aged ≥ 12 years (40).
Safety, Adverse Effects, and Contraindications
Safety evaluations of mepolizumab have raised no significant concerns so far (44–46). The most common adverse effects (incidence ≥ 5%) of mepolizumab included headache, injection site reaction (e.g., pain, erythema, swelling, itching, and burning sensation), back pain, and fatigue (40, 41, 47).
Mepolizumab is not to be used in patients with helminth infection, and whenever found, it needs to be treated before the biological drug can be prescribed. Mepolizumab is also contraindicated in the event of a hypersensitivity reaction (40, 41).
Efficacy Data
Data about the efficacy of mepolizumab were collected from several promising clinical trials conducted on adults and children aged 12 years and older including Dose-Ranging Efficacy And Safety With Mepolizumab In Severe Asthma (DREAM) (44); Mepolizumab As Adjunctive Therapy In Patients With Severe Asthma (MENSA) (45); Steroid Reduction With Mepolizumab Study (SIRIUS) (46); and Mepolizumab Adjunctive Therapy In Subjects With Severe Eosinophilic Asthma (MUSCA) (48). Most recently, a multinational, non-randomized, open-label study was conducted on children aged 6 to 11 years by Gupta et al. (NCT02377427) (49, 50).
Asthma Control
The impact on health-related quality of life has been objectified through several validated questionnaires. The Asthma Control Questionnaires (ACQ-5 and ACQ-7) have been used in most studies (44–46, 49, 50)with a minimally clinically important difference (MCID) in total score (i.e., ≥ 0.5-point reduction from baseline) detected in half of the children in the first weeks (49) and up to 1 year of treatment (50). These results resemble those of adolescents and adults with MCID from baseline achieved from 42 to 59% of population studies (see Table 1). Best results were recorded around week 56, suggesting that treatment should not be discontinued before 1 year.
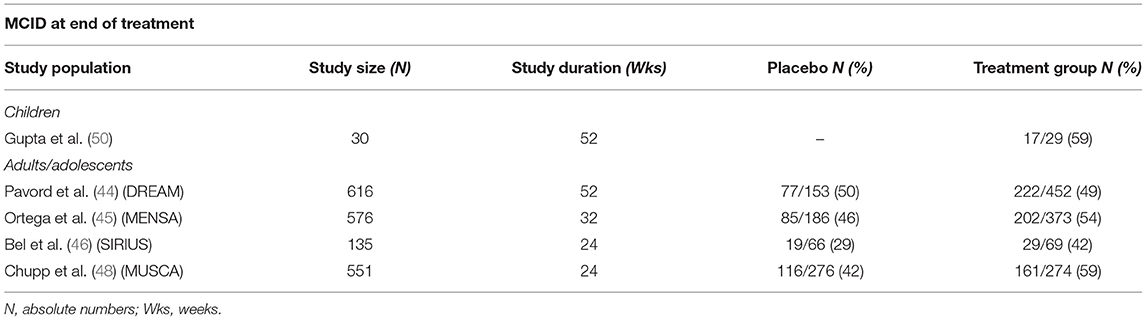
Table 1. Minimally clinically important difference (MCID) in children and adults/adolescents at end of treatment.
Another tool, the St. George's Respiratory Questionnaire (SGRQ), offers a wider perspective on treatment effectiveness and shows confirmed clinical improvement in all the domains (i.e., symptoms, activity, and impact) after 6 months of therapy compared to the placebo group (48). It is noteworthy that, despite a reduction in exacerbations risk in all the cited studies (see next paragraph), a clear subjective improvement has not always been reported as well (44).
Exacerbation Rate
Although the clinical experience with mepolizumab in pediatric asthma is often heterogeneous and with high rates of treatment discontinuation (51), this drug seems most effective in reducing exacerbations. A total of 10 children (28%) reported an exacerbation and more specifically only three patients had two exacerbations during the first 12 weeks of treatment (49). Another recently published case report showed improvement in asthma exacerbations in two children with eosinophilic nonallergic asthma treated with mepolizumab for 2 years (52). When compared to adolescents and adults, pediatric exacerbation rates were similar (53). As previously stated, a dissociation between clinical efficacy and symptoms has been noted since the first studies. We assume that the main effect of mepolizumab (i.e., reduction in blood eosinophils with modulation of eosinophilic airway inflammation) has more impact on exacerbations than asthma control perception. Thus, exacerbation risk and daily symptoms could be distinct features in patients with severe asthma.
