- Department of Pulmonary and Critical Care Medicine, Peking University People’s Hospital, Beijing, China
Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD) is a rare life-threatening disorder that can occur during childhood. All children with ROHHAD develop alveolar hypoventilation during wakefulness and sleep. The key treatment for these patients is the optimization of oxygenation and ventilation. Here, we report the case of a 5-year-old girl with suspected ROHHAD, with rapid weight gain, breathing cessation, decreased height, hypoventilation, central hypothyroidism, hyperprolactinemia, and absolute deficiency of growth hormone, and negative PHOX2B sequencing results. The presentation met the diagnostic criteria for ROHHAD syndrome. During the 5-year follow-up, she presented with progressive deterioration of the function of the hypothalamus and respiratory center, hypoxemia (PO2 < 60 mmHg), and hypercapnia [transcutaneous carbon dioxide (TcPCO2) > 70 mmHg] during the first two cycles of N3 sleep with a poor response to ventilatory support. Early diagnosis and application of non-invasive positive pressure ventilation during sleep can improve the quality of life and outcomes of patients with ROHHAD, and polysomnography and TcPCO2 should be repeated every 3–6 months to follow the progress and regulate ventilator support. Multidisciplinary care is crucial for the successful management of these patients.
Introduction
Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD) syndrome is a rare disorder commonly diagnosed in childhood that features disrupted respiratory control, autonomic nervous system regulation, and hypothalamic function (1). Young patients exhibit breathing abnormalities, snoring, pauses in breathing, and shallow breathing during sleep. Formerly called late onset-central hypoventilation syndrome (LO-CHS), it was recognized as a special type of congenital central hypoventilation syndrome until 2007 when Ize-Ludlow named it “ROHHAD” because of the existence of other features such as dramatic and rapid weight gain, central hypoventilation, hypothalamic dysfunction, abnormality of the endocrine system and negative PHOX2B sequencing results (2–4). No specific cause has been identified for ROHHAD, and etiological studies are still underway (2, 5, 6).
In this case report, we describe a 5-year-old Chinese girl with ROHHAD syndrome who underwent treatment over time to emphasize the importance of early diagnosis and nocturnal ventilatory support in such patients.
Case report
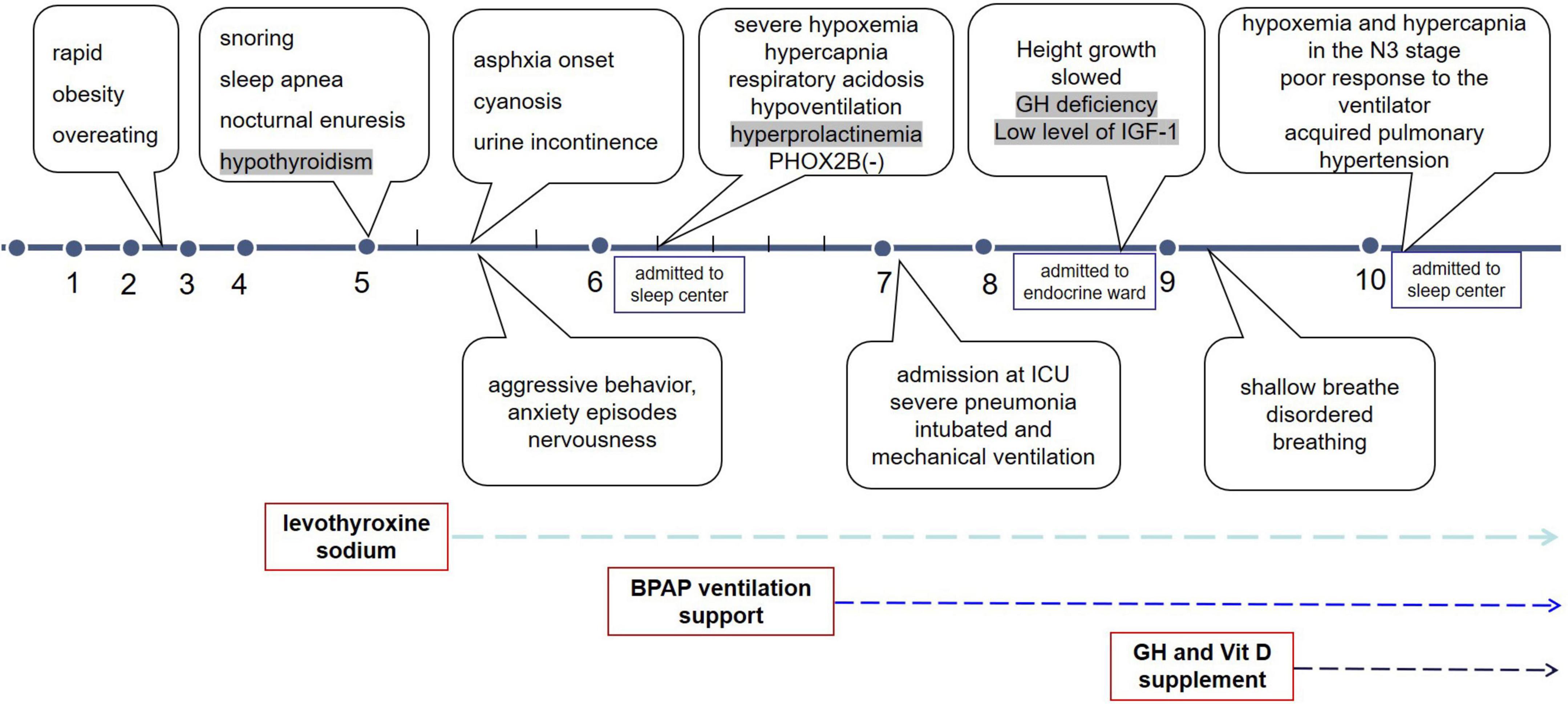