Lung Function
Most studies detected a moderate effect on pulmonary function tests and only a few studies showed a significant improvement in forced expiratory volume during the first second (FEV1) compared to the placebo group (45, 48). As a matter of fact, a clear pattern of FEV1 changes from baseline has not been detected in children (49, 50). However, pediatric data are lacking and no studies including a placebo group are available. Baseline blood eosinophils diminish in 97% of patients treated with mepolizumab, but higher levels of blood eosinophils have not been defined as predictive factors for treatment response (54). The best response in terms of FEV1 improvement has been highlighted in patients with higher baseline sputum eosinophils (55). It can be hypothesized that patients with diffused airway eosinophilic inflammation present with worse baseline lung function. In this scenario, mepolizumab has a double rationale, targeting both local and systemic eosinophils.
Data are summarized in Table 2.
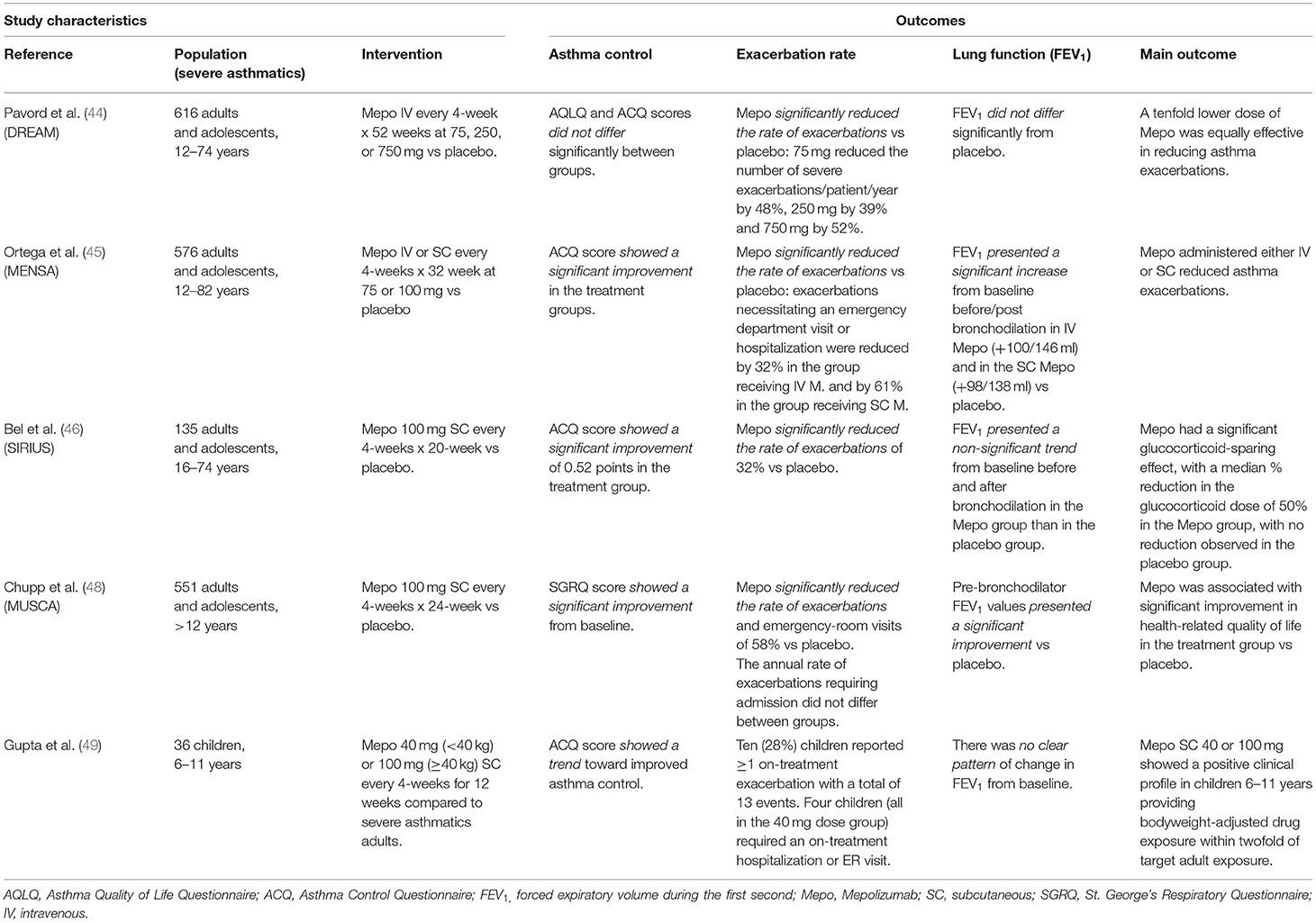
Table 2. Available studies on mepolizumab in adults, adolescents and children with summarized the main outcomes and results.
Recommendation on Discontinuation and Predictor Response Criteria
There is no validated recommendation on mepolizumab discontinuation, and GINA guidelines 2022 suggest an initial trial of at least 4 months (12). The National Institute for Health and Care Excellence (NICE) guidelines suggest reevaluating patients after 12 months and continuing the treatment if exacerbations have been reduced by ≥ 50% (56). However, several studies have reported that patients who discontinued mepolizumab showed an increase in Asthma Control Questionnaire-5 (ACQ-5) score, asthmatic attacks rate, and peripheral eosinophilia (57).
Currently, there are no standardized response criteria for mepolizumab. Blood eosinophil count and the increase in lung function are considered the main parameters of therapy response. Moreover, the improvement of quality of life and physical fitness and the reduction of exacerbations have also been reported as clinical predictor tools to evaluate treatment benefit (54, 58).
The most recent GINA guidelines published in 2022 suggested the following potential predictors of good response to anti-IL5 treatment: (1) higher blood eosinophils, (2) higher number of severe exacerbations in the previous year, (3) adult-onset asthma, (4) adult-onset asthma, (5) nasal polyposis, (6) maintenance oral corticosteroids, and (7) low lung function.
Other Indications in Children or Adolescents
To date, mepolizumab is also approved for the treatment of pediatric patients aged ≥12 years with hypereosinophilic syndrome (HES) at a dose of 300 mg every 4 weeks. The recommended patient population includes those with ≥ 6 month duration of HES without an identifiable non-hematologic secondary cause (40, 41).
Conclusions
From a clinical point of view, the main purpose of asthma treatment is to reduce symptoms and the recurrence of exacerbations. Mepolizumab proves its efficacy in the specific phenotype of asthmatic patients with intense eosinophilic airway inflammation and recurrent exacerbations. As often stated in children, we believe that FEV1 is not the most appropriate marker to detect mepolizumab's beneficial effects. Due to its capacity to target specific inflammatory type 2 mediators, mepolizumab represents a milestone in the application of personalized medicine. The prescription of mepolizumab for the treatment of severe asthma has expanded rapidly and this drug, currently used in adults, has also been registered for children, despite most of the scientific evidence in literature coming from adults. More data on efficacy in pediatric patients would be necessary to confirm its promising effects. Further clinical trials in the pediatric population are also important to prove long-term safety and the impact of this medication on the natural history of the disease. Finally, the identification of new biomarkers could be useful to predict real benefits from therapy with mepolizumab and to establish its optimal duration.