The girl was admitted at the age of 5 years and 6 months with progressive abnormal weight gain for 3 years along with snoring, sleep apnea, and cyanosis for 6 months. The patient was born at full-term via cesarean section, with a 3.55 kg birth weight and 51 cm length, which are normal parameters according to Chinese neonatal birth conditions. The neonatal and infancy periods were unremarkable. Since 2.5 years of age, she has demonstrated rapid weight gain due to overeating, and at age 5, she underwent a sleep study showing gasping, snoring, breathing cessation during sleep, and nocturnal enuresis. At that time, the patient was diagnosed with hypothyroidism and treated with levothyroxine sodium (12.5 mg). She developed behavioral changes, including aggressive behavior, episodes of anxiety, and nervousness. She showed no improvement with thyroid hormone replacement therapy.
On physical examination, she was obese with a weight of 30 kg and height of 122.5 cm; the results of other physical examinations were unremarkable. Laboratory tests showed severe hypoxia (SaO2 91%, oxygen 4 L/min) and hypercapnia (PCO2 50 mmHg) when awake. During sleep, the hypoxia became even more pronounced (minimum SaO2 60%, 4–5 L/min oxygen) without arousal reactions. Table 1 shows low free triiodothyronine (T3), low thyroxine (T4), and low thyroid-stimulating hormone (TSH) levels with a daily supplement of 12.5 mg levothyroxine, indicating central hypothyroidism. Hyperprolactinemia was also observed. Laryngoscopy, chest radiography, echocardiography, and brain magnetic resonance imaging of the hypothalamus and pituitary gland were unremarkable. Pulmonary hypertension was not observed. As alveolar hypoventilation was highly suspected, nocturnal polysomnography (PSG) with transcutaneous carbon dioxide pressure (TcPCO2) was performed. No obstructive or central apnea-hypopnea events were found, and the apnea-hypopnea index was 0 events/h. Due to severe hypoxemia (SpO2 60%) and hypercapnia (PCO2 75 mm Hg), the PSG had to be interrupted and restarted with bi-level positive airway pressure (BPAP) titration via a nasal mask (Figure 1). Normal tidal volume was guaranteed in spontaneous/timed mode with inspiratory positive airway pressure (IPAP) of 17 cmH2O, expiratory positive airway pressure (EPAP) of 5 cmH2O, and a frequency of 18 times per minute.

Figure 1. Nocturnal pulse oximetry, TcPCO2 evolution, and sleep stage presentation in PSG, starting at 1 a.m. of the BPAP pressure titration. Hypoventilation was seen (SpO2 between 60 and 98%; PCO2 maximum 75 mmHg) for 4 h during the night, accompanied by sleep structure disruption (recurrent arousal and inability to deep sleep). Sleep stage: W, wake; R, REM stage, N1, NREM stage 1; N2, NREM stage 2; N3, NREM stage 3.