Author Contributions
RC and NU discussed the writing project. RC, NU, FPe, and OF wrote the manuscript with significant support from FPo, EP, and AO. NU and RC supervised the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Licari A, Manti S, Marseglia A, De Filippo M, De Sando E, Foiadelli T et al. Biologics in children with allergic diseases. Curr Pediatr Rev. (2020) 16:140–7. doi: 10.2174/1573396315666191029123822
2. Sánchez-Borges M, Martin BL, Muraro AM, Wood RA, Agache IO, Ansotegui IJ et al. The importance of allergic disease in public health: an iCAALL statement. World Allergy Organ J. (2018) 11:8. doi: 10.1186/s40413-018-0187-2
3. Lang A, Carlsen KH, Haaland G, Devulapalli CS, Munthe-Kaas M, Mowinckel P et al. Severe asthma in childhood: assessed in 10 year olds in a birth cohort study. Allergy. (2008) 63:1054–60. doi: 10.1111/j.1398-9995.2008.01672.x
4. O'Byrne PM, Pedersen S, Schatz M, Thoren A, Ekholm E, Carlsson LG et al. The poorly explored impact of uncontrolled asthma. Chest. (2013) 143:511–23. doi: 10.1378/chest.12-0412
5. Montalbano L, Ferrante G, Montella S, Cilluffo G, Di Marco A, Bozzetto S et al. Relationship between quality of life and behavioural disorders in children with persistent asthma: a Multiple Indicators Multiple Causes (MIMIC) model. Sci Rep. (2020) 10:6957. doi: 10.1038/s41598-020-62264-9
6. Licari A, Brambilla I, Marseglia A, De Filippo M, Paganelli V, Marseglia GL. Difficult vs. severe asthma: definition and limits of asthma control in the pediatric population. Front Pediatr. (2018) 6:170. doi: 10.3389/fped.2018.00170
7. Just J, Deschildre A, Lejeune S, Amat F. New perspectives of childhood asthma treatment with biologics. Pediatr Allergy Immunol. (2019) 30:159–171. doi: 10.1111/pai.13007
8. Votto M, De Filippo M, Licari A, Marseglia A, De Amici M, Marseglia GL. Biological therapies in children and adolescents with severe uncontrolled asthma: a practical review. Biologics. (2021) 15:133–42. doi: 10.2147/BTT.S252574
9. Morris TS, Autry EB, Kuhn R. The role of biologics in the management of asthma in the pediatric patient. J Pediatr Pharmacol Ther. (2021) 26:427–36. doi: 10.5863/1551-6776-26.5.427
10. De Keyser HH, Chipps B, Dinakar C. Biologics for asthma and allergic skin diseases in children. Pediatrics. (2021) 148:e2021054270. doi: 10.1542/peds.2021-054270
11. Ahmed H, Turner S. Severe asthma in children—a review of definitions, epidemiology, and treatment options in (2019). Pediatr Pulmonol. (2019) 54:778–787. doi: 10.1002/ppul.24317
12. Global Initiative for Asthma. Global Strategy for Asthma Management Prevention. (2022). Available online at: www.ginasthma.org.
13. Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European respiratory society/american thoracic society guideline. Eur Respir J. (2020) 55:1900588. doi: 10.1183/13993003.00588-2019
14. British Thoracic Society/Scottish intercollegiate guideline network. British Guideline on the Management of Asthma. (2016). Available online at: www.brit-thoracic.org.uk (accessed April 15, 2021).
15. National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Management of Asthma. (2007). Available online at: www.nhlbi.nih.gov (accessed August 31, 2021).
16. Porcaro F, Ullmann N, Allegorico A, Di Marco A, Cutrera R. Difficult and severe asthma in children. Children (Basel). (2020) 7:286. doi: 10.3390/children7120286
17. Bush A, Cutrera R, Piacentini G, Santamaria F, Ullmann N. Editorial: difficult and severe asthma in children. Front Pediatr. (2019) 7:205. doi: 10.3389/fped.2019.00205
18. Gillette C, Rockich-Winston N, Kuhn JA, Flesher S, Shepherd M. Inhaler technique in children with asthma: a systematic review. Acad Pediatr. (2016) 16:605–15. doi: 10.1016/j.acap.2016.04.006
19. Klok T, Kaptein AA, Brand PLP. Non-adherence in children with asthma reviewed: The need for improvement of asthma care and medical education. Pediatr Allergy Immunol. (2015) 26:197–205. doi: 10.1111/pai.12362
20. Licari A, Castagnoli R, Denicolò CF, Rossini L, Marseglia A, Marseglia GL. The nose and the lung: united airway disease? Front Pediatr. (2017) 5:44. doi: 10.3389/fped.2017.00044
21. Ullmann N, Mirra V, Di Marco A, Pavone M, Porcaro F, Negro V et al. Asthma: differential diagnosis and comorbidities. Front Pediatr. (2018) 6:276. doi: 10.3389/fped.2018.00276
22. Porsbjerg C, Menzies-Gow A. Co-morbidities in severe asthma: clinical impact and management. Respirology. (2017) 22:651–661. doi: 10.1111/resp.13026
23. Licari A, Brambilla I, De Filippo M, Poddighe D, Castagnoli R, Marseglia GL. The role of upper airway pathology as a co-morbidity in severe asthma. Expert Rev Respir Med. (2017) 11:855–65. doi: 10.1080/17476348.2017.1381564
24. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. (2014) 43:343–73. doi: 10.1183/09031936.00202013
25. De Groot EP. Breathing abnormalities in children with breathlessness. Paediatr Respir Rev. (2011) 12:83-87. doi: 10.1016/j.prrv.2010.09.003
26. Wright MF, Balfour-Lynn I. Habit-tic cough: presentation and outcome with simple reassurance. Pediatr Pulmonol. (2018) 53:512-516. Epub (2018). Jan 24. doi: 10.1002/ppul.23948
27. Ghirardo S, Mazzolai M, Di Marco A, Petreschi F, Ullmann N, Ciofi Degli Atti ML et al. Biological treatments ant target therapies for pediatric respiratory medicine: not only asthma. Front Pediatr. (2022) 10:837667. doi: 10.3389/fped.2022.837667
28. Maglione M, Poeta M, Santamaria F. New drugs for pediatric asthma. Front Pediatr. (2019) 6:432. doi: 10.3389/fped.2018.00432
29. Russo D, Di Filippo P, Attanasi M, Lizzi M, Di Pillo S, Chiarelli F. Biologic therapy and severe asthma in children. Biomedicines. (2021) 9:760. doi: 10.3390/biomedicines9070760
30. Licari A, Castagnoli R, Brambilla I, Marseglia A, Tosca MA, Marseglia GL et al. Asthma endotyping and biomarkers in childhood asthma. Pediatr Allergy Immunol Pulmonol. (2018) 31:44–55. doi: 10.1089/ped.2018.0886
31. Agustí A, Bafadhel M, Beasley R, Bel EH, Faner R, Gibson PG et al. Precision medicine in airway diseases: moving to clinical practice. Eur Respir J. (2017) 50:1701655. doi: 10.1183/13993003.01655-2017
32. Kuruvilla ME, Lee FEH, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. (2019) 56:219–33. doi: 10.1007/s12016-018-8712-1
33. Lötvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. (2011) 127:355–60. doi: 10.1016/j.jaci.2010.11.037
34. Samitas K, Zervas E, Gaga M. T2-low asthma: current approach to diagnosis and therapy. Curr Opin Pulm Med. (2017) 23:48–55. doi: 10.1097/MCP.0000000000000342
35. Stokes JR, Casale TB. Characterization of asthma endotypes: implications for therapy. Ann Allergy Asthma Immunol. (2016) 117:121–5. doi: 10.1016/j.anai.2016.05.016
36. Eyerich S, Metz M, Bossios A, Eyerich K. New biological treatments for asthma and skin allergies. Allergy. (2020) Mar;75(3):546-560. doi: 10.1111/all.14027
37. Fahy JV. Type 2 inflammation in asthma-present in most, absent in many. Nat Rev Immunol. (2015) 15:57–65. doi: 10.1038/nri3786
38. Sehmi R, Smith SG, Kjarsgaard M, Radford K, Boulet LP, Lemiere C et al. Role of local eosinophilopoietic processes in the development of airway eosinophilia in prednisone-dependent severe asthma. Clin Exp Allergy. (2016) 46:793–802. doi: 10.1111/cea.12695
39. Schoettler N, Strek ME. Recent advances in severe asthma. Chest. (2020) 157:516–28. doi: 10.1016/j.chest.2019.10.009
40. Food and Drug Administration. Nucala®. Avaiable online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf (accessed February 22, 2021).
41. GSK European Commission. Approves Nucala (mepolizumab) for the treatment of children with severe asthma. Available online at: https://www.gsk.com/en-gb/media/press-releases/european-commission-approves-nucala-mepolizumab-for-the-treatment-of-children-with-severe-asthma/ (accessed February 22, 2021).