Molecular analysis for PHOX2B was inconsistent with congenital central hypoventilation syndrome.
The presence of rapid and early onset obesity, alveolar hypoventilation, hyperprolactinemia, central hypothyroidism, behavioral disorders, and absence of PHOX2B gene mutations is consistent with ROHHAD syndrome. Nocturnal BPAP ventilation and daily levothyroxine supplementation were prescribed to the patient during long-term follow-up.
At the age of 7 years, the girl was intubated in the emergency room because of severe pneumonia and was under mechanical ventilation for 45 days. When she was 8 years old, her height growth slowed, while her weight gain did not reach 10 kg per year. The growth curves of the patient are shown in Figure 2. As illustrated in Table 1, further endocrinological investigations confirmed an absolute deficiency of growth hormone (GH) and low-level insulin-like growth factor-1. A negative result of the GH provocation test indicated dysfunction of the hypothalamic-pituitary axis. No evidence of adrenal insufficiency or diabetes insipidus was found.
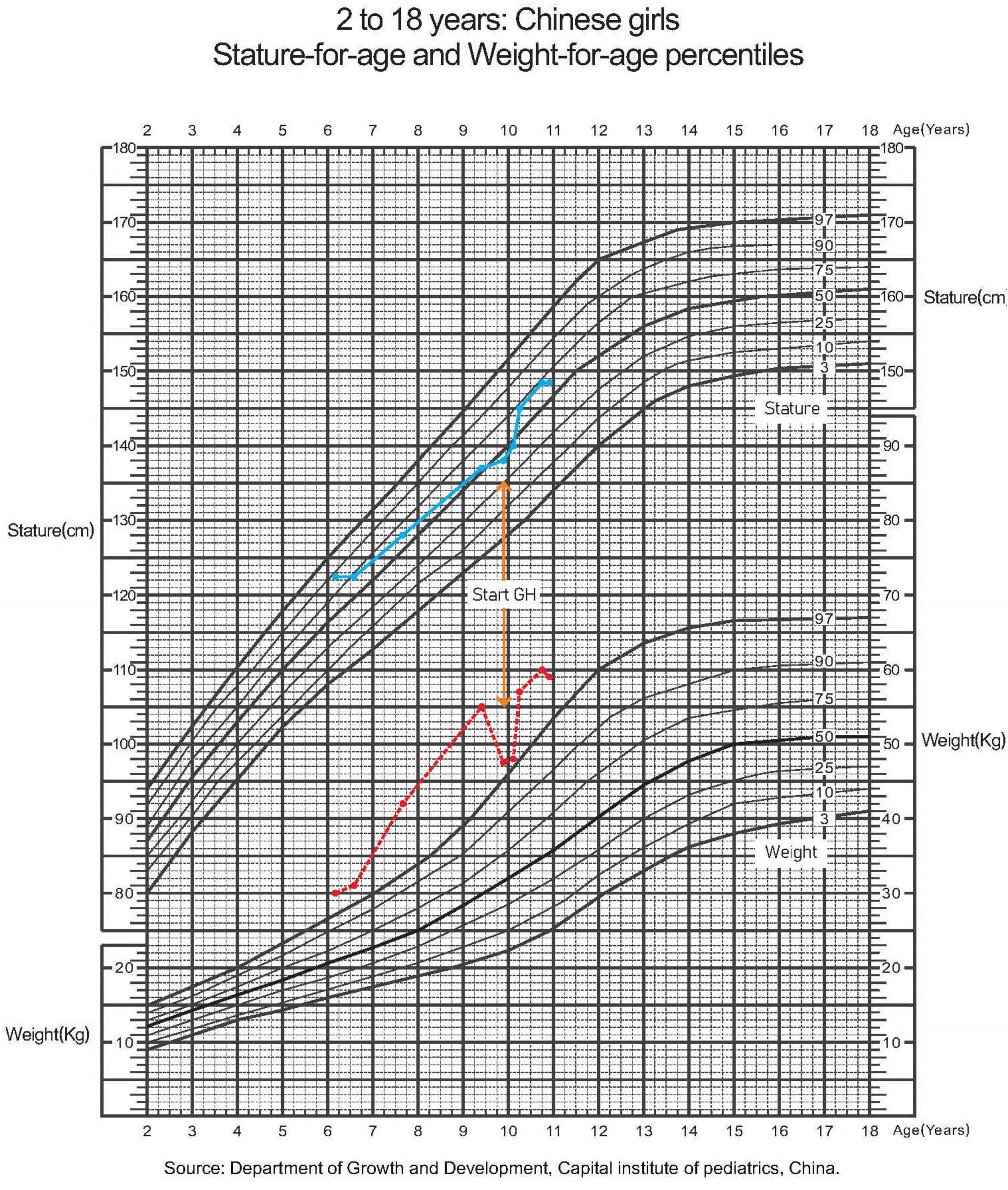
Figure 2. A growth curve showing stature and weight for age. Dotted line in red, weight; solid line in blue, height.
Her height increased by 2 cm and her weight increased by 0.5 kg in 2 months after she received regular subcutaneous injections of GH and vitamin D prescribed by an endocrinologist.
Her liver and kidney function, electrolytes, complete blood count, and urine test results were normal. Ultrasonography findings of the breast and pelvis were normal. Chest and abdominal computed tomography scans showed no evidence of neural crest tumors.
When she was 10 years and 2 months old, she visited our sleep center again with the complaint of “forgetting to breathe” and visible disordered breathing (weak and strong alternating episodes) even when she was awake. Cyanosis and urinary incontinence were also observed. A timeline of the symptom and progress was shown in Figure 3. Nocturnal SpO2 on ventilation support showed a transient drop to 70% during the first half of the night, before increasing to 95% by late midnight. An arterial blood gas test on room air revealed a pH of 7.36, PaCO2 of 55 mmHg, PaO2 of 72 mmHg, and HCO3– of 31.2 mmol/L. On admission, acquired pulmonary hypertension was detected using echocardiography. Non-invasive positive pressure ventilation (NPPV) titration was performed at night with simultaneous PSG and TcPCO2, with IPAP/EPAP set to 19/6 cmH2O and frequency set to 20 times/min. PSG studies found that the patient could not trigger an IPAP/EPAP cycle during slow-wave sleep; therefore, the backup rate was adjusted to be relatively high (20 times per minute). We set the backup rate to 20 to ensure reliable trigger ventilation and adjusted the IPAP/EPAP to increase tidal volume. Airflow, tidal volume and leaks were monitored. No significant leakages were observed. The pressure support gradually increased with the tidal volume, SpO2, and TcPCO2 improved. The final titration pressure setting was IPAP/EPAP 21/8, supplemental oxygen (2 L/min), the SpO2 reached 95%, and TcPCO2 55–65 mmHg (similar to when she was awake). The patient tolerated the procedure well and did not experience nocturia. However, hypoxemia (SpO2 < 90%) and hypercapnia (TcPCO2 > 70 mmHg) still appeared during stage N3 of the first two sleep cycles (Supplementary Figure 1) without much change in ventilator settings. The progression of the disease was presumed to be related to further deterioration of the hypothalamus and dysfunction of the respiratory center.
Discussion
In this case, we report the management of a 5-year-old girl with rapid and early onset obesity, alveolar hypoventilation, hypothalamic dysfunction (hyperprolactinemia, central hypothyroidism, GH deficiency), and behavioral disorders (irritability and aggression) in her first visit, presenting with convincing evidence of a ROHHAD diagnosis. This is the first reported case of ROHHAD in the Chinese population. ROHHAD syndrome is a life-threatening disease in children, with death occurring at an average age of approximately 10 years (3). Central hypoventilation caused by a deficit in breathing control is the most lethal feature of ROHHAD and carries a high risk of cardiorespiratory arrest, even during treatment. A systematic review of the clinical manifestations of ROHHAD by Lee et al. involved 46 studies with 158 patients; the calculated median age of onset was 4 years, and the prevalence of the disease in girls was twice that in boys (7). The diagnosis of ROHHAD was based on a cooperative consultation with multidisciplinary experts. Diagnosis is often missed or delayed, as in our case. Although the girl presented with rapid weight gain as early as 2 years of age, she was diagnosed at the age of 5. However, the effects of this delay on brain growth and development remain unknown.
Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome may be clinically heterogeneous. Most commonly, the patient initially presents with dramatic and rapid weight gain associated with hyperphagia and varying degrees of hypoventilation and autonomic nervous system dysregulation. Hypothalamic dysfunction is manifested by high prolactin levels, low thyroid hormone levels, GH deficiency, and early or late puberty among other abnormalities. Dhondt et al. also reported a case of ROHHAD with a hypocretin-1 deficiency that displayed the classic features of narcolepsy with cataplexy, which broadened hypothalamus dysfunction and expands the phenotype of ROHHAD (8). A subset of patients (52.1%) with ROHHAD will develop neuroendocrine tumors, such as ganglioneuromas or ganglioneuroblastomas. Some researchers have recommended that this phenotype be termed ROHHADNET (7, 9).
Breathing abnormalities are present in almost all patients (2). Children may snore or show breathing pauses during sleep, without the perception of dyspnea, which can be easily ignored. All children with ROHHAD develop alveolar hypoventilation with very shallow breathing during sleep (both nap and night). In more severely affected patients with ROHHAD, hypoventilation is visible when they are not only asleep but also awake. In patients with ROHHAD, even if breathing is sufficient during the daytime, there is a lack of normal responsiveness to aggravated hypoxia and hypercapnia during sleep and wakefulness. Carroll et al. found that patients with ROHHAD showed a diminished tidal volume and inspiratory drive response to a hypoxic hypercapnia test, combined with a lack of behavioral perception of asphyxia, indicating a blunted chemosensory response (10). Our patient showed a progression to central hypoventilation. When challenged by a respiratory infection or anesthesia, these patients can suddenly develop respiratory failure and require mechanical ventilation (11, 12); as in our case, intubation and mechanical ventilation were required during an episode of pneumonia. According to published articles, the proportion of patients with ROHHAD receiving mechanical ventilation is 42–47%, and of those, NPPV is required in 53% of patients (2, 7). In a review of 51 patients with ROHHAD, 69% required artificial ventilation via tracheostomy, with a mean age of 3.8 years, and 31% required 24-h NPPV support (13). Recently, Reppucci et al. reviewed six children with suspected ROHHAD with a median age of 7.2 years of nocturnal hypoventilation onset; five of six patients accepted BPAP without tracheostomy, which is similar to our study results (14). Historically, invasive ventilation through tracheostomy has been the first line of treatment. However, with the development of sleep medicine, non-invasive ventilation with both frequency and volume backup mode has become more available, and treatment during sleep can improve wake-time ventilation or delay the progression of the disease. Undiagnosed or untreated children with ROHHAD may present with complications of chronic hypoventilation, including pulmonary hypertension, cor pulmonale, seizures, or developmental delays. Once a diagnosis is suspected, a full respiratory assessment is required during wakefulness and sleep, and BPAP titration may be performed. Although the patient in this case report underwent several PSG tests and BPAP titrations, chronic hypoventilation was difficult to completely eliminate because of the inconsistent use of BPAP and blunted chemosensory response.
In the present case report, hypoventilation due to ROHHAD was severe during NREM sleep, especially in stage N3 of the first two sleep cycles, showing frequent and significant oxygen desaturation and CO2 retention. This phenomenon is in contrast to other sleep-related breathing disorders, such as obstructive sleep apnea, and other sleep-related hypoventilations, which are often related to REM (15). Some studies have reported that hypoventilation in CHS/LO-CHS is more apparent in NREM sleep than in REM sleep, with a smaller increase in respiratory rate, greater decrease in minute ventilation, infrequent arousal, and frequent central sleep apnea during NREM sleep than during REM sleep (16, 17). Goldbart et al. found that children with ROHHAD syndrome have suppressed slow-wave activity power and shallower slow-wave activity slopes during the first two sleep cycles, which may explain the hypoventilation in stage N3 during the first half of the night (18). In addition, previous reports have indicated that hypothyroidism contributes to central hypoventilation, and obvious hypoxic and hypercarbia disorders are observed in untreated thyroid dysfunction (19). Therefore, for patients with ROHHAD, continuous hypothyroidism treatment and regular evaluation of thyroid function are required. Annual echocardiography, ambulatory cardiac monitoring, neurocognitive evaluations, urine tests, and imaging of neural crest tumors are also needed. To ensure optimal oxygenation and ventilation, continued patient education is needed to improve PAP adherence and PSG must be repeated every 3–6 months during follow-up. Anesthesia is challenging in patients with ROHHAD. For anesthetic management of patients with ROHHAD, Chandrakantan and Poulton reported the use of short-acting anesthetics with minimal respiratory effects combined with anxiolytics. Continuous electrocardiography, SpO2, and end-tidal carbon dioxide monitoring should be performed even with conscious sedation in brief procedures (20).