42. Pelaia C, Vatrella A, Busceti MT, Gallelli L, Terracciano R, Savino R et al. Severe eosinophilic asthma: from the pathogenic role of interleukin-5 to the therapeutic action of mepolizumab. Drug Des Devel Ther. (2017) 11:3137–44. doi: 10.2147/DDDT.S150656
43. Comberiati P, McCormack K, Malka-Rais J, Spahn J.D. Proportion of severe asthma patients eligible for mepolizumab therapy by age and age of onset of asthma. J Allerg Clinic Immunol Pract. (2019) 19:2689–96. doi: 10.1016/j.jaip.2019.05.053
44. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. (2012) 380:651–9. doi: 10.1016/S0140-6736(12)60988-X
45. Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. (2014) 371:1198–207. doi: 10.1056/NEJMoa1403290
46. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. (2014) 371:1189–97. doi: 10.1056/NEJMoa1403291
47. Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. (2007) 176:1062–71. doi: 10.1164/rccm.200701-085OC
48. Chupp GL, Bradford ES, Albers FC, Bratton DJ, Wang-Jairaj J, Nelsen LM et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. (2017) 5:390–400. doi: 10.1016/S2213-2600(17)30125-X
49. Gupta A, Ikeda M, Geng B, Azmi J, Price RG, Bradford ES et al. Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype. J Allergy Clin Immunol. (2019) 144:1136–1342. doi: 10.1016/j.jaci.2019.08.005
50. Gupta A, Pouliquen I, Austin D, Price RG, Kempsford R, Steinfeld J et al. Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma. Pediatr Pulmonol. (2019) 54:1957–67. doi: 10.1002/ppul.24508
51. Wetzke M, Funken D, Ahrens F.O, Gappa M, Hansen G, Koerner-Rottberg C, et al. Mepolizumab treatment in severe pediatric asthma: first multicentric real-world data. Klin Pediatr. (2022) 22:2234. doi: 10.1055/a-1717-2234
52. Tosca M.A., Girosi D, Sacco O, Bernardini R, Ciprandi G. Steroid-sparing effect of mepolizumab in children with severe eosinophilic nonallergic asthma. Allergol Immunopathol (Madr). (2021) 49:113–6. doi: 10.15586/aei.v49i5.466
53. Gupta A, Steinfeld J, Price R, Azmi J, Bradford E, Yancey S. Mepolizumab for severe eosinophilic asthma: a comparison of efficacy in children, adolescents, and adults. Eur Respir J. (2018) 51:PA5447. doi: 10.1183/13993003.congress-2018.PA5447
54. Drick N, Seeliger B, Welte T, Fuge J, Suhling H. Anti-IL-5 therapy in patients with severe eosinophilic asthma—clinical efficacy and possible criteria for treatment response. BMC Pulm Med. (2018) 18:119. doi: 10.1186/s12890-018-0689-2
55. Schleich F, Graff S, Nekoee H, Moermans C, Henket M, Sanchez C et al. Real-word experience with mepolizumab: does it deliver what it has promised? Clin Exp Allergy. (2020) 50:687–95. doi: 10.1111/cea.13601
56. Mepolizumab for Treating Severe Refractory Eosinophilic Asthma. NICE Technology Appraisal Guidance [TA431]. Available online at: https://www.nice.org.uk/guidance/ta431 (accessed April 20, 2021).
57. Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. (2017) 9:CD010834. doi: 10.1002/14651858.CD010834.pub3
Keywords: asthma, biologics, treatment, children, adolescents, mepolizumab, anti-interleukin-5, antibodies
Citation: Ullmann N, Peri F, Florio O, Porcaro F, Profeti E, Onofri A and Cutrera R (2022) Severe Pediatric Asthma Therapy: Mepolizumab. Front. Pediatr. 10:920066. doi: 10.3389/fped.2022.920066
Received: 14 April 2022; Accepted: 20 May 2022;
Published: 01 July 2022.
Edited by:
Mario Barreto, Sapienza University of Rome, ItalyReviewed by:
Garry M. Walsh, University of Aberdeen, United KingdomKestutis Malakauskas, Lithuanian University of Health Sciences, Lithuania
Zorica Momcilo Zivkovic, University Hospital Center Dr Dragiša Mišović, Serbia
Copyright © 2022 Ullmann, Peri, Florio, Porcaro, Profeti, Onofri and Cutrera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Ullmann, bmljb2xhLnVsbG1hbm5Ab3BiZy5uZXQ=
 Nicola Ullmann
Nicola Ullmann Francesca Peri
Francesca Peri Olivia Florio
Olivia Florio Federica Porcaro
Federica Porcaro Elisa Profeti1
Elisa Profeti1 Alessandro Onofri
Alessandro Onofri Renato Cutrera
Renato Cutrera