The etiopathogenesis of ROHHAD is unknown; however, an immune-mediated process is suspected in some cases. Several reports of immunoglobin therapy or immunosuppressive drugs (rituximab and cyclophosphamide) in patients with ROHHAD report a transient or partial improvement, but the target and long-term efficacy are unclear (21–23). These therapies were not prescribed to our patients as there was insufficient evidence of their value.
In conclusion, we described the presentation and chronic management of a patient with ROHHAD with non-invasive mechanical ventilation at the time of diagnosis. We believe that early recognition and application of NPPV during sleep is vital to slow the deterioration of respiratory function and improve the quality of life. Multidisciplinary care is crucial for the successful diagnosis and management of these patients.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Review Committee of Peking University. Written informed consent to participate in this case study was provided by the participants’ legal guardian.
Author contributions
XD, ZG, and FH contributed to the study concept and design and reviewed the manuscript. RZ collected the medical information of the participant and completed draft manuscript. XD was the guarantor of the manuscript and took responsibility for the integrity of the data. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.919921/full#supplementary-material
References
1. Patwari PP, Wolfe LF. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation: review and update. Curr Opin Pediatr. (2014) 26:487–92. doi: 10.1097/mop.0000000000000118
2. Ize-Ludlow D, Gray JA, Sperling MA, Berry-Kravis EM, Milunsky JM, Farooqi IS, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation presenting in childhood. Pediatrics. (2007) 120:179–88. doi: 10.1542/peds.2006-3324
3. Katz ES, McGrath S, Marcus CL. Late-onset central hypoventilation with hypothalamic dysfunction: a distinct clinical syndrome. Pediatr Pulmonol. (2000) 29:62–8.
4. Proulx F, Weber ML, Collu R, Lelievre M, Larbrisseau A, Delisle M, et al. Hypothalamic dysfunction in a child: a distinct syndrome? Report of a case and review of the literature. Eur J Pediatr. (1993) 152:526–9. doi: 10.1007/bf01955066
5. Thaker VV, Esteves KM, Towne MC, Brownstein CA, James PM, Crowley L, et al. Whole exome sequencing identifies RAI1 mutation in a morbidly obese child diagnosed with ROHHAD syndrome. J Clin Endocrinol Metab. (2015) 100:1723–30. doi: 10.1210/jc.2014-4215
6. Rand CM, Patwari PP, Rodikova EA, Zhou L, Berry-Kravis EM, Wilson RJ, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation: analysis of hypothalamic and autonomic candidate genes. Pediatr Res. (2011) 70:375–8.
7. Lee JM, Shin J, Kim S, Gee HY, Lee JS, Cha DH, et al. Rapid-onset obesity with hypoventilation, hypothalamic, autonomic dysregulation, and neuroendocrine tumors (ROHHADNET) syndrome: a systematic review. Biomed Res Int. (2018) 2018:1–17. doi: 10.1155/2018/1250721
8. Dhondt K, Verloo P, Verhelst H, Van Coster R, Overeem S. Hypocretin-1 deficiency in a girl with ROHHAD syndrome. Pediatr. (2013) 132:788–92. doi: 10.1542/peds.2012-3225
9. Bougneres P, Pantalone L, Linglart A, Rothenbuhler A, Le Stunff C. Endocrine manifestations of the rapid-onset obesity with hypoventilation, hypothalamic, autonomic dysregulation, and neural tumor syndrome in childhood. J Clin Endocrinol Metab. (2008) 93:3971–80. doi: 10.1210/jc.2008-0238
10. Carroll MS, Patwari PP, Kenny AS, Brogadir CD, Stewart TM, Weese-Mayer DE. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD): response to ventilatory challenges. Pediatr Pulmonol. (2015) 50:1336–45. doi: 10.1002/ppul.23164
11. Kocaay P, Siklar Z, Camtosun E, Kendirli T, Berberoglu M. ROHHAD syndrome: reasons for diagnostic difficulties in obesity. J Clin Res Pediatr Endocrinol. (2014) 6:254–7. doi: 10.4274/Jcrpe.1432
12. Özcan G, Özsu E, Şiklar Z, Çobanoğlu N. A rare cause of sleep-disordered breathing: ROHHAD syndrome. Front Pediatr. (2020) 8:573227. doi: 10.3389/fped.2020.573227
13. Chew HB, Ngu LH, Keng WT. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation and autonomic dysregulation (ROHHAD): a case with additional features and review of the literature. BMJ Case Rep. (2011) 2011:bcr0220102706. doi: 10.1136/bcr.02.2010.2706
14. Reppucci D, Hamilton J, Yeh EA, Katz S, Al-Saleh S, Narang I. ROHHAD syndrome and evolution of sleep disordered breathing. Orphanet J Rare Dis. (2016) 11:106. doi: 10.1186/s13023-016-0484-1
15. Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. (2000) 162:682–6. doi: 10.1164/ajrccm.162.2.9908058
16. Huang J, Colrain IM, Panitch HB, Tapia IE, Schwartz MS, Samuel J, et al. Effect of sleep stage on breathing in children with central hypoventilation. J Appl Physiol. (2008) 105:44–53. doi: 10.1152/japplphysiol.01269.2007
17. Fraigne JJ, Orem JM. Phasic motor activity of respiratory and non-respiratory muscles in REM sleep. Sleep. (2011) 34:425–34. doi: 10.1093/sleep/34.4.425
18. Goldbart AD, Arazi A, Golan-Tripto I, Levinsky Y, Scheuerman O, Tarasiuk A. Altered slow-wave sleep activity in children with rapid-onset obesity with hypothalamic dysregulation, hypoventilation, and autonomic dysregulation syndrome. J Clin Sleep Med. (2020) 16:1731–5. doi: 10.5664/jcsm.8678
19. Milla CE, Zirbes J. Pulmonary complications of endocrine and metabolic disorders. Pediatr Respir Rev. (2012) 13:23–8. doi: 10.1016/j.prrv.2011.01.004
20. Chandrakantan A, Poulton TJ. Anesthetic considerations for rapid-onset obesity, hypoventilation, hypothalamic dysfunction, and autonomic dysfunction (ROHHAD) syndrome in children. Paediatr Anaesth. (2013) 23:28–32. doi: 10.1111/j.1460-9592.2012.03924.x
21. Huppke P, Heise A, Rostasy K, Huppke B, Gärtner J. Immunoglobulin therapy in idiopathic hypothalamic dysfunction. Pediatr Neurol. (2009) 41:232–4. doi: 10.1016/j.pediatrneurol.2009.03.017
22. Paz-Priel I, Cooke DW, Chen AR. Cyclophosphamide for rapid-onset obesity, hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome. J Pediatr. (2011) 158:337–9. doi: 10.1016/j.jpeds.2010.07.006
23. Marpuri I, Ra E, Naguib MN, Vidmar AP. Weight management in youth with rapid-onset obesity with hypothalamic dysregulation, hypoventilation, autonomic dysregulation, and neural crest tumor (ROHHAD-NET): literature search and case report. J Pediatr Endocrinol Metab. (2021) 35:543–8. doi: 10.1515/jpem-2021-0600
Keywords: ROHHAD syndrome, hypoventilation, obesity, hypothalamic dysfunction, noninvasive positive pressure ventilation
Citation: Zhao R, Dong X, Gao Z and Han F (2022) Case Report: Considerations of nocturnal ventilator support in ROHHAD syndrome in chronic care of childhood central hypoventilation with hypothalamus dysfunction. Front. Pediatr. 10:919921. doi: 10.3389/fped.2022.919921
Received: 14 April 2022; Accepted: 04 August 2022;
Published: 31 August 2022.
Edited by:
Ron Rubenstein, Washington University in St. Louis, United StatesReviewed by:
Ajay Kasi, Emory University, United StatesSalvador Ibanez-Mico, Hospital Universitario Virgen de la Arrixaca, Spain
Copyright © 2022 Zhao, Dong, Gao and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaosong Dong, ZG9uZ3hpYW9zb25nQHBrdXBoLmVkdS5jbg==; Fang Han, aGFuZmFuZzFAaG90bWFpbC5jb20=
 Rui Zhao
Rui Zhao Xiaosong Dong
Xiaosong Dong Zhancheng Gao
Zhancheng Gao Fang Han
Fang Han